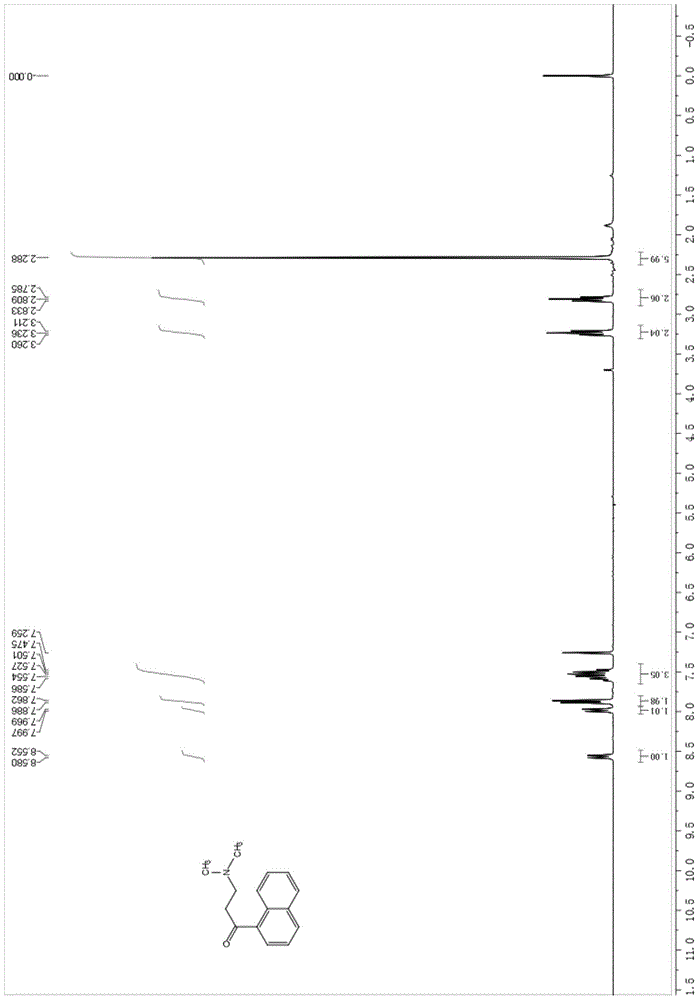
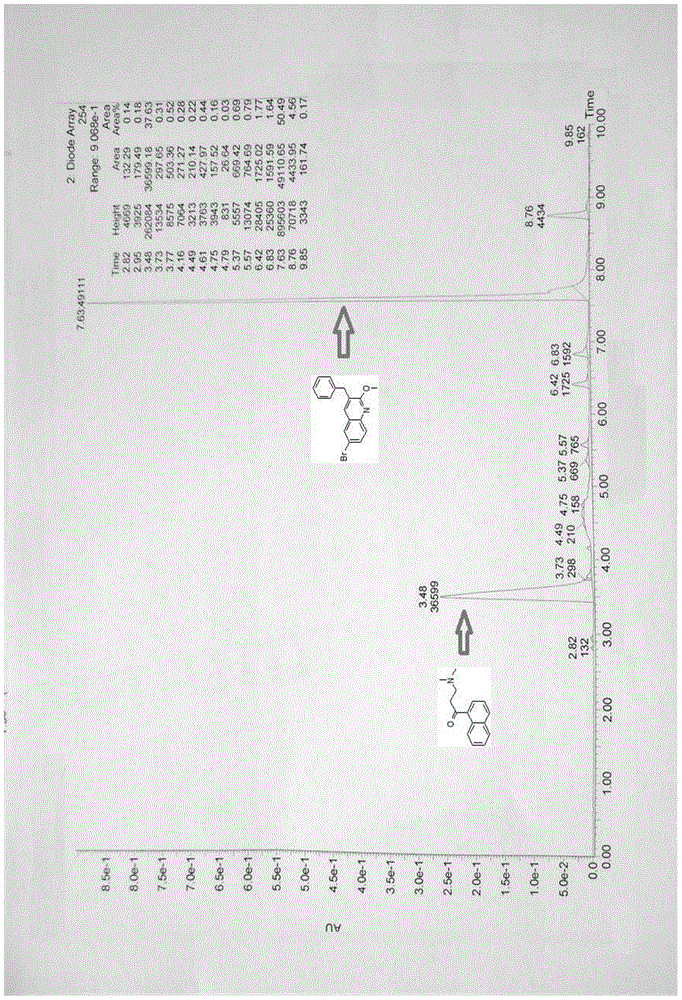
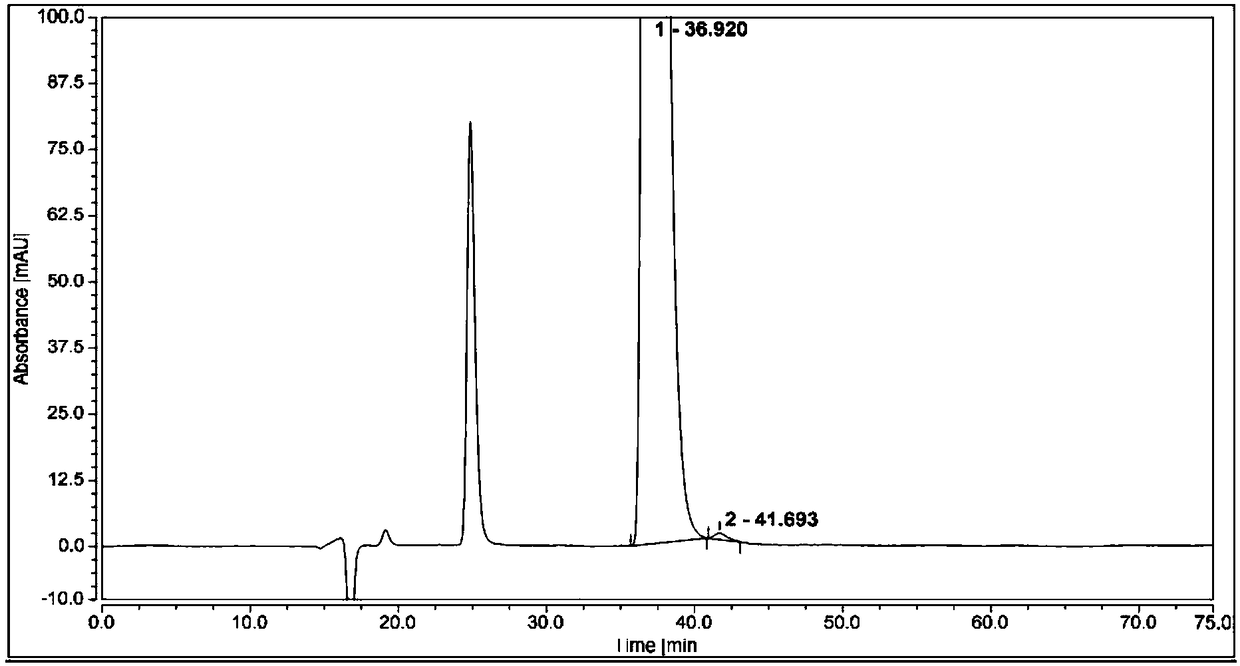
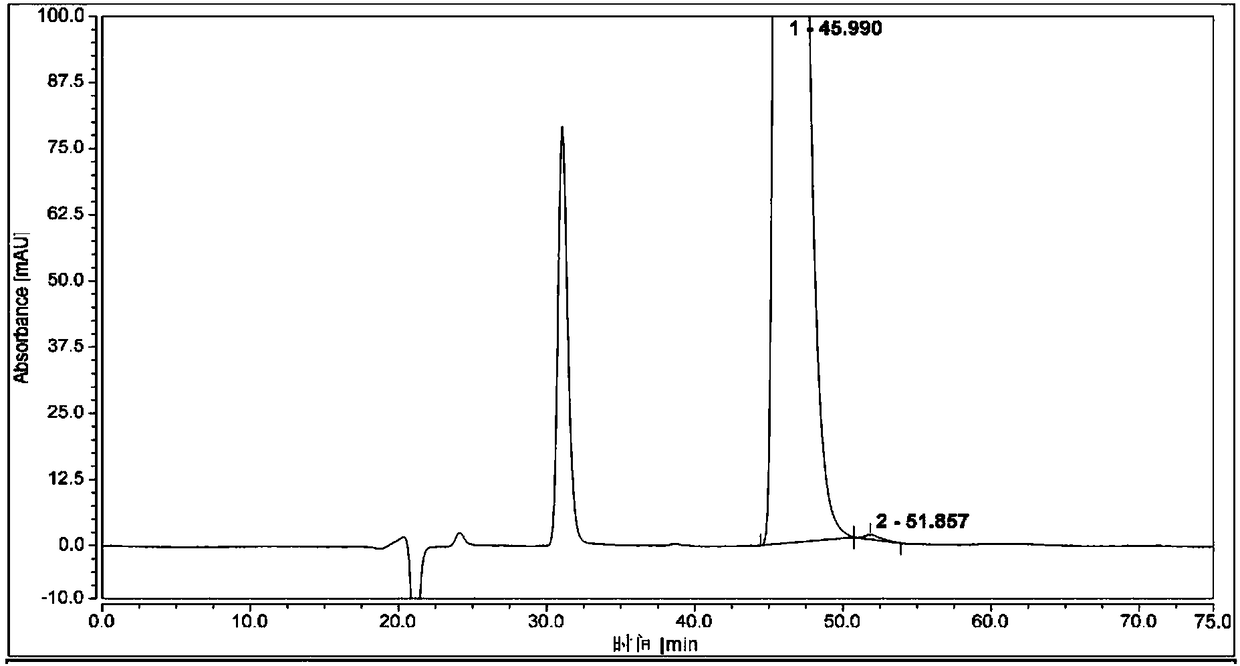
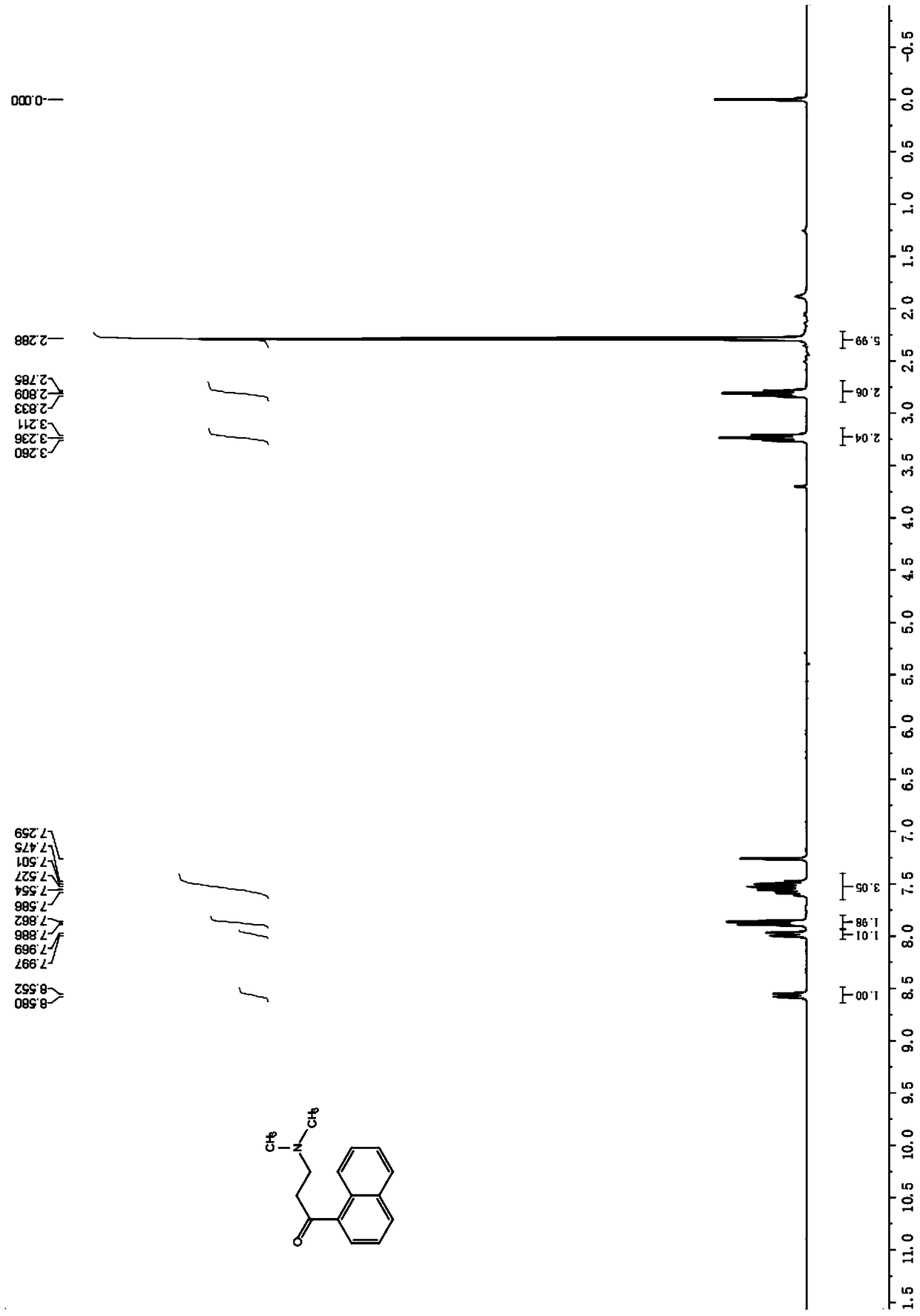
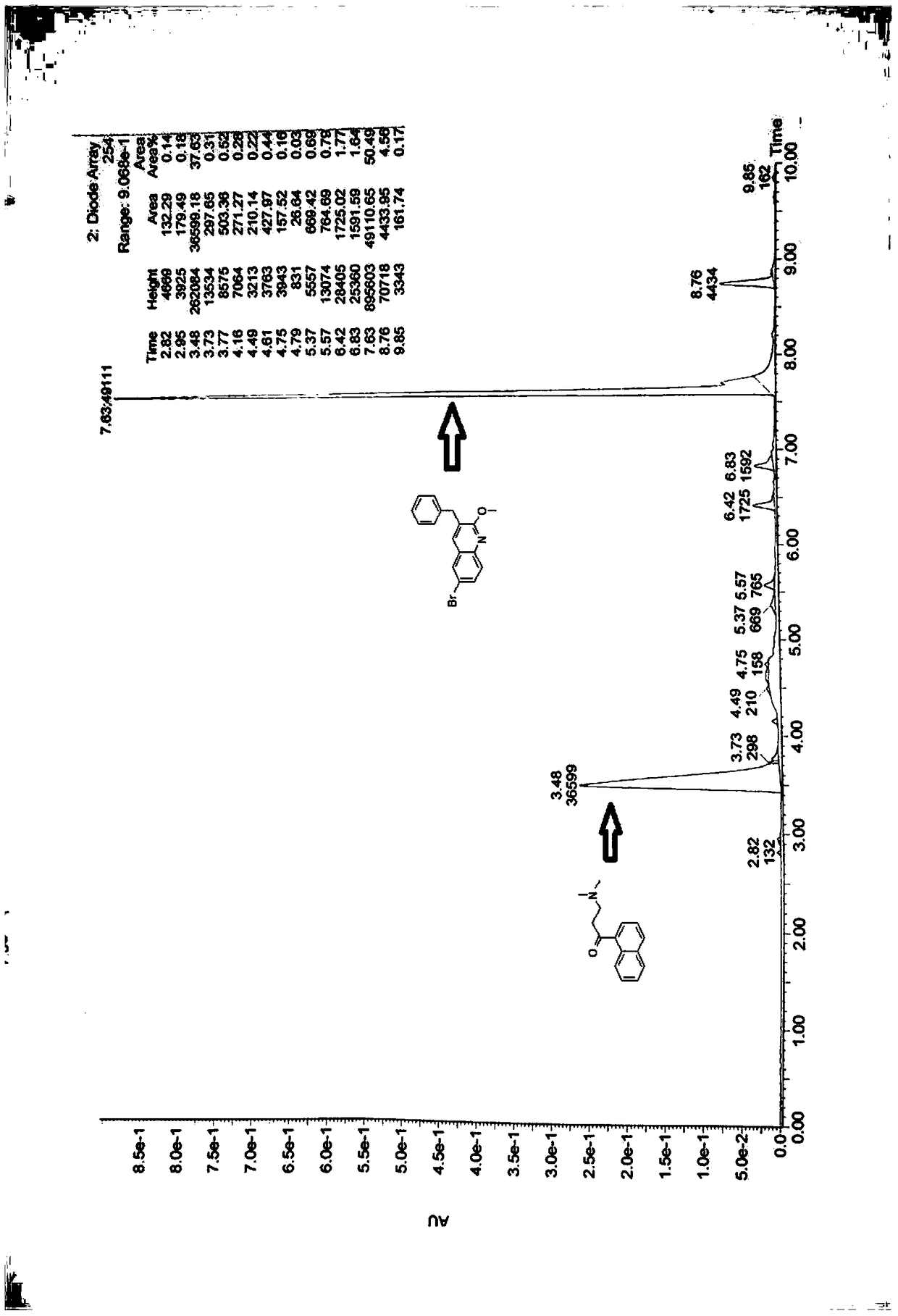
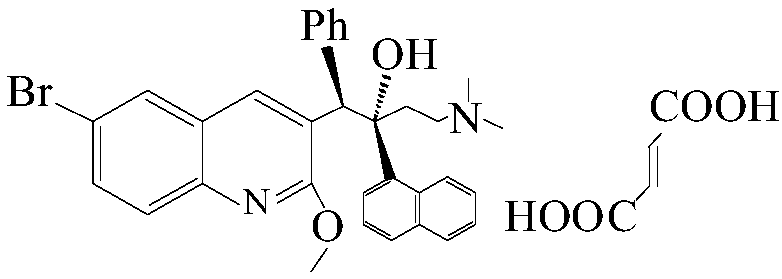
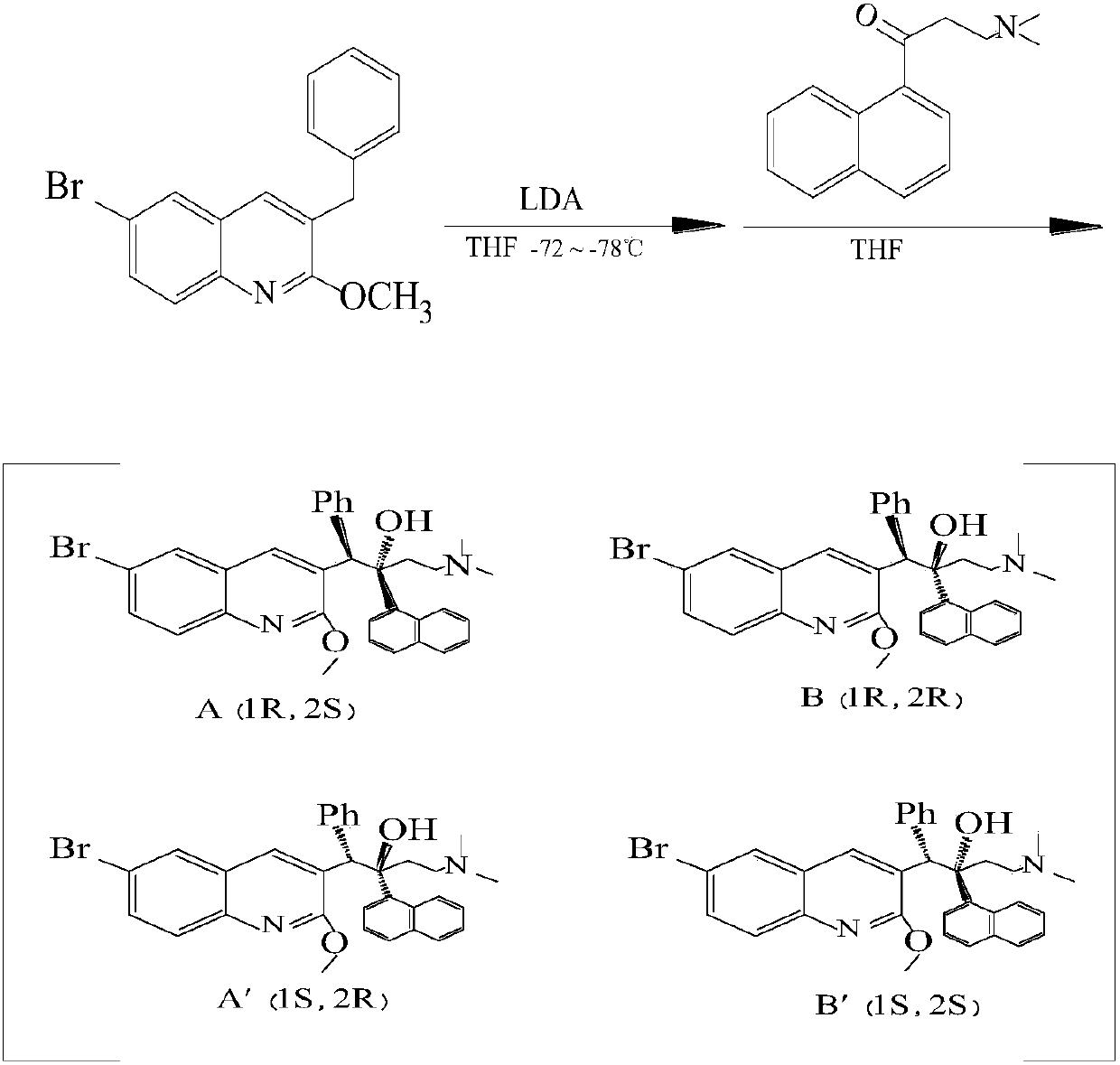
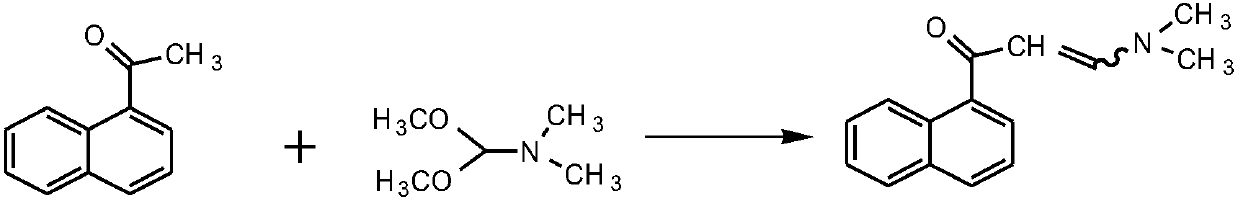
