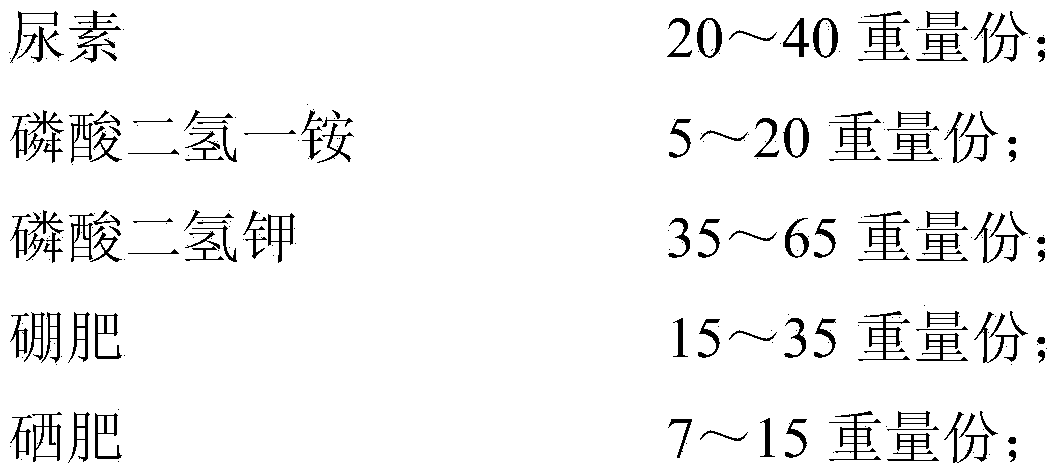Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
108 results about "Pyrazinamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pyrazinamide is used with other medications to treat tuberculosis (TB).
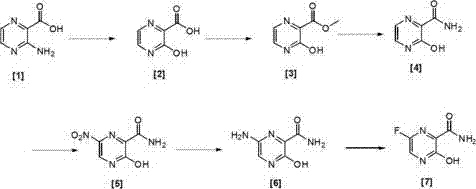
Preparation method of 6-fluoro-3-hydroxy-2-pyrazinamide
ActiveCN102775358AConvenient sourceSimple and fast operationOrganic chemistryProcess conditionsReaction step
The invention relates to a preparation method of 6-fluoro-3-hydroxy-2-pyrazinamide, which is characterized by comprising the following steps: by using methyl 3-amino-2-pyrazinecarboxylate as the initial raw material, hydroxylating, esterifying, aminating, nitrifying, reducing and fluorizing to obtain the 6-fluoro-3-hydroxy-2-pyrazinamide. The preparation method of 6-fluoro-3-hydroxy-2-pyrazinamide has the advantages of easily purchased initial raw material, mild reaction conditions and high yield, is simple to operate, and is suitable for industrial production. The technical route is short, the operation is simple, and the technical conditions are easy to control. The yields of all the six reaction steps are high. The technical conditions are mild and easy to control. The invention is suitable for an industrial production device, and can operate stably.
Owner:SHANDONG QIDU PHARMA
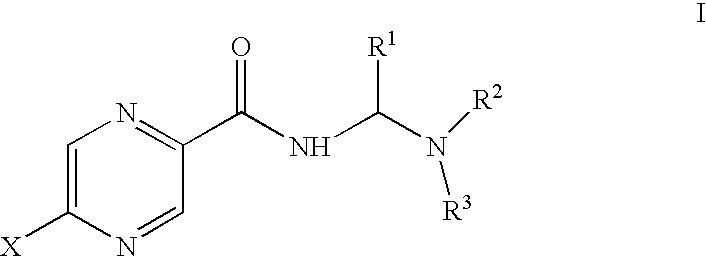
Aminomethylene amide analogs of pyrazinamide with intracellular antimycobacterial activity against pyrazinamide-resistant mycobacteria combined with a rifamycin
InactiveUS6399607B1Delay disintegration and absorptionSuitable for manufactureBiocideCarbohydrate active ingredientsDiseaseAryl
Methods for treating diseases involving pyrazinamide-resistant mycobacteria comprise administering to a mammal in need of treatment a therapeutically effective amount of a combination of rifamycin and a compound of formula I:whereinR1 is hydrogen haloalkyl, or lower alkyl;R2 and R3 are independently chosen from alkyl, substituted alkyl, cycloalkyl, aryl, substituted aryl, alkylaryl and substituted alkylaryl, or R2 and R3 taken together form a five- or six-membered heterocyclic or substituted heterocyclic ring; andX is hydrogen, halogen, or lower alkyl;or a pharmaceutically acceptable salt thereof.
Owner:RES FOUND STATE UNIV OF NEW YORK THE
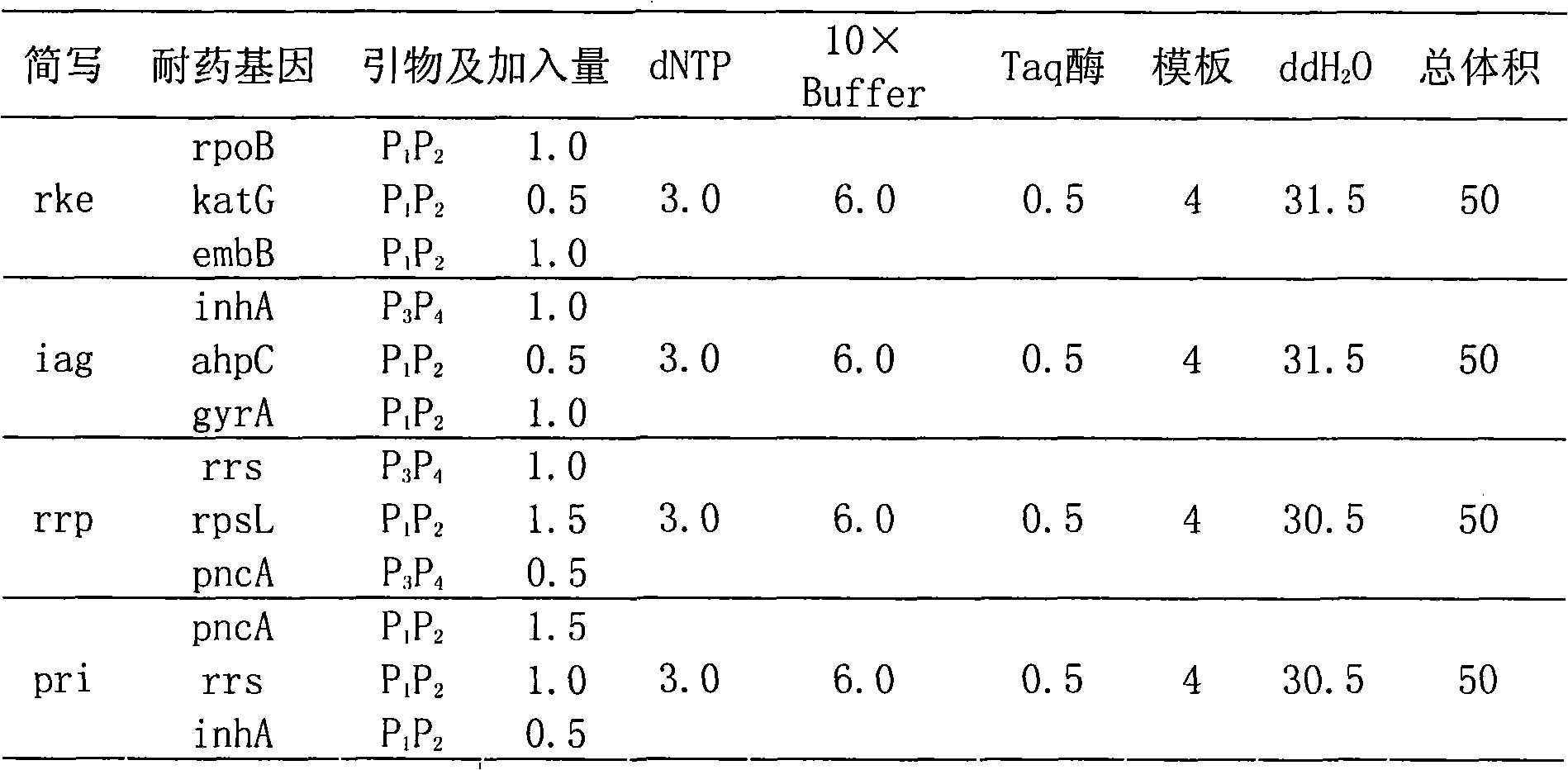
Drug-resistance gene film chip for detecting mycobacterium tuberculosis
InactiveCN101580879AHigh sensitivityTo achieve the purpose of detectionNucleotide librariesMicrobiological testing/measurementRpoBBiotin
A drug-resistance gene film chip for detecting mycobacterium tuberculosis is prepared by designing 54 probe spotting on a nylon film by aiming at the mutant sites of mycobacterium tuberculosis rpoB, kagG, embB, inhA, ahpC, gyrA, rrs, rpsL and pncA gene; 12 pairs of specific primers with biotin-labeled at 5' end are utilized; a sample DNA is subjected to triple PCR and amplified to form a large amount of gene fragment products with biotin; the amplified products and the probes on the film chip carry out specific hybridization; and then film washing, enzyme-linking and color reaction are carried out, thus preparing the chip. The gene film chip and the detection method thereof can detect the common gene mutations of the mycobacterium tuberculosis on the drug resistance of drugs such as isoniazid, rifampicin, streptomycin, ethambutol, pyrazinamide, quinolone and the like at one step, and are applicable to extracorporeal detection sputum sample, clinical isolation strains and mycobacterium tuberculosis multi-drug resistant gene in the organization sample.
Owner:GUANGXI MEDICAL UNIVERSITY
Slow released compound antituberculotic preparation
