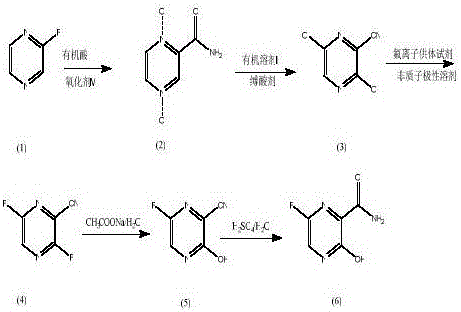
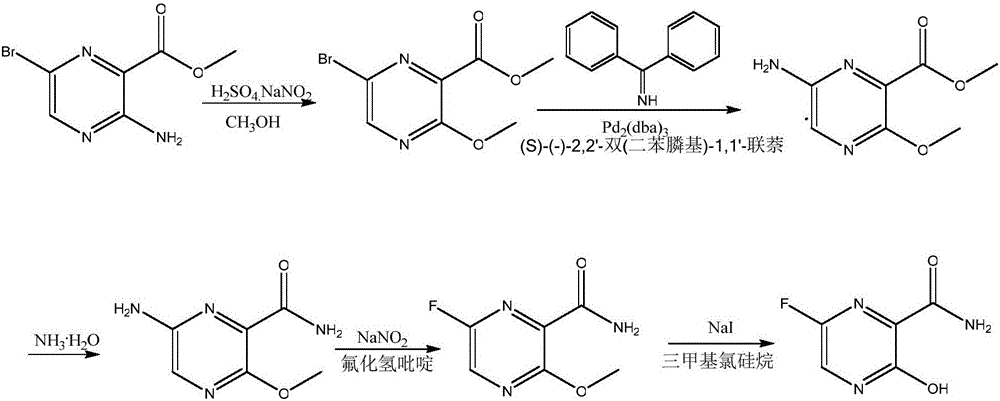
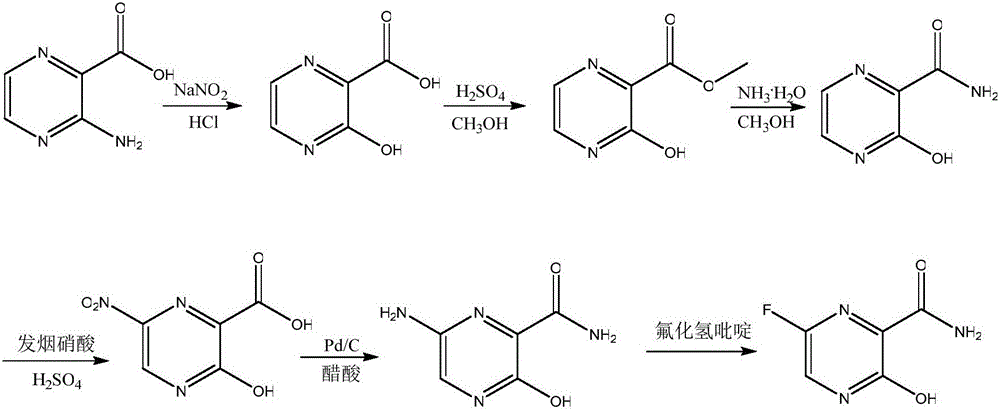
Favipiravir synthesis process
A technology of synthesis process and process route, applied in the field of medicine and chemical industry, can solve the problems of troublesome industrial processing, cumbersome operation, low yield, etc., and achieve the effects of good industrialization value, simple synthesis process and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1) Preparation of 1.4-dioxypyrazinamide (2): Control the temperature at -5 to 5°C, mix 5.25 g of 2-cyanopyrazine (1) with 29.95 g of glacial acetic acid and 45.30 g of 30% hydrogen peroxide, Heating to 95°C, reflux reaction for 22h, TLC showed no raw material, 40°C reduced pressure rotary evaporation to remove solvent, added 15ml water, reduced pressure rotary evaporation, repeated several times to remove glacial acetic acid, added 15ml water, added hot chloroform extraction ( 15ml*3), the water layer was spin-dried under reduced pressure, recrystallized with 90% methanol, filtered, and the filter cake was vacuum-dried to obtain 4.45g of white powder 1,4-dioxopyrazinamide (2), with a melting point greater than 300°C and a yield of 57.43%.
[0033] 2) Preparation of 3,6-dichloro-2-cyanopyrazine (3): 6.20 g of 1.4-dioxypyrazinamide (2) was added to 24.53 g of redistilled phosphorus oxychloride and mixed evenly, Stir at 50°C for 50min, heat up to 70°C for 1h, cool to room...
Embodiment 2
[0038]1) Preparation of 1.4-dioxypyrazinamide (2): control the temperature at 0-5° C., disperse 4.92 g of pyrazinamide (1) in 5 ml of ethyl acetate, and mix m-chloroperoxybenzoic acid (85%) with 17.92 g was dissolved in 35ml of ethyl acetate, washed once with saturated brine, the organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was added dropwise to the pyrazinamide ethyl acetate solution, and reacted at room temperature for 24 hours after the dropwise addition, TLC After the reaction is shown, filter, wash the filter cake with ethyl acetate (10ml*3), recrystallize the filter cake with 90% methanol, and vacuum dry to obtain 5.23g of white powder 1,4-dioxypyrazinamide (2) , the yield is 84.37%, and the melting point is greater than 300°C.
[0039] 2) Preparation of 3,6-dichloro-2-cyanopyrazine (3): Mix 6.20 g of 1.4-dioxypyrazinamide (2) with 10 mL of chlorobenzene and 22.08 g of phosphorus oxychloride, and heat up to Stir at 50°C for 50min, he...
Embodiment 3
[0044] 1) Preparation of 1.4-dioxypyrazinamide (2): Control the temperature at 0-5° C., mix 2.46 g of pyrazinamide (1) with 12 g of 98% trifluoroacetic acid and 18 g of 30% hydrogen peroxide, and heat up to 95 ℃, reflux reaction for 22 hours, TLC showed no raw material, 40 ℃ vacuum rotary evaporation to remove the solvent, add 5ml of water, add hot chloroform after vacuum rotary evaporation (10ml*3), the water layer was spin-dried under reduced pressure, 90% Methanol was recrystallized, filtered, and the filter cake was vacuum-dried to obtain 2.12 g of white powdery 1,4-dioxypyrazinamide (2), with a melting point greater than 300° C. and a yield of 68.40%.
[0045] 2) Preparation of 3,6-dichloro-2-cyanopyrazine (3): Mix 2.46 g of 1.4-dioxypyrazinamide (2) with 5 mL of chlorobenzene and 12.1 g of phosphorus oxychloride, and heat up to Stir at 50°C for 50min, heat up to 70°C for 1h, cool to room temperature, add 1.52g of pyridine, heat up to 110°C after addition, reflux for 8h, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com