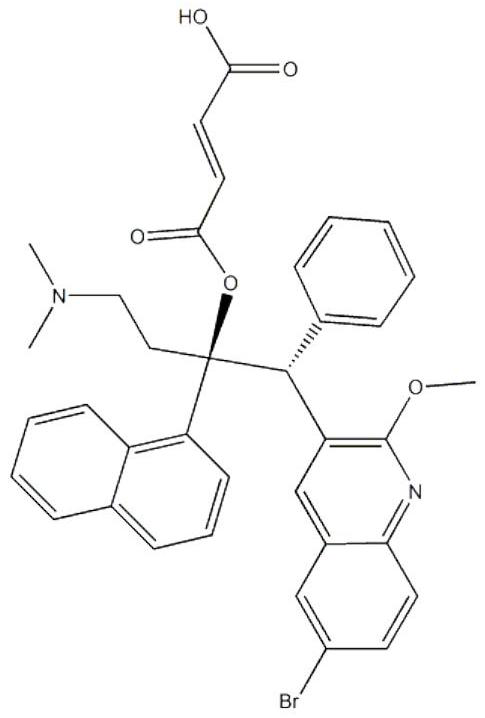
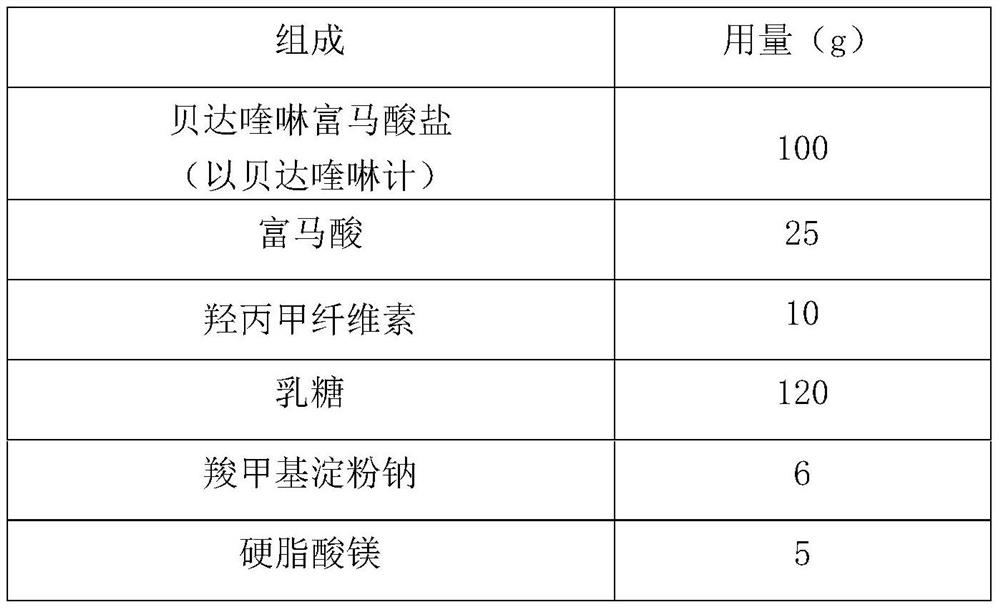
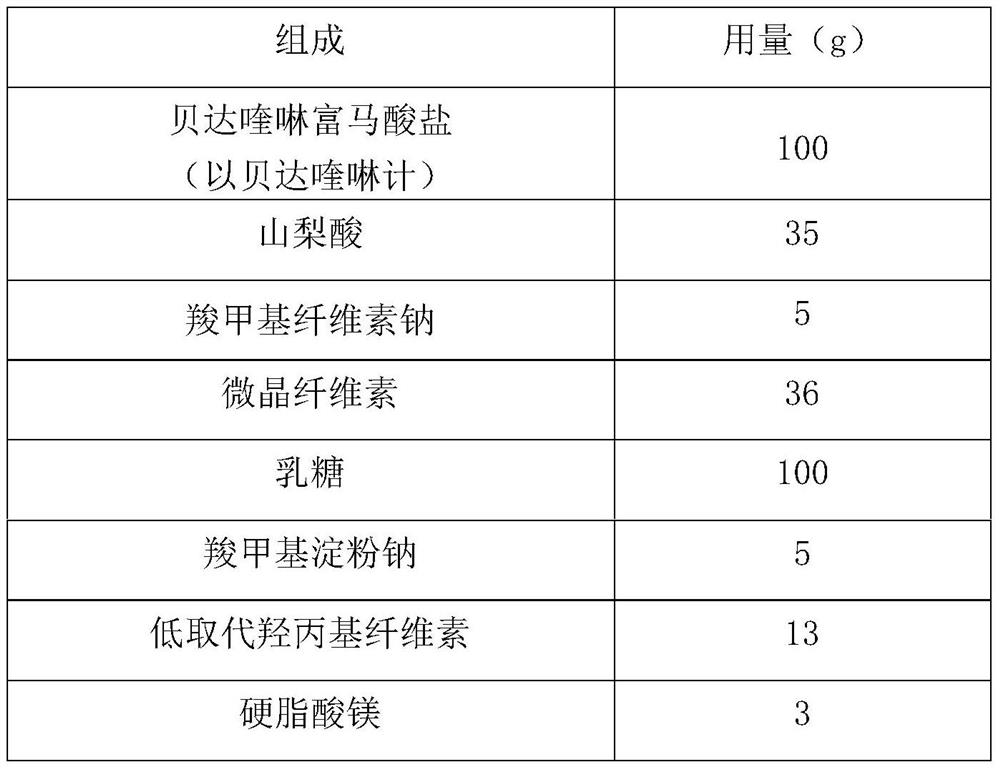
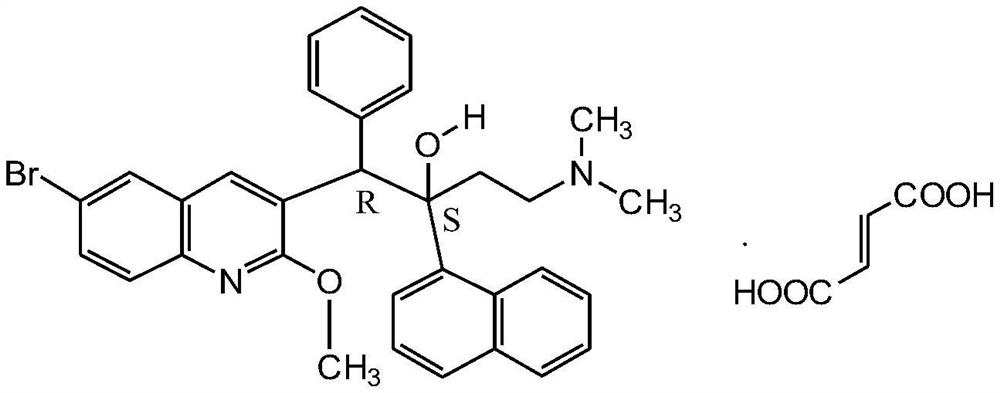
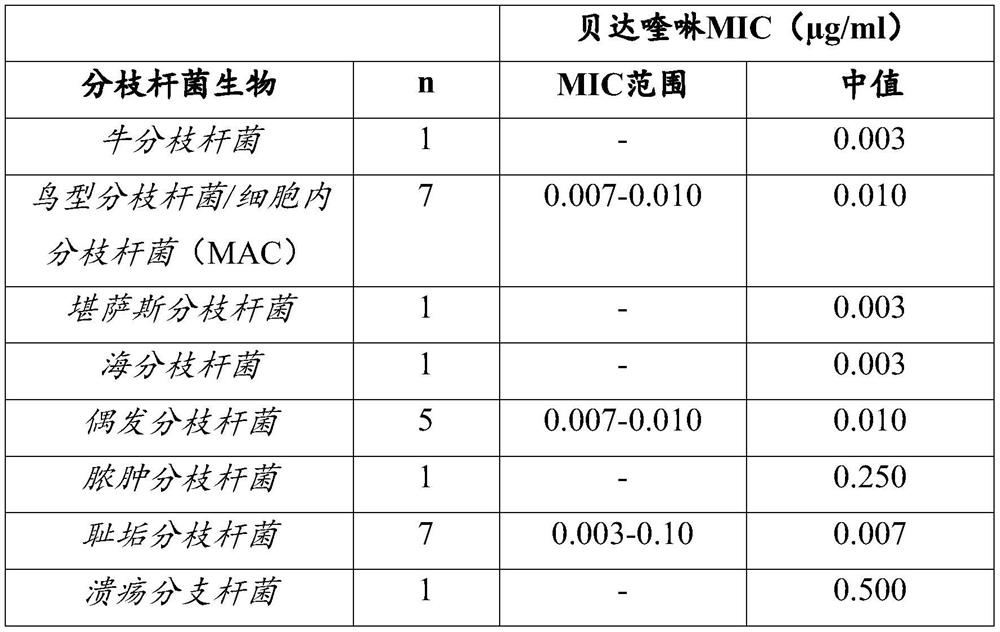
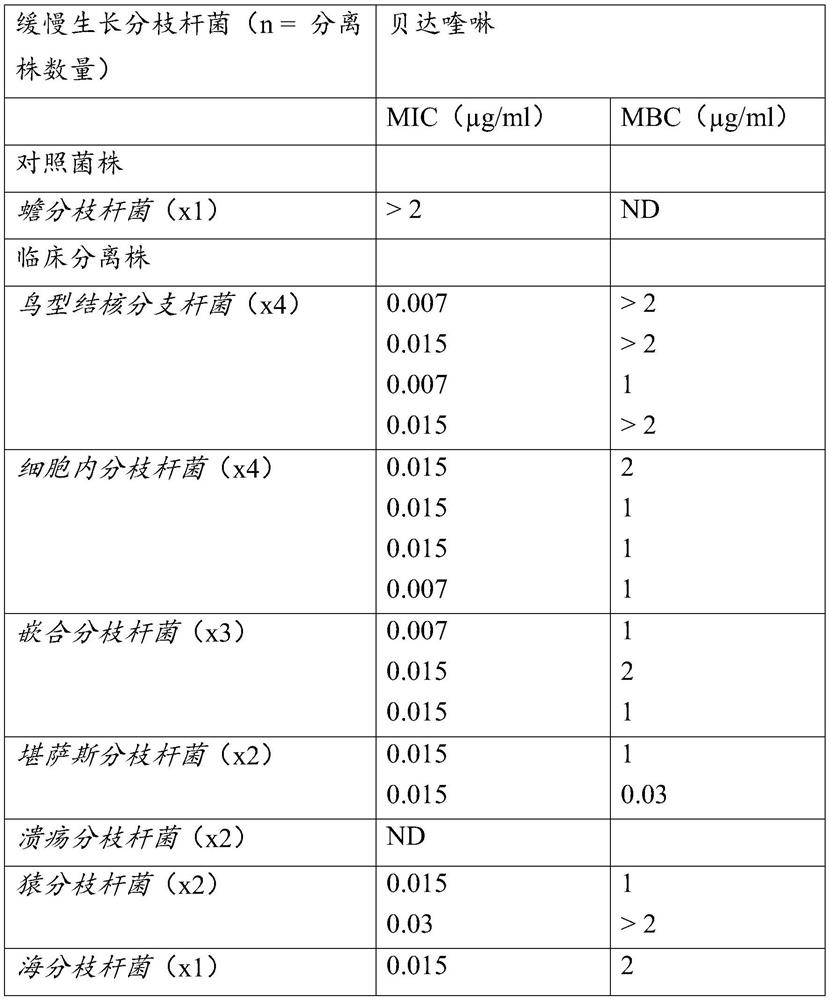
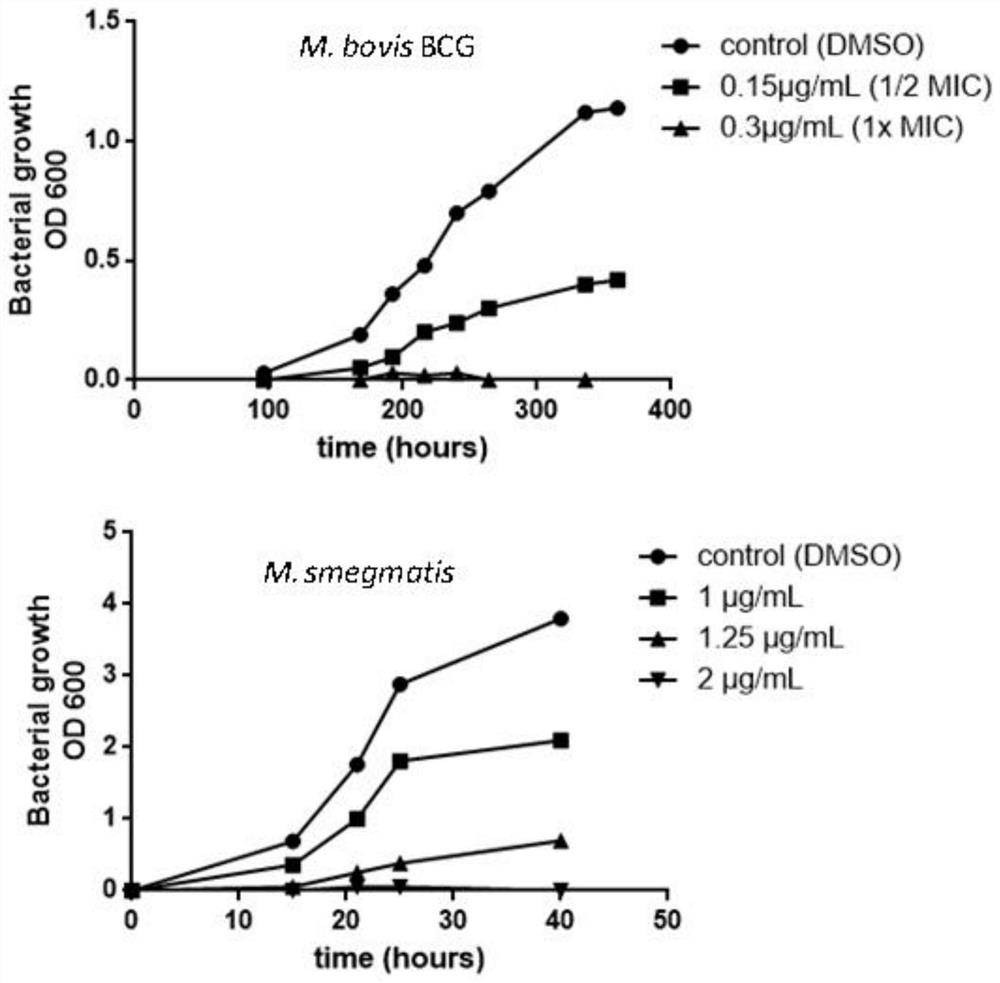
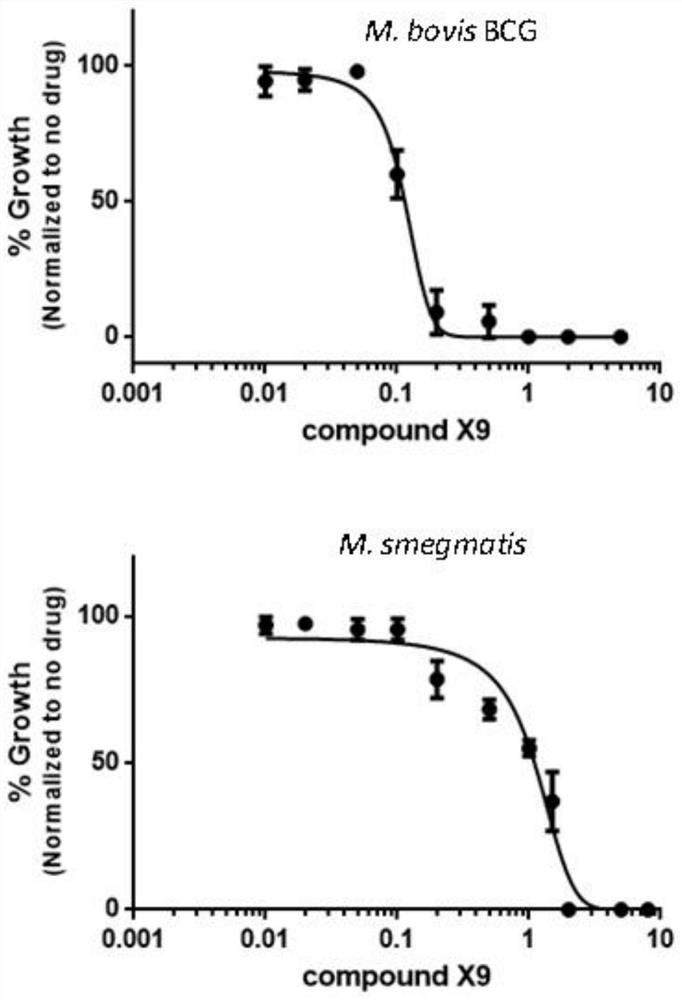