The slow released compound antituberculotic preparation contains at least one of rifampicin, pyrazinamide, kanamycin, isoniazide, rifapentine, etc. The slow released preparation is slow released injection or slow released implanting agent. The slow released injection consists of slow released microsphere and solvent, the slow released microsphere contains slow releasing supplementary material and antituberculotic, and the solvent is special solvent containing suspending agent carboxymethyl cellulose sodium and of viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is EVAc, PLA, PLGA, sebacic acid copolymer, etc. The slow released compound antituberculotic preparation is set or injected into local tuberulosis focus to treat various kinds of intractable tuberulosis, and has medicine releasing period up to 30-40 days, less systemic toxicity and unique curative effect.
Owner:JINAN SHUAIHUA PHARMA TECH
Joint detection method for drug resistance of mycobacterium tuberculosis and pyrazinamide in clinical sample
ActiveCN102925554AExclude non-specific amplification resultsStrong specificityMicrobiological testing/measurementMicroorganism based processesEnzyme GeneCell free
The invention relates to a joint detection method for the drug resistance of mycobacterium tuberculosis and the pyrazinamide thereof in a clinical sample, comprising the following steps of: A, extracting DNA (deoxyribonucleic acid) in the sample; B, detecting the mycobacterium tuberculosis via fluorescent quantitative PCR (polymerase chain reaction) amplification for a tuberculosis genome segment containing total-length pyrazinamide enzyme genes (pncA); and C, detecting the drug resistance of pyrazinamide for the mycobacterium tuberculosis positive sample, wherein the steps for detecting the drug resistance of the pyrazinamide are as follows: 1, performing PCR reaction once by taking the fluorescent quantitative PCR product as a template, so as to add an in-vitro expression element; 2, adding the PCR product in a cell-free expression system, so as to express a pyrazinamide enzyme; and 3, comparing the pyrazinamide enzyme activity of the sample with a standard strain, so as to give the drug resistance result of pyrazinamide. According to the invention, joint detection for the drug resistance of mycobacterium tuberculosis and the pyrazinamide thereof in the sample is realized by combining fluorescent quantitative PCR amplification for pncA genes with the enzyme activity of the in-vitro expressed pyrazinamide enzyme, without the need of bacterial culture and a DNA sequencer.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Disease prevention and frost resistance fruit selenium-enriching nutritional agent for fruit trees and preparation method of disease prevention and frost resistance fruit selenium-enriching nutritional agent
InactiveCN103449931AGood for balanced growthPromote growthFertilizer mixturesNutritional statusPhosphate
The invention discloses a disease prevention and frost resistance fruit selenium-enriching nutritional agent for fruit trees and a preparation method of the disease prevention and frost resistance fruit selenium-enriching nutritional agent. The nutritional agent consists of the following components in part by weight: 20-40 parts of urea, 5-20 parts of ammonium dihydrogen phosphate, 35-65 parts of potassium dihydrogen phosphate, 15-35 parts of a boron fertilizer, 7-15 parts of a selenium fertilizer, 3-8 parts of salicylic acid, 1-3 parts of captopril, 4-10 parts of humic acid, 3-5 parts of pyrazinamide, 2-6 parts of metronidazole, 10-20 parts of paclobutrazol, 0.4-1 part of methylglutarate and 0.5-2 parts of disopyramide phosphate. The selenium-enriching nutritional agent disclosed by the invention has strong pertinence, can be used for solving multiple prominent problems existing in the planting of the fruit trees simultaneously, and has multiple effects such as disease prevention, rotten fruit prevention, frost resistance, dehiscent fruit prevention, fruit dropping prevention, fruit plumpness promotion, nutrition, selenium enrichment and the like.
Owner:新疆久业富硒农业科技开发有限公司
6-fluorine-3-hydroxy-2-pyrazinamide synthetic method
The invention relates to a compound 6-fluorine-3-hydroxy-2-pyrazinamide synthetic method. The method comprises the following steps that a compound of a formula (IV) is used for preparing a compound of a formula (III) through amine ester exchange, the compound of the formula (III) is used for preparing a compound of a formula (II) through a fluorization reaction, and reaction equations are specified in the description, wherein the substituent group R is C1-4 alkoxy groups, X and Y are Cl or Br, and substituent groups X and Y can be identical or different. The preparation method for synthesizing a starting material 6-bromine (chlorin)-3-amino pyrazine formic ether is simple and low in cost; the synthetic line is simple and short, the method is simple, and key products can be obtained only through four-step reactions; reaction conditions of each step are mild, strong-corrosion and high-toxicity reagents are prevented from being adopted, and environmental pollution is reduced.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Myricetin pharmaceutical eutectic crystal and preparation method thereof
ActiveCN103819440AImprove solubilityHigh dissolution rateOrganic chemistryHydrogenDrugs preparations
The invention discloses a myricetin pharmaceutical eutectic crystal and a preparation method thereof. The myricetin pharmaceutical eutectic crystal uses myricetin as the active pharmaceutical ingredients, uses theine, nicotinamide, pyrazinamide or 4-cyanopyridine as the precursors, and is a myricetin-theine eutectic crystal, a myricetin-nicotinamide eutectic crystal, a myricetin-pyrazinamide eutectic crystal or a myricetin-4-cyanopyridine eutectic crystal formed through intermolecular hydrogen bonds. The eutectic crystals are prepared by adopting the solution mediating crystal transformation method. The myricetin pharmaceutical eutectic crystal provided by the invention inherits the pharmacological activity of the myricetin; meanwhile, the solubleness, the dissolution rate and the stability of the myricetin pharmaceutical eutectic crystal are all significantly improved as compared with those of the myricetin; the myricetin pharmaceutical eutectic crystal facilitates the development of pharmaceutic preparation and the wide applications of the myricetin in the field of medicine.
Owner:SHANGHAI UNIV OF T C M
Preparation method for 6-fluoro-3-hydroxyl-2-pyrazinamide
The invention belongs to the field of chemical synthesis, and specifically relates to a preparation method for 6-fluoro-3-hydroxyl-2-pyrazinamide. According to the invention, 6-fluoro-3-hydroxyl-2-pyrazinamide is prepared by using 6-bromo-3-amino-2-pyrazinamide as a raw material through four reaction steps of amino protection, halogen replacement, deprotection and azidation. The invention has the following advantages: procurement or preparation of the raw material is convenient; the preparation method is simple and convenient to operate; and the prepared 6-fluoro-3-hydroxyl-2-pyrazinamide has high yield and is applicable to industrial production.
Owner:QINGDAO HUANGDAO HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Process for preparing compound antituberculous preparation
InactiveCN102920707AReduce exposureImprove bioavailabilityAntibacterial agentsOrganic active ingredientsHydrazoneBULK ACTIVE INGREDIENT
The invention provides a process for preparing a compound antituberculous preparation. Active ingredients are rifampicin and isoniazide and one of mixtures of isoniazide + pyrazinamide and isoniazide + pyrazinamide + gatifloxacin. The process includes that the rifampicin and a part of pharmaceutic adjuvant are sieved and mixed to be subjected to dry granulating, or rifampicin and any other active ingredients except for isoniazide are mixed with corresponding amount of pharmaceutic adjuvant to be subjected to the dry granulating, then the isoniazide, residual active ingredients and corresponding quantity of pharmaceutic adjuvant are sieved and mixed to be subjected to dry granulating or wet granulating, and finally the residual pharmaceutic adjuvant and two types of granules are mixed for a secondary tabletting to obtain the preparation. The process for preparing the compound antituberculous preparation has the advantages that by means of granulation step by step, the contact between the rifampicin and isoniazide is reduced, the amount of impurity hydrazone generated through reaction between the isoniazide and the rifampicin can be effectively reduced, the bioavailability of the isoniazide and the rifampicin is greatly improved, and the medication effectiveness and safety for mass patients with tuberculosis are guaranteed.
Owner:SHENYANG PHARMA UNIVERSITY
Near infrared spectrum damage-free analysis method for anti-tuberculosis drugs
InactiveCN101101260AQuick checkNon-destructive testingColor/spectral properties measurementsSpecial data processing applicationsInfraredAction spectrum
The invention discloses method of testing effective constituent of near infrared light detection anti tubercular agent. Using the basis of anti tubercular agent action spectrum near infrared light and the measurand information, getting effective constituent (rifampicin,isoniazide or pyrazinamide ) of which by anti tubercular agent action spectrum near infrared light and their background information; achieving the lossless detection the content of effective constituent (rifampicin,isoniazide or pyrazinamide ) in the anti tubercular agent near infrared light complex background by the multi element normalized method of chemometrics. It settles the problem of high cost, long period, medicament cannot use after analyzing etc when analyzing the anti tubercular agent effective constituent in existence, establishing the fast, high pass mete, lossless and needed on-line analysis green medicament analytic method for the anti tubercular agent. The advantages is that the sample pre-processing is easy, the detection is fast and undamaged, the detection time of each sample is shortage of two min; the result is credibility, the error is less than5%.
Owner:JILIN UNIV
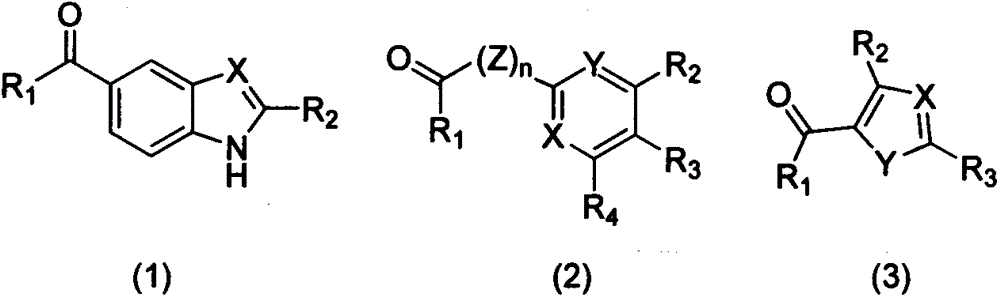
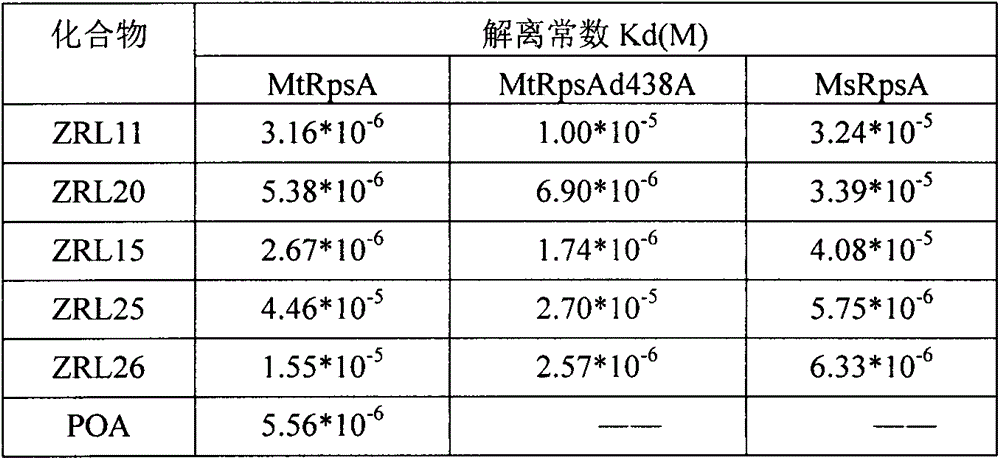
Medical application of three carboxyl substituted aromatic compounds in treating pyrazinamide-resistance tuberculosis and tuberculosis
InactiveCN106109467AInhibit Mycobacterium tuberculosisInhibitory activityAntibacterial agentsOrganic active ingredientsRibosomal protein E-L30Carboxyl radical
The invention discloses three carboxyl substituted aromatic compounds (shown as Formulas (1-3)), which can antagonize ribosome proteins S1 (RpsA) of a ribosome 30S small subunit, effectively inhibit the growth of mycobacterium tuberculosis and pyrazinamide-resistance mycobacterium tuberculosis and can be used for preparing drugs for treating pyrazinamide-resistance tuberculosis and tuberculosis (as shown in Description).