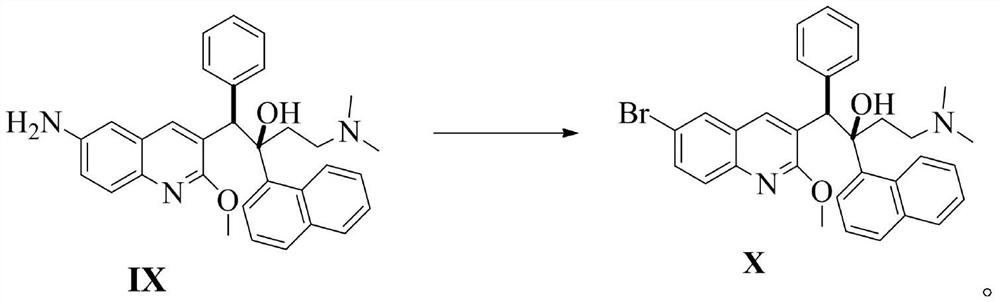
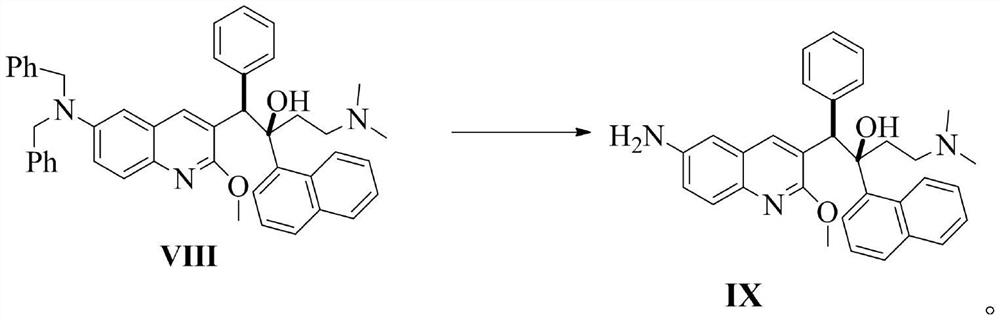
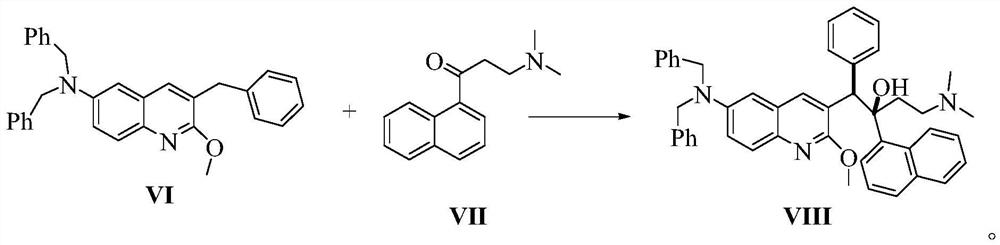
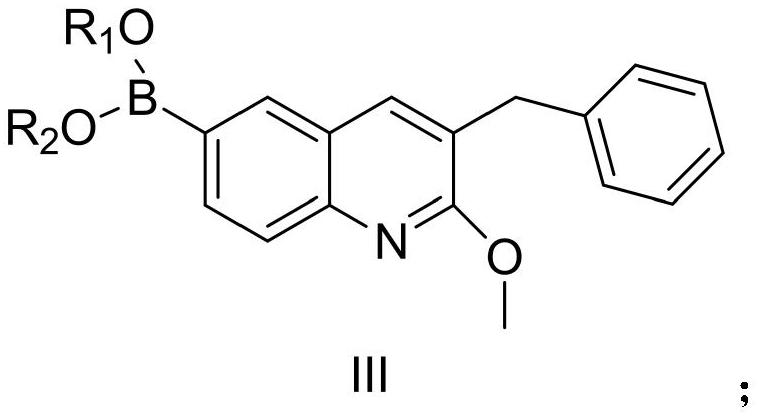
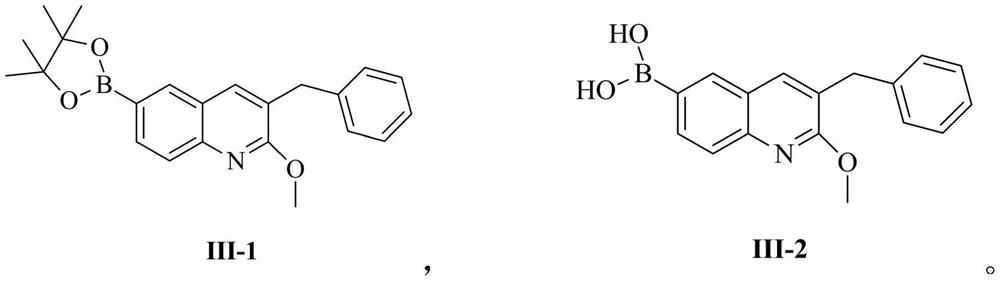
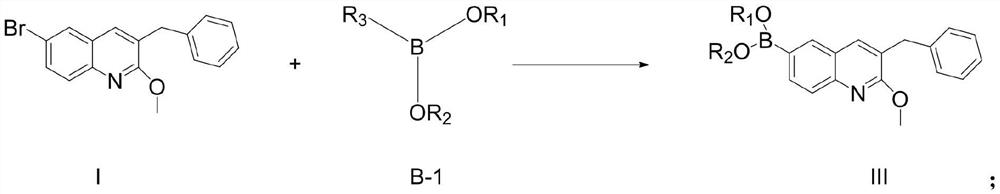
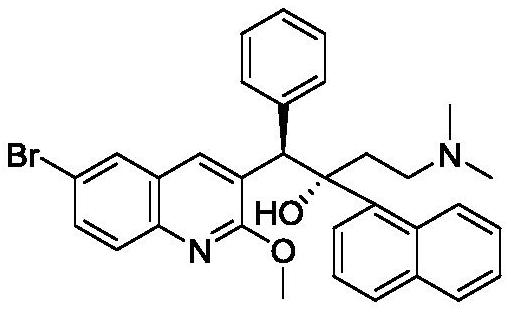
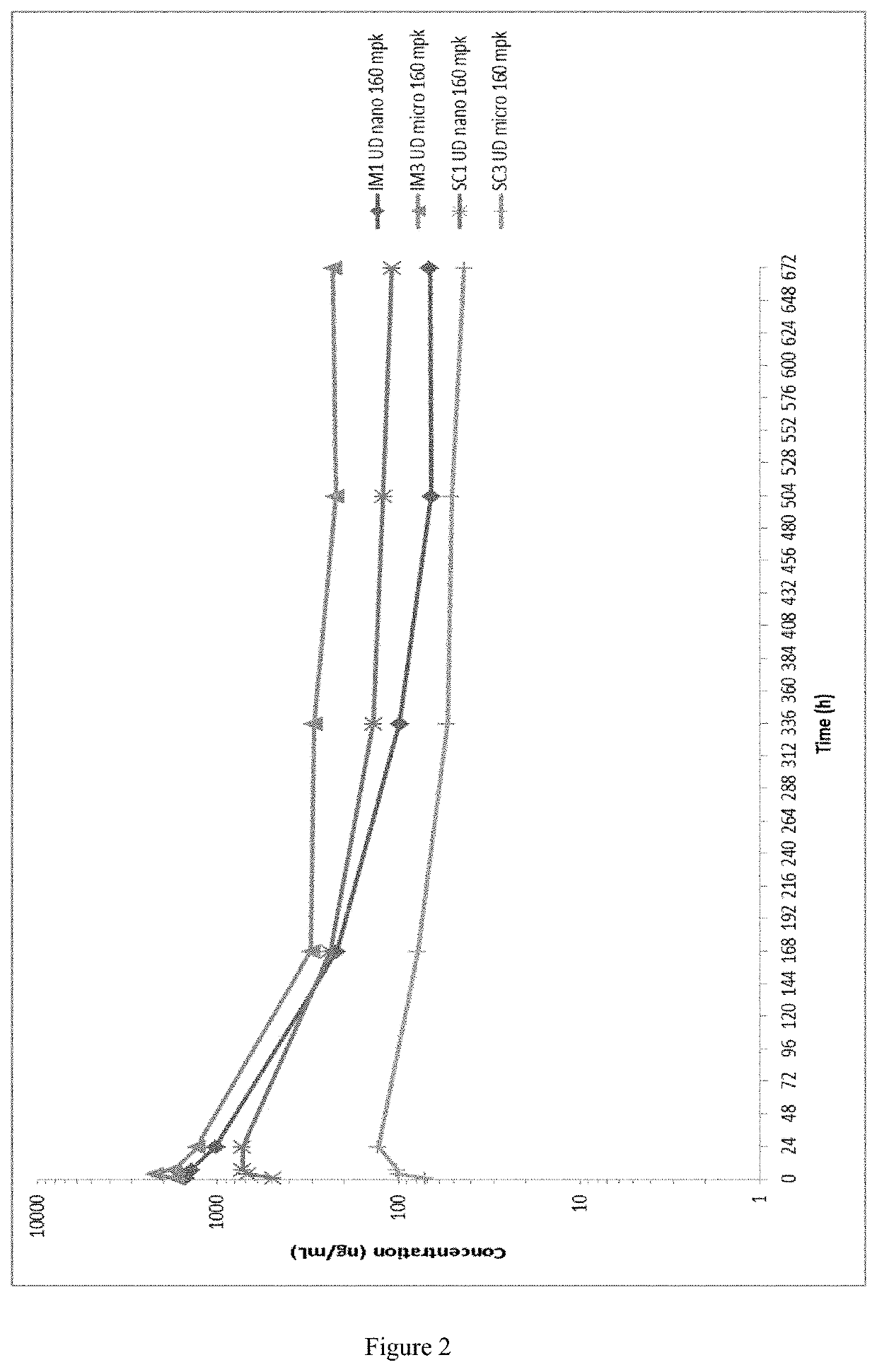
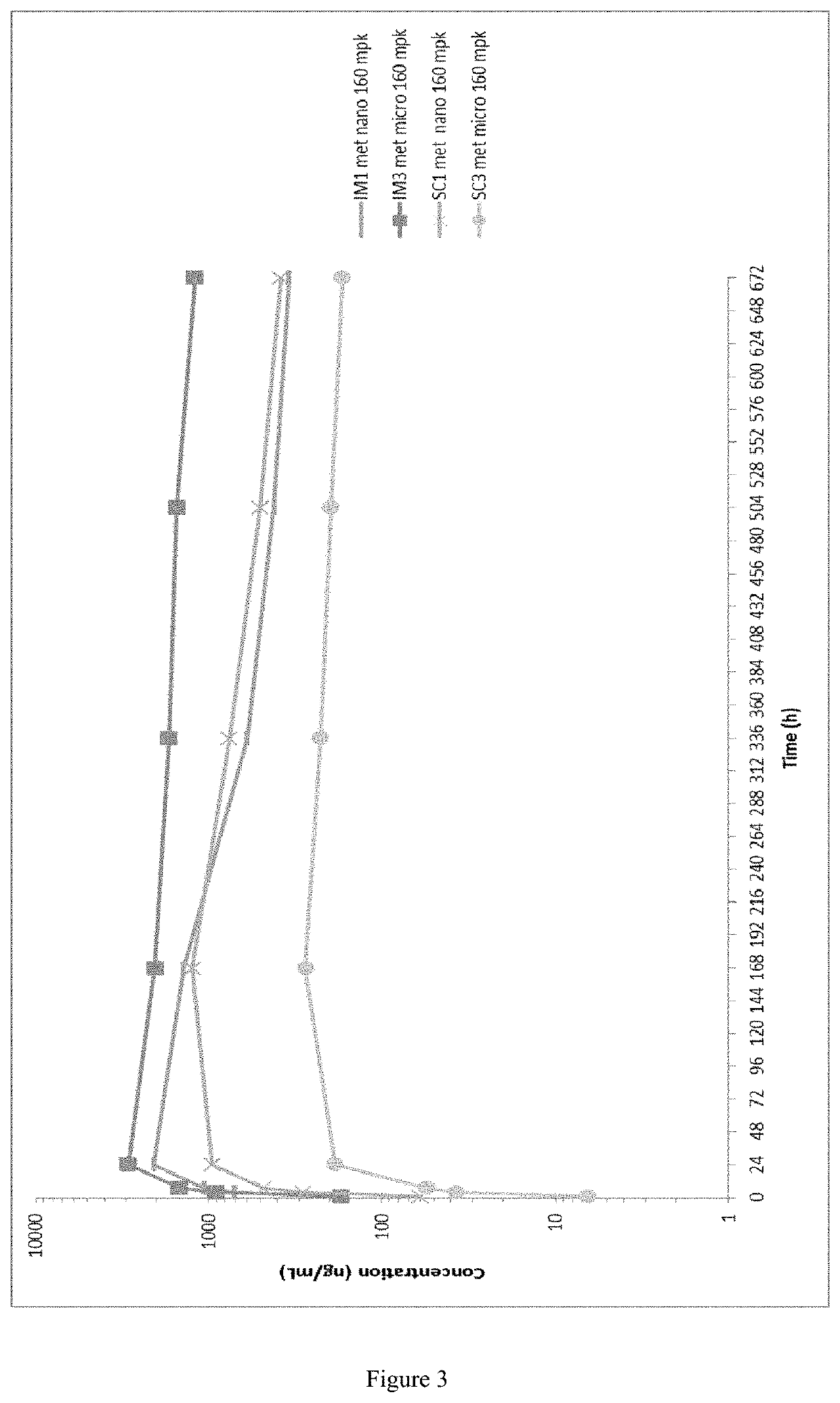