Owner:CHINA PHARM UNIV
New low side effect pharmaceutical composition containing antituberculosis drugs
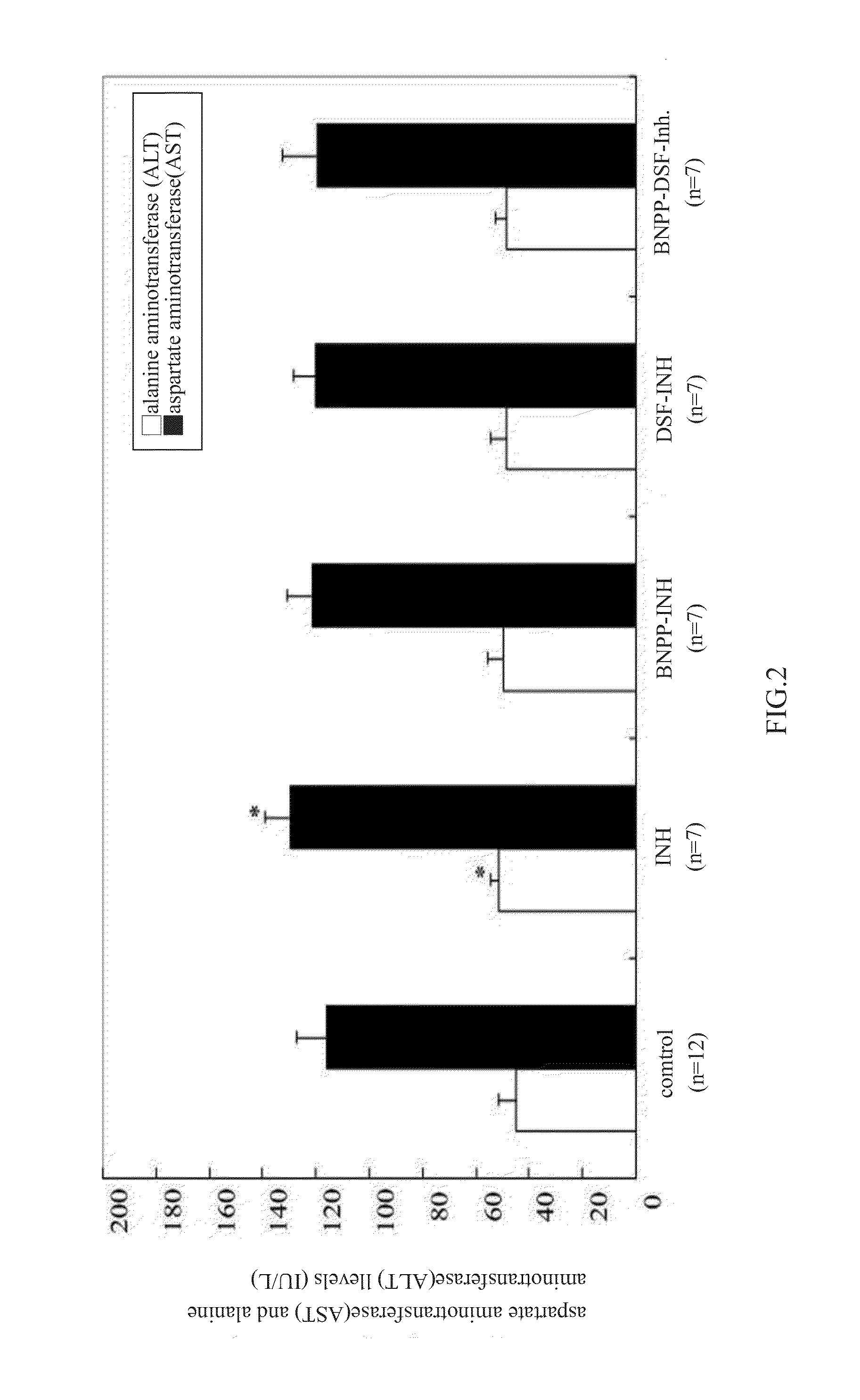
ActiveUS20140038921A1Reduce isoniazidReduce pyrazinamide induced hepatotoxicityAntibacterial agentsBiocideDiseaseSide effect
A pharmaceutical composition for treating tuberculotic diseases with no side effect / low side effect is provided by the present invention, which pharmaceutically effective amount of one or more compounds chosen from isoniazid, rifampin, pyrazinamide and ethambutol, and pharmaceutically effective amount of substances which can reduce the side effect of the antituberculosis agents.
Owner:INT EDUCATION FOUND
Triple compound microsphere vascular targeted embolization sustained-release preparation containing antituberculous drug as well as preparation method and application of preparation
ActiveCN104324032AExcellent anti-tuberculosis effect in vitro and in vivoReduce concentrationAntibacterial agentsOrganic active ingredientsAntituberculous drugHemoptyses
The invention relates to a triple compound microsphere vascular targeted embolization sustained-release preparation containing an antituberculous drug as well as a preparation method and application of the preparation. The sustained-release agent comprises a carrier and drugs, wherein the drugs are coated with the carrier; the carrier is sodium alginate or chitosan, and the drugs are triple antituberculous compound drugs including rifampicin, isoniazid and pyrazinamide or moxifloxacin. The three antituberculous drugs are matrix drug solutions, the sodium alginate or chitosan is a carrier solution, the matrix drug solutions and the carrier solutiona are mixed to prepare a solution, the polymer solution containing drugs is dispersed into fogdrops with a certain diameter by adopting a high-voltage electrostatic droplet mode, and the fogdrops are sprayed into a solidifying liquid to prepare antituberculous drug microspheres under the action of calcium ions. The embolization sustained-release preparation can be used for treating tuberculosis, massive hemoptysis of pulmonary tuberculosis, tuberculosis cavity, renal tuberculosis, osteoarticular tuberculosis, genital tuberculosis, tuberculosis of thyroid gland, tuberculosis of cervical lymph nodes, tuberculosis of pericardium, tuberculosis of chest wall, pleural tuberculosis and other kinds of tuberculosis in a body.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY +1
Isoliquiritigenin pyrazinamide eutectic crystal and preparation method thereof
The invention belongs to the technical field of medicine eutectic crystal, and concretely discloses an isoliquiritigenin pyrazinamide eutectic crystal and a preparation method thereof. According to the method, isoliquiritigenin and pyrazinamide are used as raw materials; a solvent auxiliary grinding method or a solvent suspension method is used for preparing the isoliquiritigenin pyrazinamide eutectic crystal. The obtained eutectic crystal has a novel crystal form, is favorable for novel preparation development, and promotes the application of the isoliquiritigenin to medicine clinics.