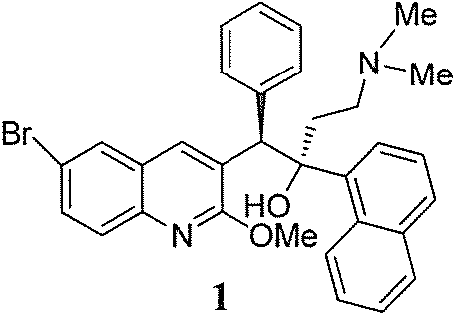
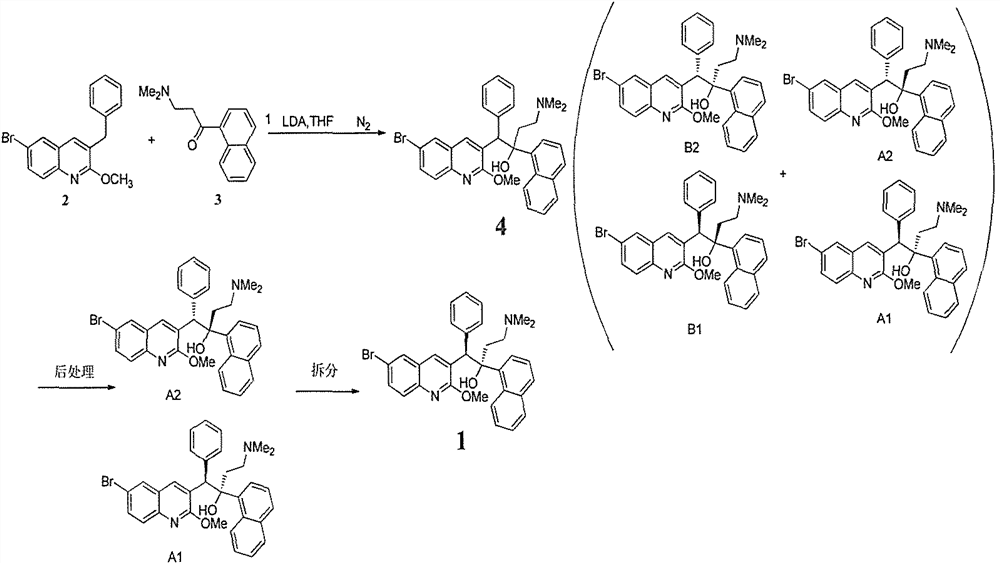
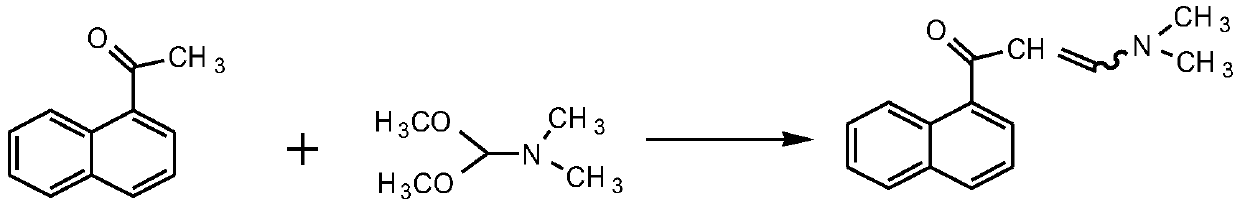
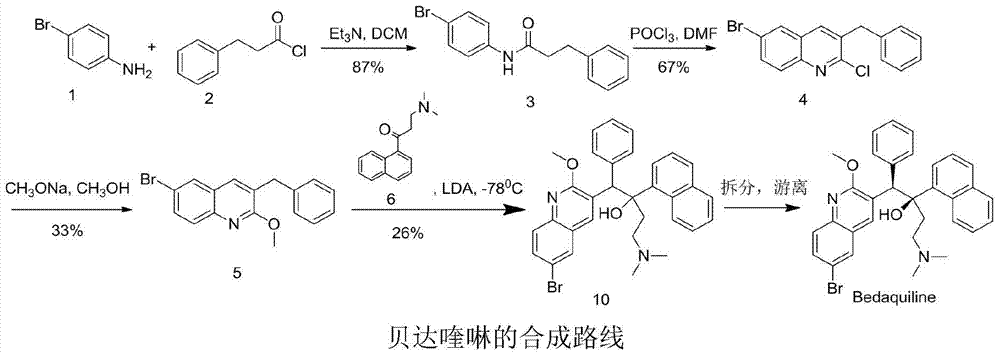
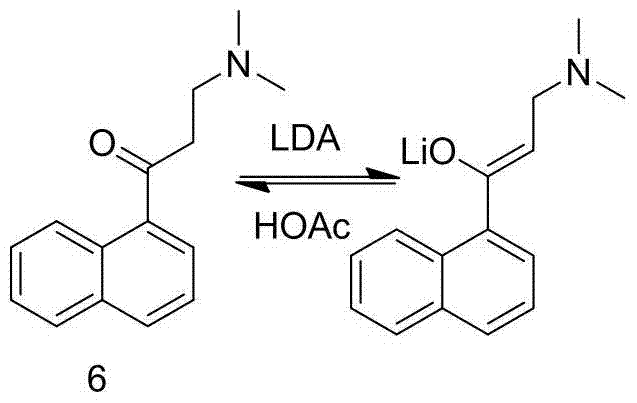
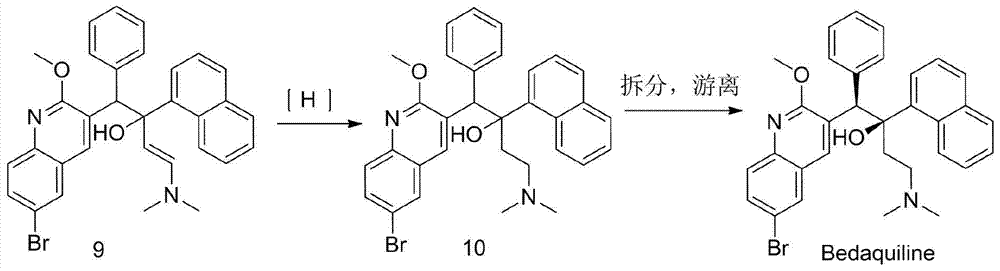
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
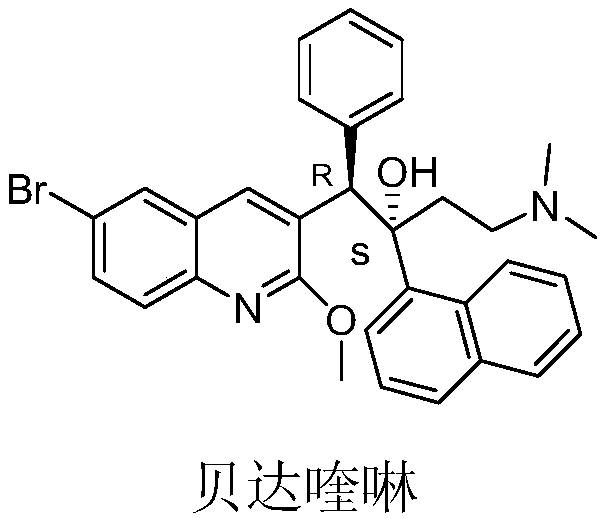
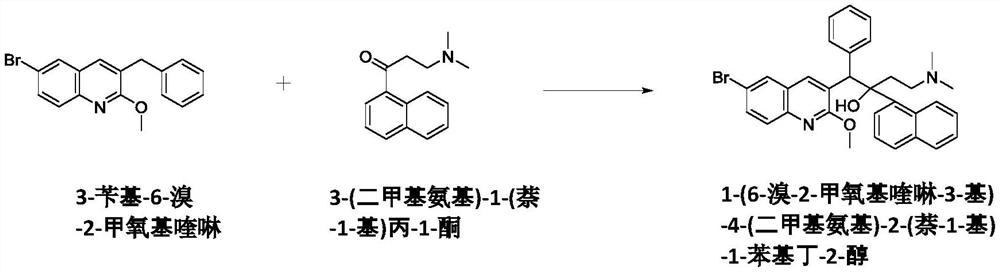
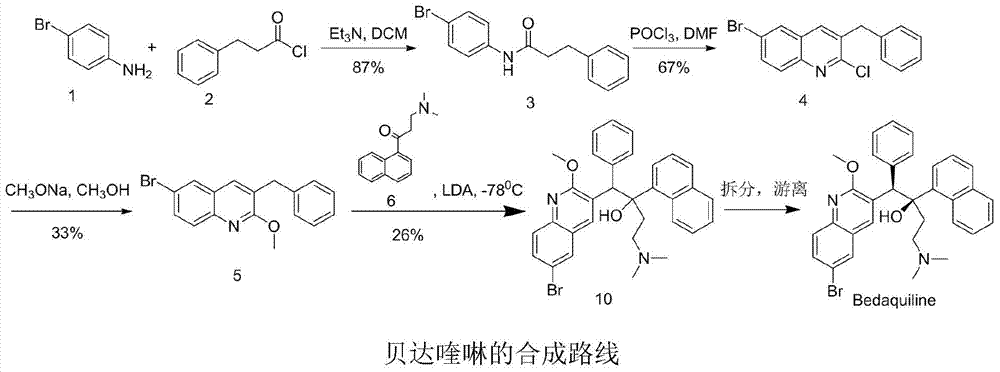
33 results about "Bedaquiline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
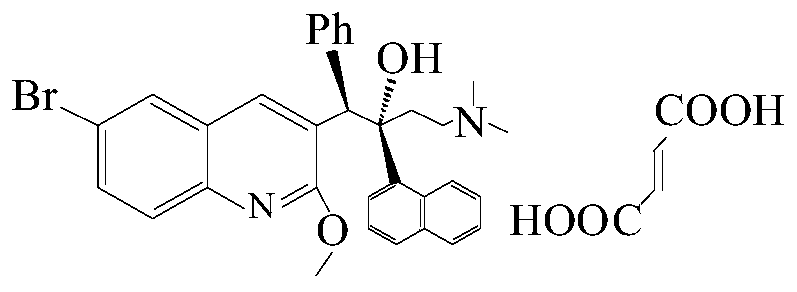
This medication must be used with other medications to treat active multi-drug-resistant tuberculosis (TB) of the lungs in people with limited treatment options.