Owner:CHINA PHARM UNIV +1
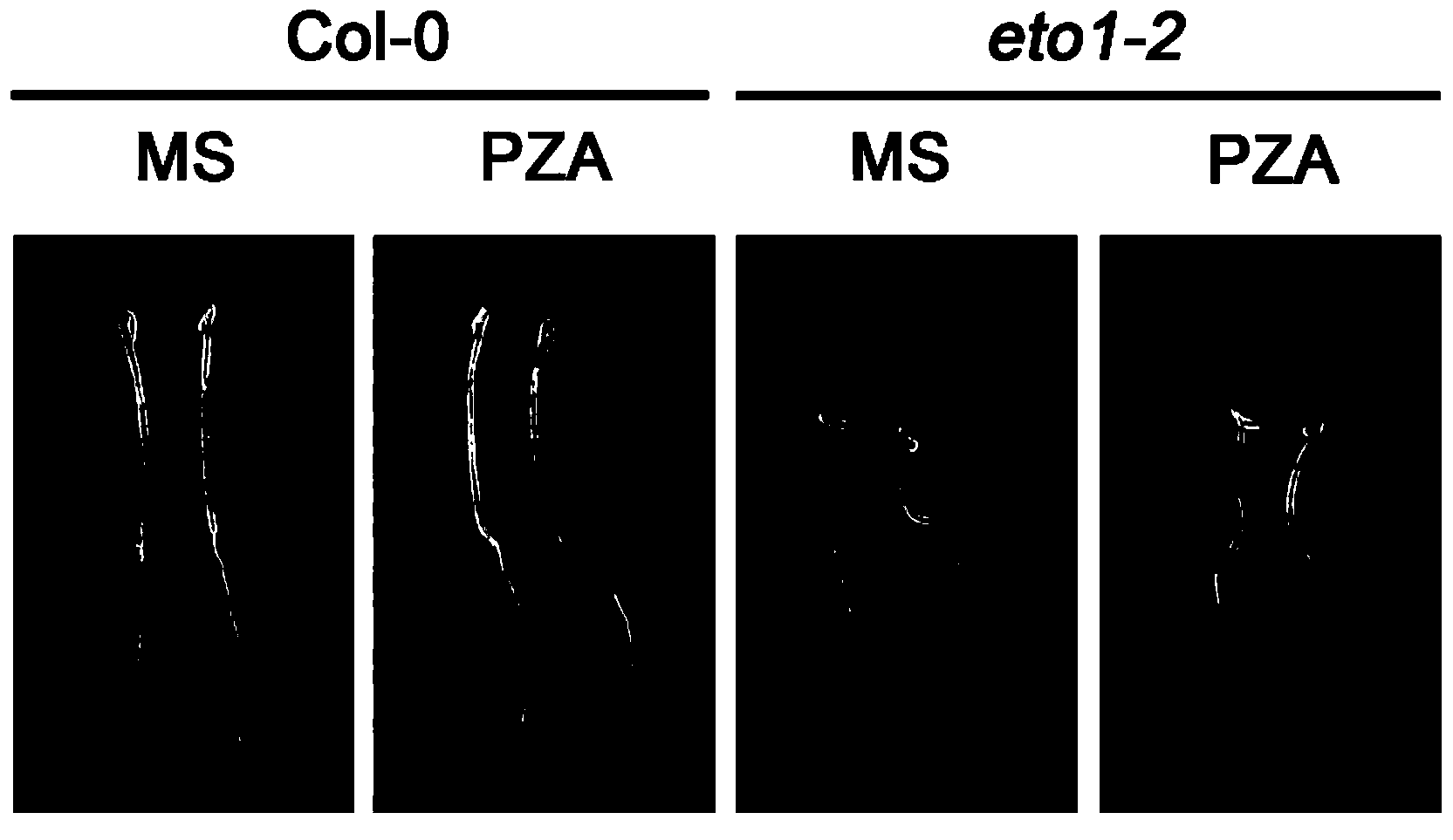
Small molecular inhibitor pyrazinamide used in plant ethylene synthesis and application thereof
ActiveCN103524434AInhibit synthesisStrong specificityPlant growth regulatorsBiocideACC oxidaseStructural formula
The invention discloses a small molecular inhibitor pyrazinamide (shortened as PZA) used in plant ethylene synthesis. The small molecular inhibitor pyrazinamide is characterized by being a specific inhibitor of ACC oxidase (ACO), and being capable of inhibiting conversion of an ethylene synthesis precursor ACC into ethylene in the plant ethylene synthesis; and the pyrazinamide has a structural formula shown as a figure in the abstract, and has the molecular weight of 123.12. The invention further provides a culture medium which contains the pyrazinamide and can inhibit the plant ethylene synthesis. The invention further provides application of the small molecular inhibitor pyrazinamide used in the plant ethylene synthesis to plant growth and development regulation and refreshment during plant transportation. The inhibitor pyrazinamide can obviously inhibit the plant ethylene synthesis, has a stronger effect than known ethylene synthesis inhibitors, and is convenient in popularization and application.
Owner:PEKING UNIV
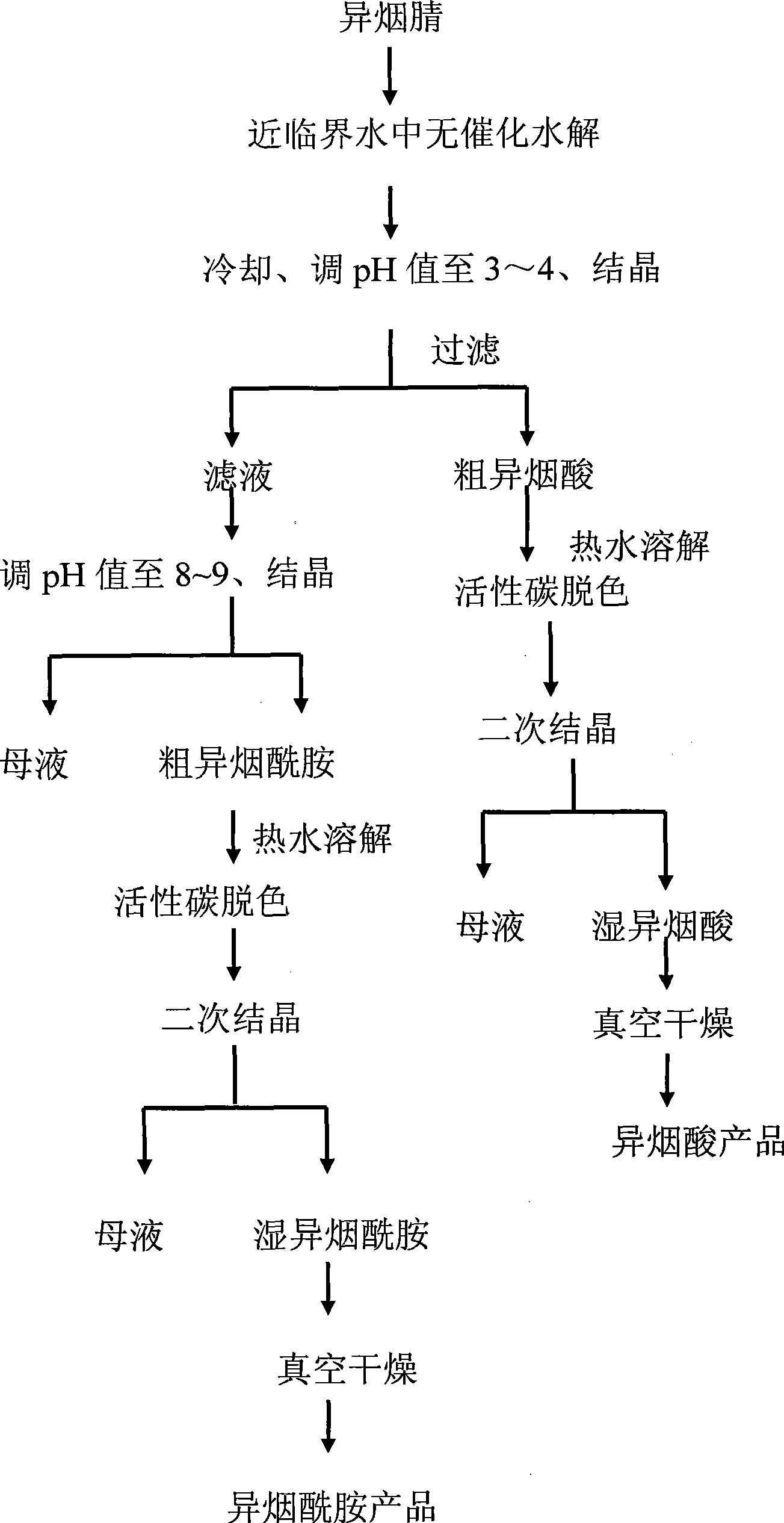
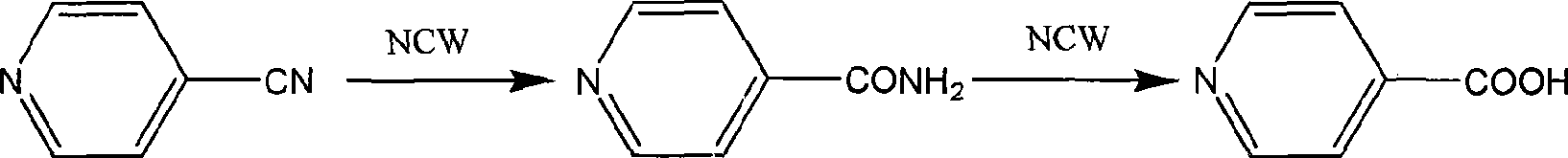
Method for preparing isonicotinic acid and pyrazinamide by non-catalytic hydrolyzing isonicotinonitrile in near-critical water medium
InactiveCN101475527AYield adjustableSolve the pollution problemOrganic chemistryExhaust valveFiltration
The invention discloses a method for simultaneously preparing pyrazinamide and isonicotinic acid through uncatalyzed hydrolysis of isonicotinic nitrile in a near critical water medium. The method comprises the following steps: adding deionized water and the isonicotinic nitrile into a high-pressure reaction kettle, stirring the mixture, raising the temperature to the boiling point at normal pressure, and opening an exhaust valve for 2 to 5 minutes; closing the exhaust valve, continuously raising the temperature to be between 200 and 300 DEG C, and hydrolyzing for 10 to 15 minutes; cooling hydrolysate, adjusting the pH value of the hydrolysate to be between 3 and 4, performing crystallization and filtration on the hydrolysate and obtaining coarse isonicotinic acid and filtrate; making the coarse isonicotinic acid subjected to hot-water dissolution, activated carbon decolorization, secondary crystallization and vacuum drying and obtaining isonicotinic acid products; adjusting the pH value of the filtrate to be between 