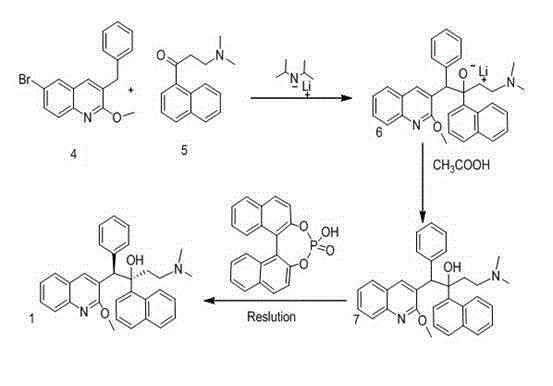
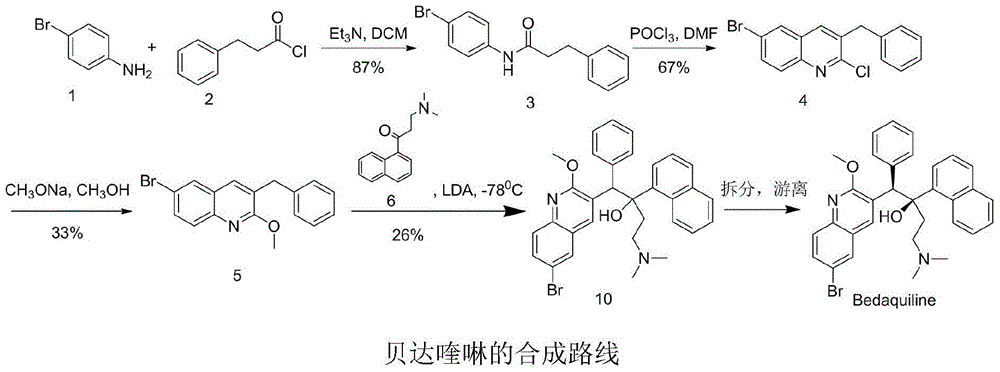
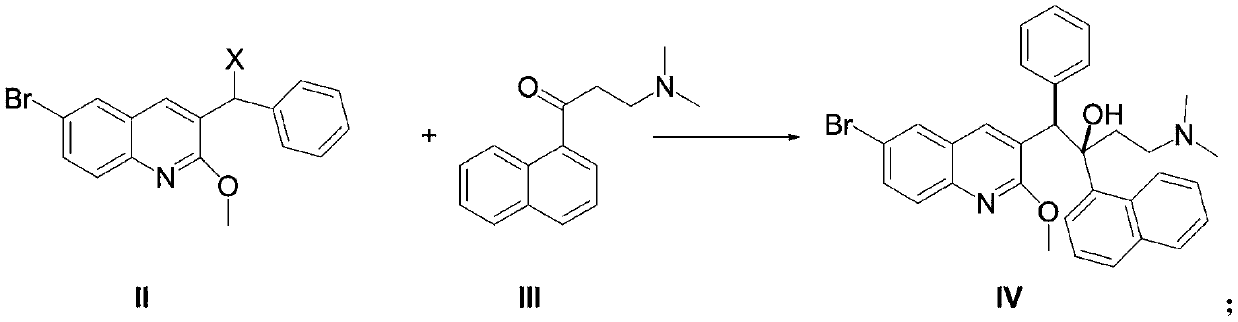
New synthesis route and method of bedaquiline racemate
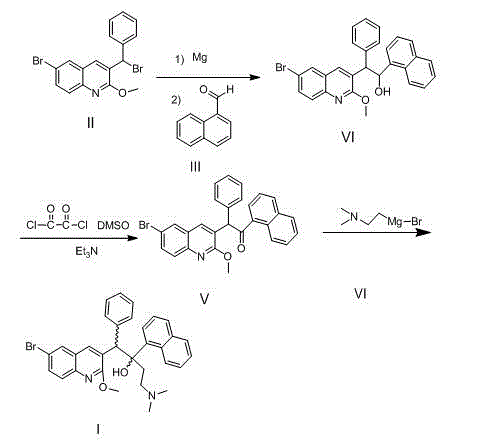
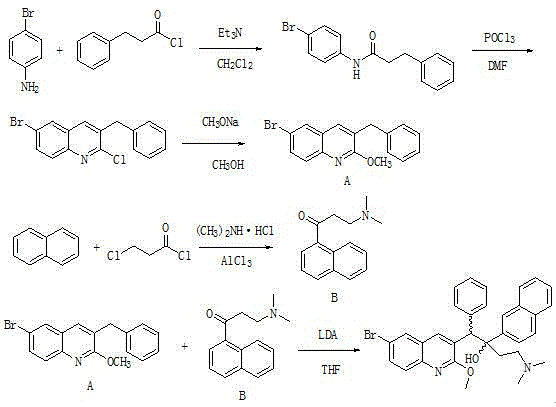
The invention relates to a new synthesis route and method of a bedaquiline racemate, and belongs to the medical technical field. The method comprises the steps: (1) carrying out a reaction of a starting material 3-bromobenzyl-6-bromo-2-methoxyquinoline (II) with metal magnesium in THF to prepare a Grignard reagent, then adding 1-naphthaldehyde (III) in the prepared Grignard reagent, carrying out a reflux reaction to obtain a compound (IV) 2-(6-bromo-2-methoxyquinoline-3-yl)-1-(naphthalen-1-yl)-2-phenylethanol; (2) undergoing swern oxidation of the compound (IV) to obtain a carbonyl compound 2-(6-bromo-2-methoxyquinoline-3-yl)-1-(naphthalen-1-yl)-2-phenyl ethyl ketone (a compound V); and (3) carrying out a Grignard reaction of the compound (V) with the Grignard reagent (VI) to obtain the bedaquiline racemate (I). The method is simple in route, is mild in reaction conditions, low in cost, high in yield, and suitable for industrialized production application.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +1
Preparation method for bedaquiline
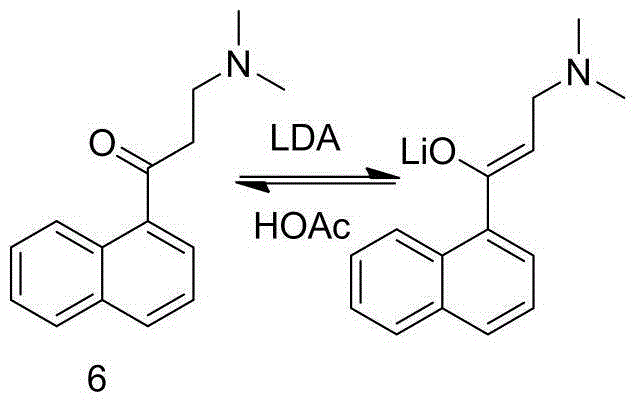
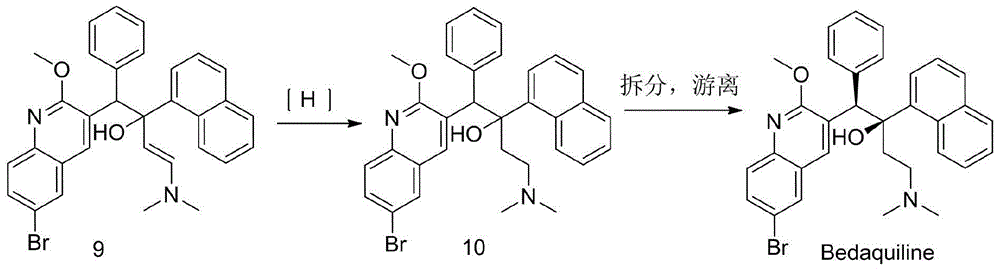
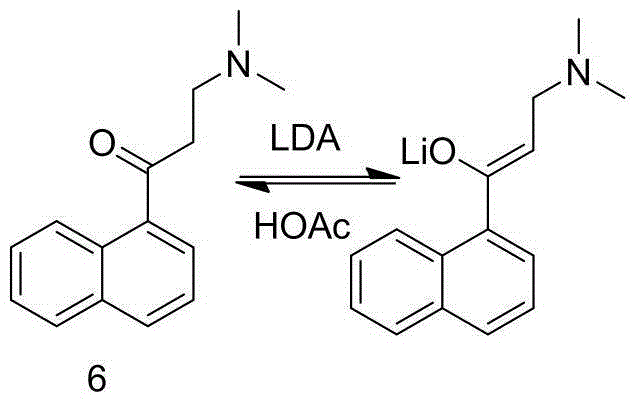
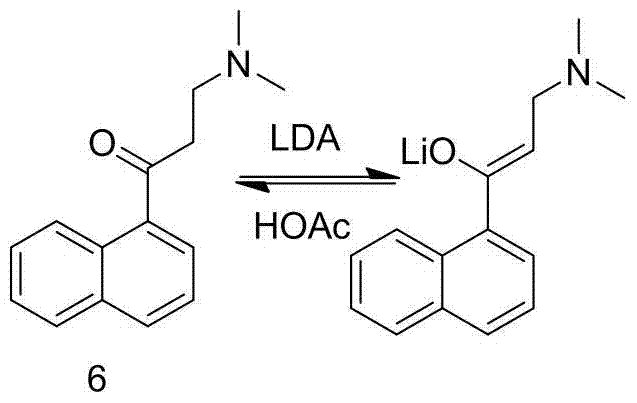
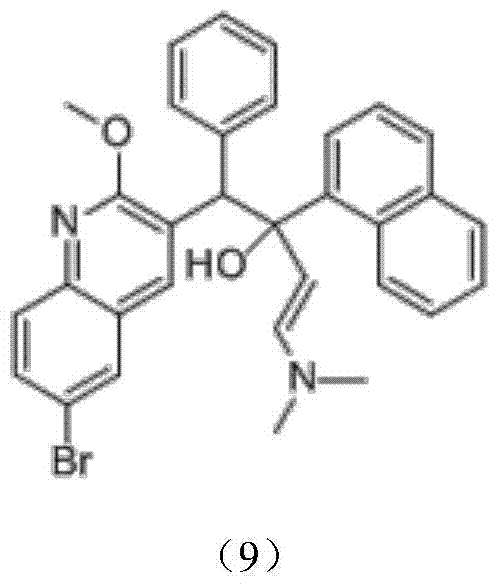
The invention discloses a preparation method for bedaquiline. The preparation method comprises the following steps: enabling a compound (9) to be reacted with a reducing agent in a solvent; and then collecting racemate of bedaquiline from a reaction product. The preparation method has the advantages that the compound (9) is a novel compound which has not been reported in literature; the racemate of bedaquiline is prepared from a compound (8) and the compound (9); the obtained product is greatly increased in yield (greater than 47%) which is remarkably greater than the yield (26%) in the original patent; and the obtained racemate of bedaquiline is high in purity, stable and controllable in quality, and beneficial for subsequent resolution reaction, and has relatively great positive effects and relatively high practical application value. The reaction formula is shown as follows: a FORMULA as shown in the description.