8 and 9, performing crystallization and obtaining coarse pyrazinamide; and making the coarse pyrazinamide subjected to hot-water dissolution, activated carbon decolorization, secondary crystallization and vacuum drying and obtaining pyrazinamide products. The method does not add any catalyst during reaction, solves the pollution problem of acid and alkali catalyzed hydrolysis, has simple and green process, and has high purity and yield of the products.
Owner:ZHEJIANG UNIV
Stabilized short-course chemotherapy (SCC) anti-tuberculosis drug compositions
A stabilized oral powder or granule mixture made from at least two different anti-microbial tuberculosis drugs (e.g., rifampacin, isoniazid, ethambutol, pyrazinamide), for a short-course therapy; the powder can be consumed by mixing in a glass of water or juice and assures that each of the various drugs is in fact consumed by the tuberculosis patient.
Owner:SAPTE VINAY RAMAKANT
Compound antitubercular preparation containing gatifloxacin, and preparation method thereof
InactiveCN102198138APromote degradationReduced anti-TB efficacyAntibacterial agentsOrganic active ingredientsCurative effectTherapeutic effect
The invention relates to a new compound antitubercular preparation containing gatifloxacin, and a preparation method thereof. The compound antitubercular preparation comprises rifampicin, isoniazide, pyrazinamide and gatifloxacin. Each unit of preparation contains 100-150mg of rifampicin, 60-120mg of isoniazide, 200-400mg of pyrazinamide and 100-200mg of gatifloxacin. The addition of gatifloxacin in the preparation can shorten the course of treatment of tuberculosis and improve the treatment effect; and degradation of the rifampicin can be reduced through dry method granulating tabletting, thus improving the bioavailability of rifampicin and improving the curative effect of the medicament. The compound preparation can be prepared into common tablets, double-layer coated-core tablets or pellet-core tablets.
Owner:SHENYANG PHARMA UNIVERSITY
Process for preparation of anti-tubercular combination and pharmaceutical composition prepared therefrom
This invention relates to a process for preparing a pharmaceutical composition comprising four antitubercular drugs: rifampin or a pharmaceutically acceptable salt thereof, isoniazid or a pharmaceutically acceptable salt thereof, pyrazinamide or a pharmaceutically acceptable salt thereof and ethambutol or a pharmaceutically acceptable salt thereof, wherein rifampin and isoniazid are in separate layers. The invention also provides a pharmaceutical composition prepared therefrom having advantageous stability and bioavailability.
Owner:TAIWAN BIOTECH +1
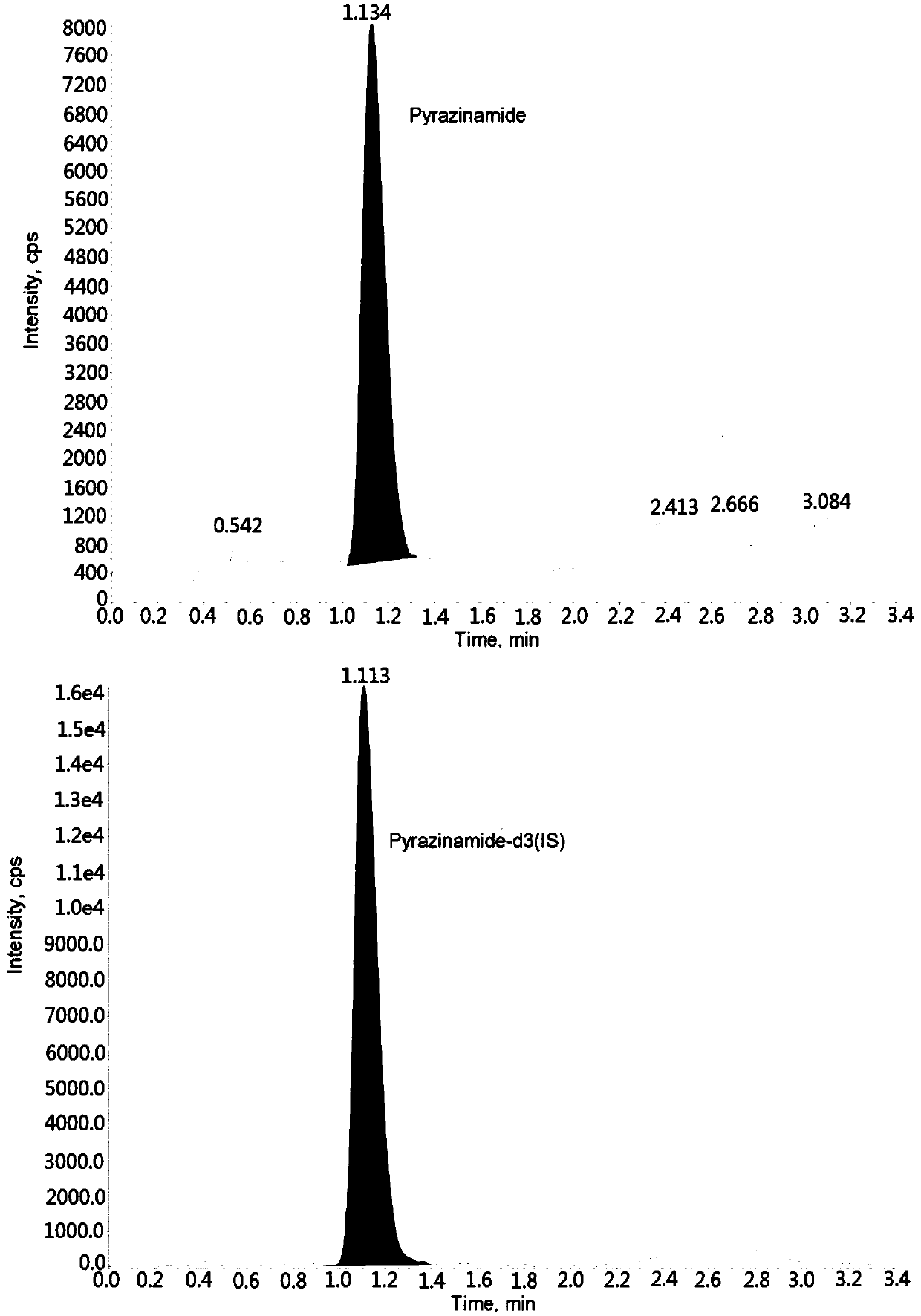
Method for measuring pyrazinamide concentration in plasma through hygroplasm combination
InactiveCN109682915AThe pretreatment method is simpleSuitable for routine determinationComponent separationOrganic solventMass spectrometry detector
The invention discloses a method for measuring pyrazinamide concentration in plasma through hygroplasm combination. A hygroplasm combination system is adopted for measurement. The method comprises thesteps that firstly, a to-be-measured sample is taken, a certain quantity of mixed organic solvent is added for conducting extraction twice, and after pretreatment, through chromatographic column separation, a mass spectrometry detector is used for detection. The method is quick to use, accurate, high in sensitivity and easy and convenient to operate, and the basis is provided for plasma concentration measurement of pyrazinamide. The linear range of a plasma standard curve is within 0.1-20 mug / mL, the within-run precision and between-run precision RSD are smaller than + / -15%, and the method issuitable for measurement of pyrazinamide concentration in the plasma.
Owner:徐州立兴佳正医药科技有限公司
Method for detecting content of impurities in isoniazid or medicinal composition thereof
The invention relates to a method for detecting the content of impurities in isoniazid or a medicinal composition thereof. The method comprises the following steps: 1, preparing an impurity reference substance solution, namely precisely weighing a proper amount of isonicotinic acid and an isonicotinic acid reference substance, dissolving with water, and quantitatively diluting to serve as isonicotinic acid and the isonicotinic acid reference substance solution; 2, preparing a test substance solution, namely weighing a proper amount of a test substance, adding water to dissolve isoniazid, diluting, filtering and taking subsequent filtrate as a test substance solution; 3, preparing a reference solution, namely precisely weighing the test substance solution, and diluting for 100 times to serve as a reference solution; and 4, carrying out a detection method, namely precisely weighing 10mu l of the impurity reference substance solution, 10mu l of the test sample solution and 10mu l of the reference solution respectively, respectively injecting into a liquid chromatograph, recording a chromatogram map, and calculating contents of isonicotinic acid, pyrazinamide and other impurities by adopting a peak area method according to the chromatogram map.
Owner:SHENYANG HONGQI PHARMA
Pyrazinamide derivatives as well as preparation method and uses thereof
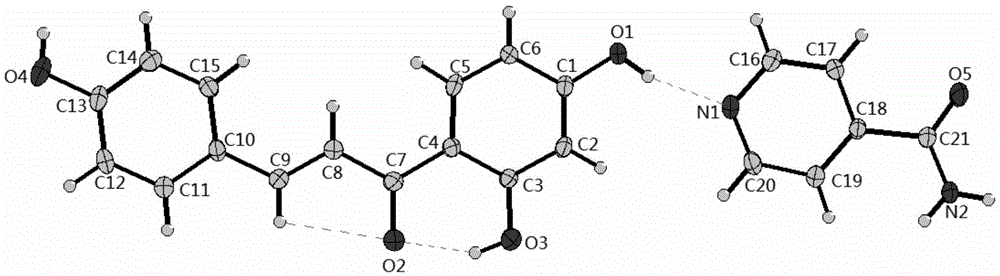
InactiveCN101182312AEasy to makeHigh yieldOrganic chemistrySurface/boundary effectAtomic force microscopyInfrared
The present invention relates to a novel pyrazinamide derivative and more particularly a hydrated crystal of N, N'-(4-nitrogen heterocyclic-1, 7-heptyl) double-pyrazinamide compound, the structure and characteristic of which are mensurated by X-ray single-crystal diffraction, X-ray powder diffraction, thermogravimetric analysis, infrared spectrum, nuclear magnetism and element analysis; a molecular formula of the hydrated crystal is C8H18.50N3.50O5, the crystal belongs to the orthorhombic system, a space group is P2<1>2<1>2, unit cell parameters are that a is 11.164, b is 26.05, c is 4.582, and alpha, beta and gamma are equal to 90 degrees; unit cell size is that V is 1332.4 <3>. The preparation process of the hydrated crystal is simple; the crystal has high yield and purity and good stability. The pyrazinamide derivative can form a two-component crystal with the water absorption quantity of 29.56 percent, and the absorption of organic compound towards water molecule has the reversibility, which ensures that the compound can be applied to the recycling technology of worn atomic force microscopy silicon tips.