Owner:CHINA NAT MEDICINES GUORUI PHARMA +1
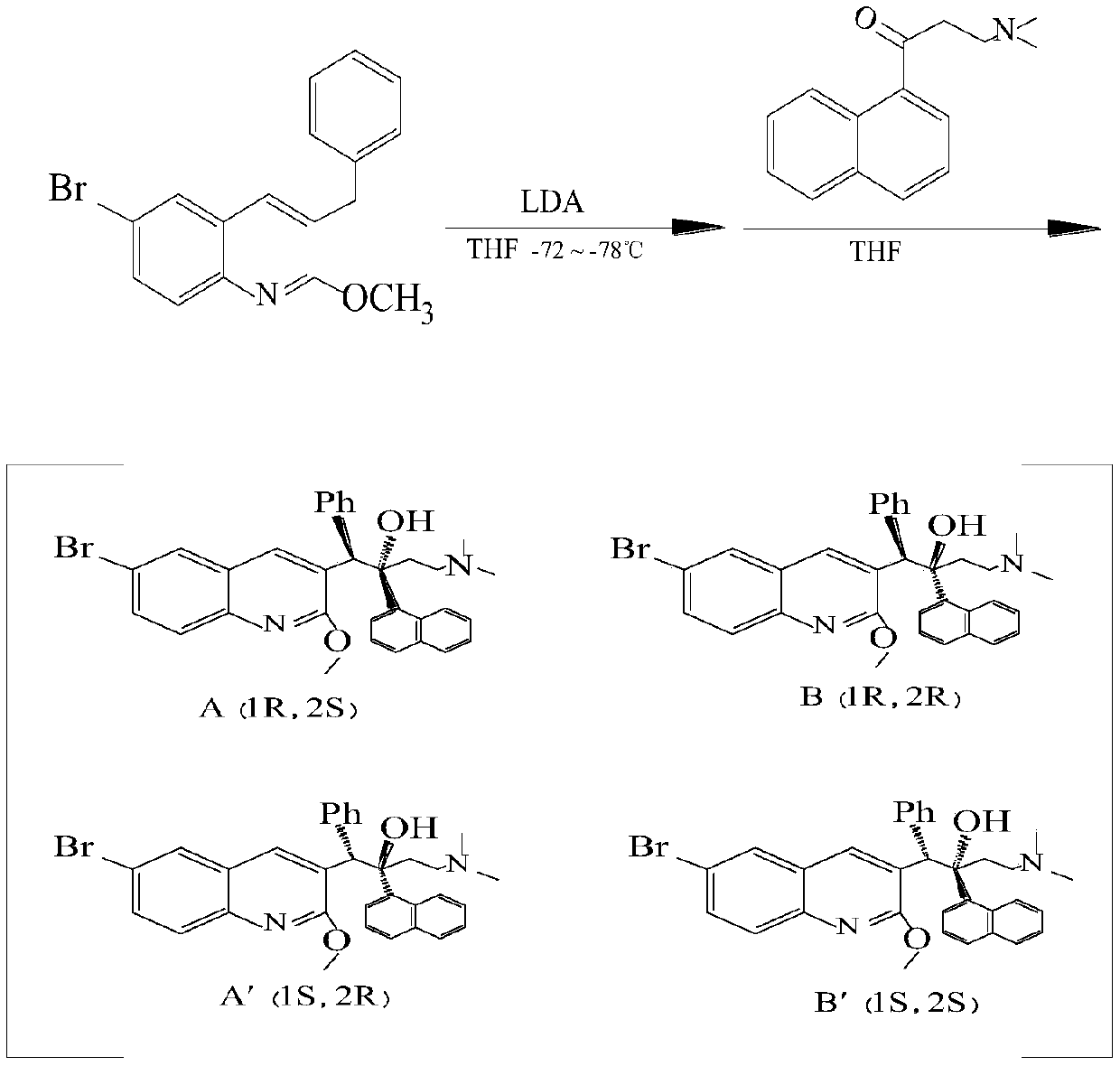
Method for high efficiency production of Bedaquiline
ActiveCN105198808AReduce yieldAvoid wastingOrganic chemistryOrganic compound preparationCompound aQuinolizine
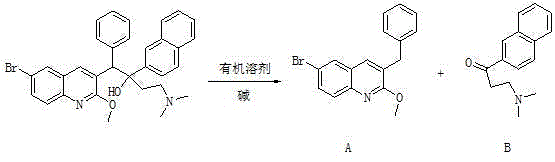
The invention relates to a method for high efficiency production of Bedaquiline. The method comprises that through optical resolution of 1-(6-bromo-2-methoxy-3-quinolyl)-4-dimethylamino-2-(1-naphthyl)-1-phenyl-2-butanol, Bedaquiline and other isomers are obtained, the other isomers undergo a reaction under the action of an alkali to produce key intermediate compounds A and B, and the key intermediate compounds A and B are separated and undergo a reaction to produce Bedaquiline. The method realizes high efficiency production of Bedaquiline, prevents large waste of materials, saves a cost and is suitable for large scale industrial production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Intermediate for preparing bedaquiline, preparation method therefor and application thereof
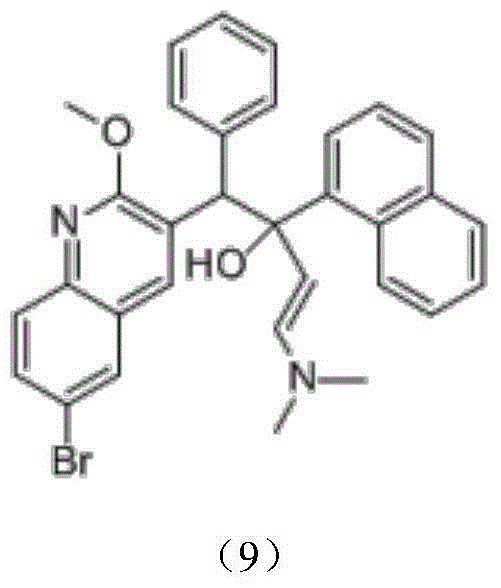
The invention discloses an intermediate for preparing bedaquiline and a preparation method therefor. The intermediate disclosed by the invention has the advantages that the intermediate avoids hydrogenation and enolization of an alpha-site in the intermediate, reduces occurrence of side reactions, and increases the conversion rate of raw materials and the total yield of reaction, and is suitable for large-scale industrial production. The intermediate for preparing bedaquiline is characterized by being a compound with a structural formula (9) or an optical isomer thereof: FORMULA is shown in the description.
Owner:CHINA NAT MEDICINES GUORUI PHARMA +1
Method for recycling and utilizing Bedaquiline stereochemical isomers
ActiveCN105017147AIncrease profitSolve the reuse problemOptically-active compound separationOrganic racemisationBromineQuinoline
The invention relates to a method for recycling and utilizing Bedaquiline stereochemical isomers. The isomers comprise (alpha R, beta R), (alpha S, beta S), and (alpha R, beta S)-6-bromine-alpha-[2-(dimethyl amidogen) ethyl]-2-methoxy-alpha-1-naphthyl-beta-phenyl-3-quinoline ethanol. The recycled isomers can generate X and Y with the high conversion rate, and X and Y can be used for synthesizing Bedaquiline again. According to the process method, the production cost of the Bedaquiline can be greatly lowered, and the utilization rate of raw materials can be improved. See the formula in the specification.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI
Method for detecting anti-tuberculosis drug in serum by ultra-high performance liquid chromatography-tandem mass spectrometry technology
InactiveCN111766311AHigh sensitivityStrong specificityComponent separationAntituberculosis drugPyrazine
The invention discloses a method for detecting an anti-tuberculosis drug in serum by an ultra-high performance liquid chromatography-tandem mass spectrometry technology. The antitubercular drug comprises cycloserine (CYS), pyrazinamide (PZN), isoniazid (INZ), p-aminosalicylic acid (P-ASA), ethiisonicotinamide (ETN), ethambutol (ETB), clofazimine (CFM), bedaquiline (BDQ), rifampicin (RFP), rifbutine (RFB) and rifapentine (RFT). The method includes: detecting the content of the antituberculosis drug in the pretreated serum by adopting an ultra-high performance liquid chromatography-tandem mass spectrometry method, performing quantifying by utilizing a mass spectrometry isotope internal standard method, establishing a calibration curve by taking the concentration ratio of the standard substance to the internal standard substance as an X axis and the peak area ratio of the standard substance to the internal standard substance as a Y axis, and calculating the concentration of the target drug in the serum; the method is high in sensitivity, strong in specificity and simple in pretreatment process, separation and detection of the anti-tuberculosis drugs in serum are completed within 5 min, and a simple and rapid detection method is provided for clinical concentration monitoring of the anti-tuberculosis drugs.