Owner:TIANJIN NORMAL UNIVERSITY
Preparation method of 6-bromine-3-hydroxyl-2-pyrazinamide
The invention belongs to the field of chemical synthesis and particularly relates to a reparation method of 6-bromine-3-hydroxyl-2-pyrazinamide. The method comprises the steps of converting 3-hydroxyl-2-pyrazinecarboxylic acid methyl ester which is taken as an initial material into 6-bromine-3-hydroxyl-2-pyrazinecarboxylic acid methyl ester, and then carrying out ammonolysis, so that the 6-bromine-3-hydroxyl-2-pyrazinamide can be prepared with high yield by simple and convenient operations. The preparation method has the advantages that the 3-hydroxyl-2-pyrazinecarboxylic acid methyl ester which is cheap in price, and convenient to purchase in markets is taken as the initial material, the method is simple and convenient and easy to implement, the selected solvent range is wide, a solvent is easy to remove, the yield is high, and the method can be applicable to industrial production.
Owner:SHANDONG UNIV +1
Combination antibacterial composition and short-course antibacterial regimen
The present invention relates to therapeutic combinations of anti-bacterial agents linezolid, bedaquiline and pretomanid, and optionally with pyrazinamide, in a short-course oral dosage regimen for the treatment of tuberculosis.
Owner:THE GLOBAL ALLIANCE FOR TB DRUG DEV
Method for simultaneously detecting five anti-tuberculosis drugs in blood plasma via UPLC-MS/MS method
The invention discloses a method for simultaneously detecting five anti-tuberculosis drugs (including rifampin, rifabutin, Pyrazinamide, ethambutol and isoniazid) in blood plasma via a UPLC-MS / MS method. Blank blood plasma is weighed precisely, and added with standard working solutions mixed with a series of standard substances, standard working solutions of isotope internal standards in one to one correspondence are added, pre-treatment is carried out in a protein precipitation method, the UPLC-MS / MS method is used for analysis, chromatograms of different samples are obtained, and a standardcurve is established by taking the ratio of an object to be measured and the corresponding internal standard peak area as the abscissa and the concentration of the object to be measured as the ordinate; and blood plasma to be measured is weighed precisely, the standard working solutions of isotope internal standards in one to one correspondence are added, pre-treatment is carried out in the protein precipitation method, the UPLC-MS / MS method is used for analysis, chromatograms of different samples are obtained, and the concentration of the blood plasma sample is calculated by using the standard curve. The method is simple and rapid in operation and high in sensitivity, accuracy and precision, the matrix effect is low, and can satisfy requirements for monitoring the drug concentration of five anti-tuberculosis drugs in clinical application.
Owner:MENGCHAO HEPATOBILIARY HOSPITAL OF FUJIAN MEDICAL UNIV
Preparation method of cefalonium
ActiveCN104725403ARaw materials are easy to getEasy to operateOrganic chemistryIodination reactionSolvent
The invention relates to a preparation method of cefalonium. According to the preparation method, raw materials (cefalotin and pyrazinamide) react at a low temperature to obtain the product cefalonium. The preparation method particularly comprises the following steps: dissolving cefalotin acid into an organic acid, carrying out carboxyl protection by using a silanization protection reagent and then carrying out iodination reaction on reaction products and iodotrimethylsilane; then carrying out amination reaction on the reaction product from the former step and pyrazinamide; and finally carrying out deprotection by alcoholysis, regulating the pH value at a low temperature and crystalizing to obtain cefalonium. According to the preparation method of cefalonium, cefalotin acid is protected by the silanization protection reagent in an organic solvent and then reacts with iodotrimethylsilane; the reaction time is short, the reaction conditions are mild, the reaction is complete and no side reaction is almost generated; due to adoption of a mixed solvent crystallization method, the characteristics of high drying speed, light color and high yield can be achieved; in addition, the used solvent can be recycled and the amount of generated sewage can be reduced; therefore, the preparation method of cefalonium has remarkable economic and environmental benefits and facilitates industrial production.
Owner:QILU SYNVA PHARMA
Method for detecting anti-tuberculosis drug in serum by ultra-high performance liquid chromatography-tandem mass spectrometry technology
InactiveCN111766311AHigh sensitivityStrong specificityComponent separationAntituberculosis drugPyrazine
The invention discloses a method for detecting an anti-tuberculosis drug in serum by an ultra-high performance liquid chromatography-tandem mass spectrometry technology. The antitubercular drug comprises cycloserine (CYS), pyrazinamide (PZN), isoniazid (INZ), p-aminosalicylic acid (P-ASA), ethiisonicotinamide (ETN), ethambutol (ETB), clofazimine (CFM), bedaquiline (BDQ), rifampicin (RFP), rifbutine (RFB) and rifapentine (RFT). The method includes: detecting the content of the antituberculosis drug in the pretreated serum by adopting an ultra-high performance liquid chromatography-tandem mass spectrometry method, performing quantifying by utilizing a mass spectrometry isotope internal standard method, establishing a calibration curve by taking the concentration ratio of the standard substance to the internal standard substance as an X axis and the peak area ratio of the standard substance to the internal standard substance as a Y axis, and calculating the concentration of the target drug in the serum; the method is high in sensitivity, strong in specificity and simple in pretreatment process, separation and detection of the anti-tuberculosis drugs in serum are completed within 5 min, and a simple and rapid detection method is provided for clinical concentration monitoring of the anti-tuberculosis drugs.
Owner:南京品生医学检验实验室有限公司
Pyrazinamide tablet and preparation method thereof
InactiveCN108542888AImprove yieldHigh speedAntibacterial agentsOrganic active ingredientsCarboxymethyl starchSucrose measurement
The invention provides a pyrazinamide tablet and a preparation method thereof. The pyrazinamide tablet is prepared from the following components in parts by weight: 250 parts of pyrazinamide, 24 partsof filler, 12 parts of saccharose, 18 parts of microcrystalline cellulose, 8 parts of hydroxypropylcellulose, 20 parts of 18% pulp starch, 2 parts of polysorbate-80, 1 part of lubricant, 4 parts of sodium carboxymethyl starch, and 1 part of binding agent. The pyrazinamide tablet prepared according to the preparation method overcomes the defects that in the prior art, the pyrazinamide tablet is prone to crushing in the preparation process and the tablet is out of shape, the finished product ratio and the tabletting speed of the pyrazinamide tablet are improved, and the production efficiency ofthe pyrazinamide tablet is remarkably improved.
Owner:JIANGSU SIHUAN BIOENGINEERING PHARM CO LTD
Kit and method for detecting antituberculous drugs and metabolites thereof in sample
PendingCN114354804AExpand the types of testingIncrease varietyComponent separationMetaboliteAntituberculous drug
The invention particularly provides a kit and a method for detecting antituberculous drugs and metabolites thereof in a sample. The kit is used for detecting antituberculous drugs and metabolites thereof in a sample, and comprises a calibration product, a quality control product, an instrument quality control product and an isotope internal standard product, both the calibration material and the quality control material contain rifampicin, isoniazide, rifapentine, pyrazinamide, ethambutol, clofazimine, cycloserine, moxifloxacin, levofloxacin, linezolid, acetyl isoniazide, bedaquiline and deacetylrifampicin; the instrument quality control product comprises a methanol solution containing rifampicin, isoniazid, rifapentine, pyrazinamide, ethambutol, clofazimine, cycloserine, moxifloxacin, levofloxacin, linezolid, acetyl isoniazid, bedaquiline and deacetylrifampicin; the isotope internal standard substance contains an internal standard substance corresponding to a substance contained in the calibrator. The kit disclosed by the invention can be used for detecting the concentrations of the antituberculous drugs and metabolites thereof in various sample types.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com