Owner:南京品生医学检验实验室有限公司
Kit and method for detecting antituberculous drugs and metabolites thereof in sample
PendingCN114354804AExpand the types of testingIncrease varietyComponent separationMetaboliteAntituberculous drug
The invention particularly provides a kit and a method for detecting antituberculous drugs and metabolites thereof in a sample. The kit is used for detecting antituberculous drugs and metabolites thereof in a sample, and comprises a calibration product, a quality control product, an instrument quality control product and an isotope internal standard product, both the calibration material and the quality control material contain rifampicin, isoniazide, rifapentine, pyrazinamide, ethambutol, clofazimine, cycloserine, moxifloxacin, levofloxacin, linezolid, acetyl isoniazide, bedaquiline and deacetylrifampicin; the instrument quality control product comprises a methanol solution containing rifampicin, isoniazid, rifapentine, pyrazinamide, ethambutol, clofazimine, cycloserine, moxifloxacin, levofloxacin, linezolid, acetyl isoniazid, bedaquiline and deacetylrifampicin; the isotope internal standard substance contains an internal standard substance corresponding to a substance contained in the calibrator. The kit disclosed by the invention can be used for detecting the concentrations of the antituberculous drugs and metabolites thereof in various sample types.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
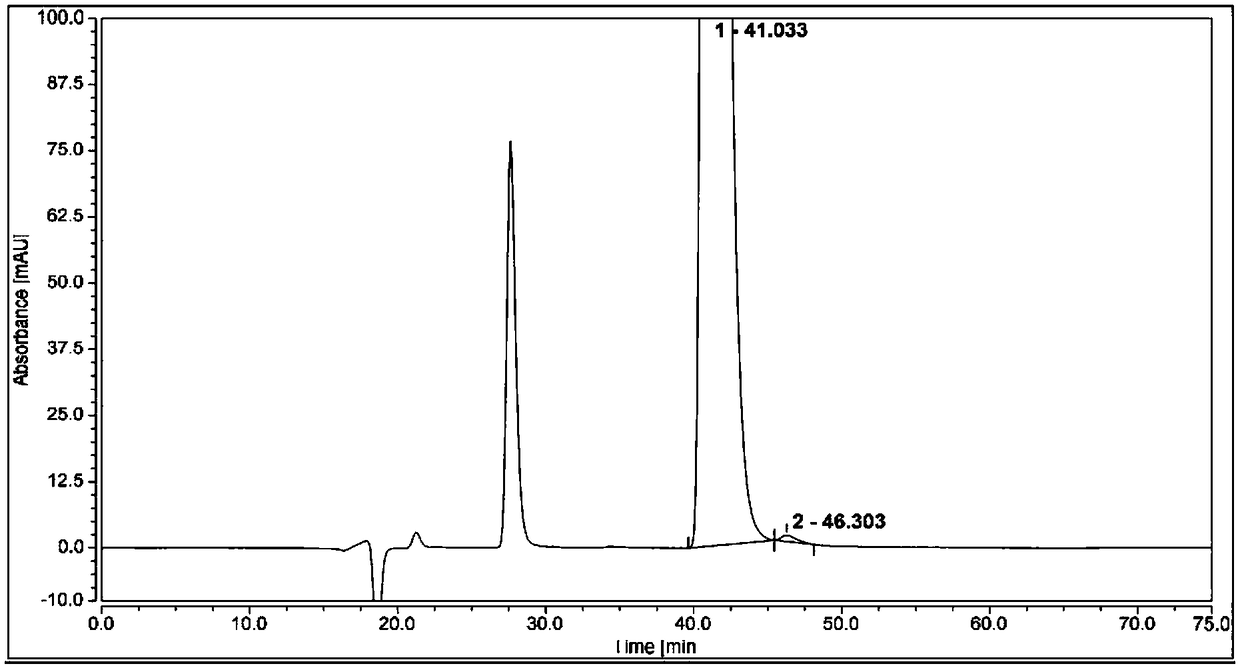
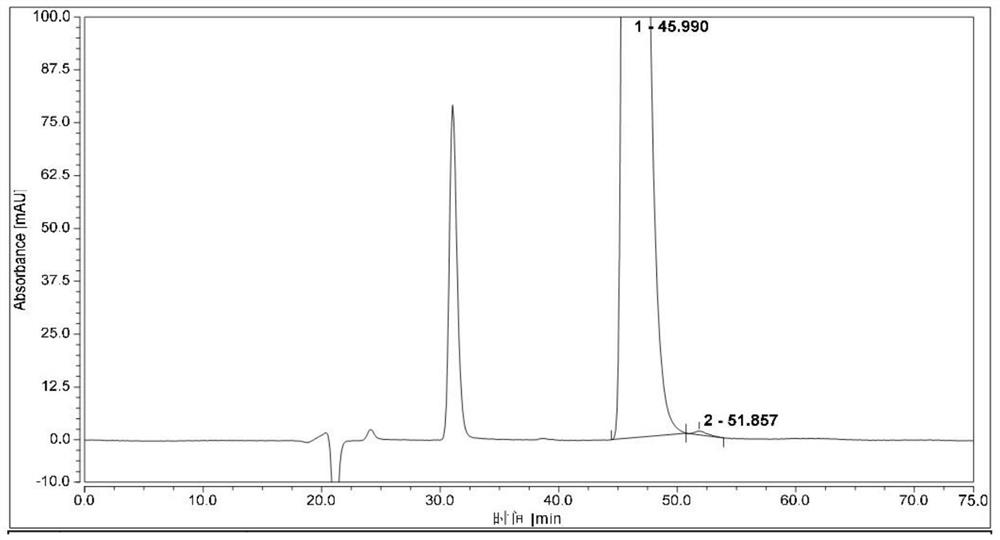
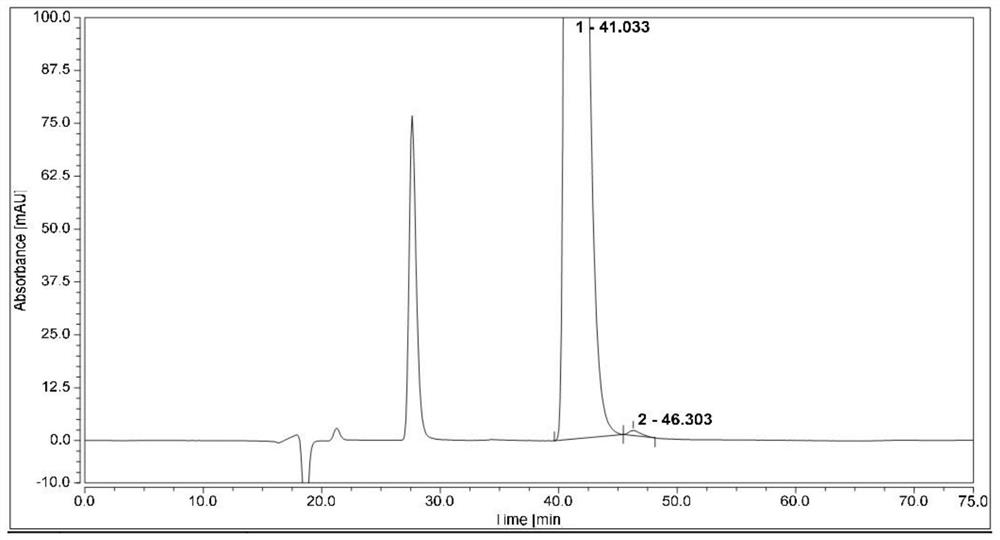
Method for separating and analyzing optical isomers of bedaquiline
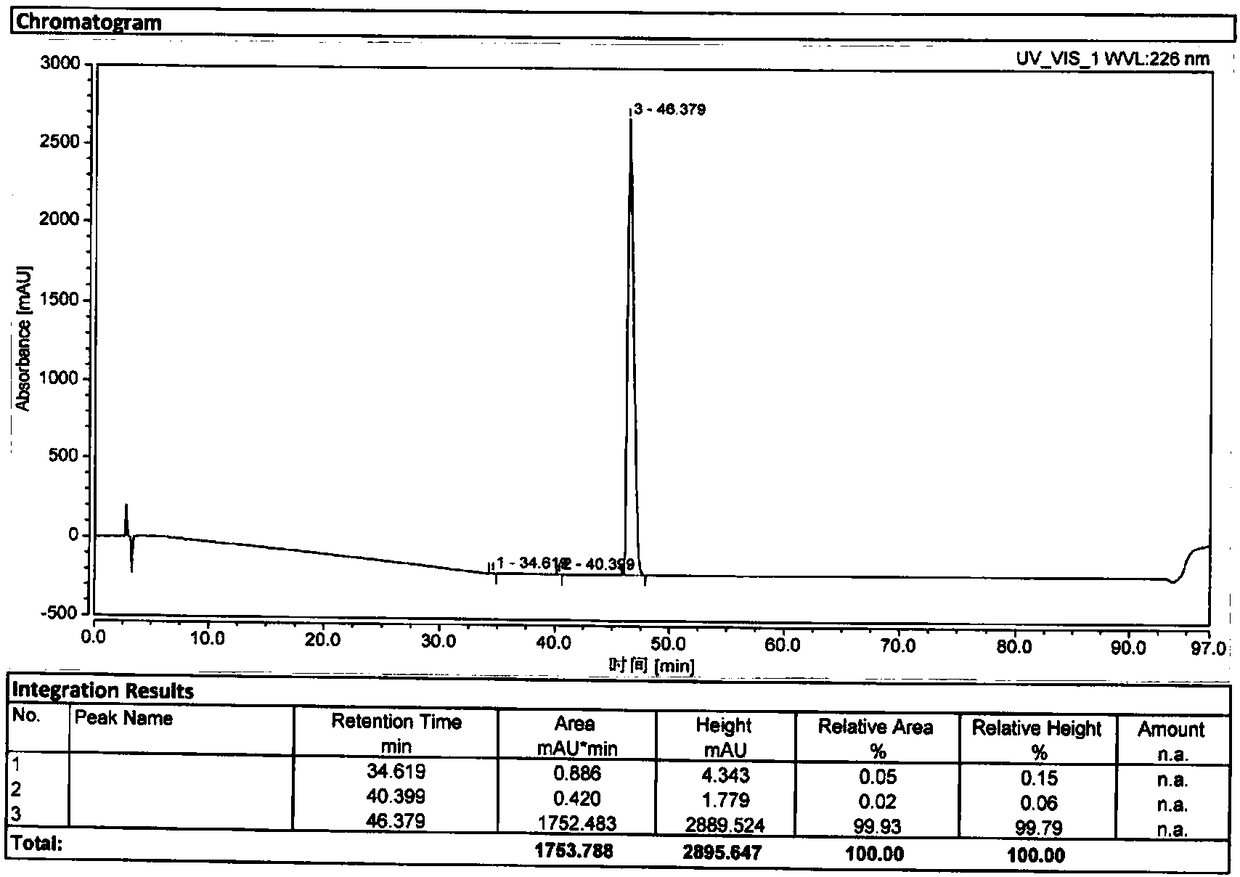
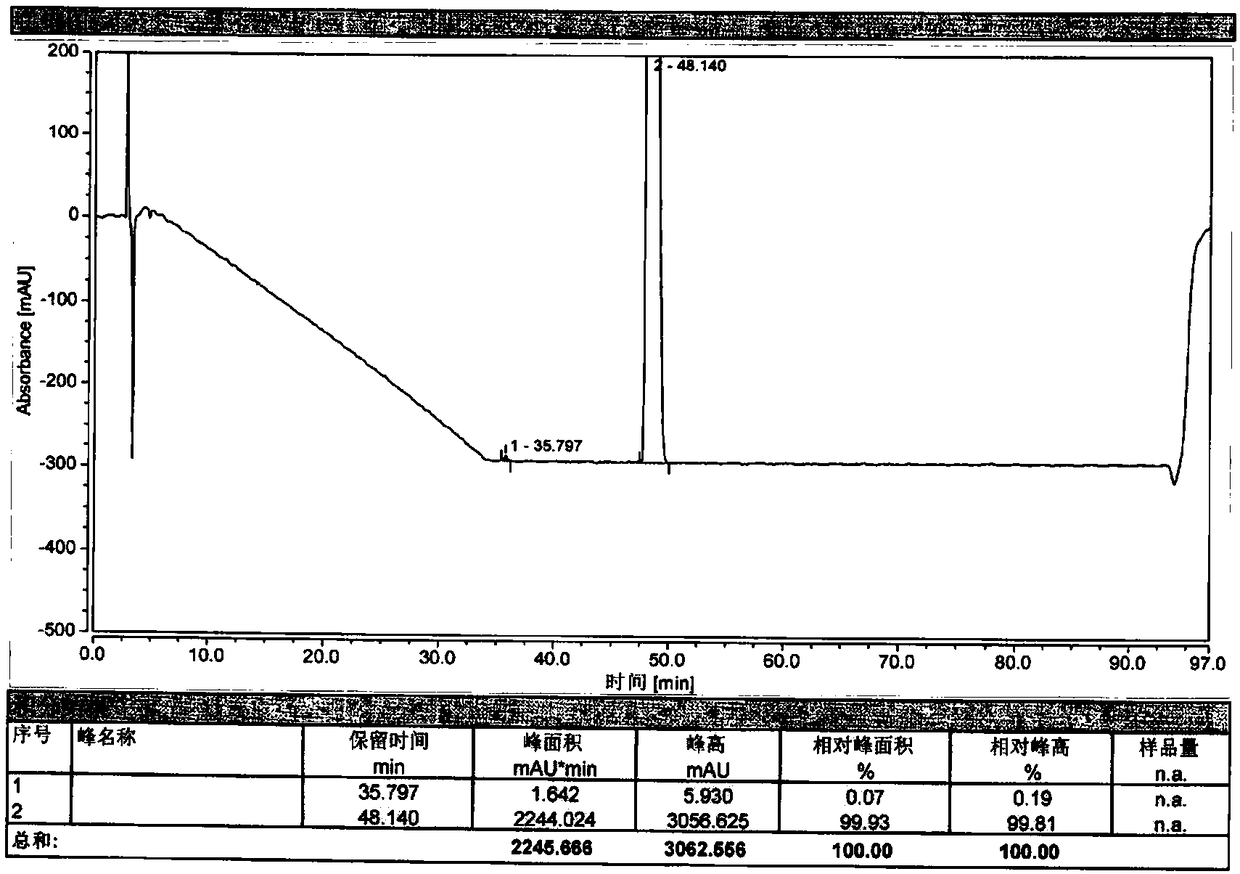
The invention relates to a method for separating and analyzing optical isomers of bedaquiline, belonging to the field of liquid chromatography. The method is reversed-phase high-performance liquid chromatography; a selected chromatographic column is an amphoteric ion exchange chiral column; the column temperature of the chromatographic column is 9-20 DEG C; a mobile phase is a mixed solution of 2mL to 3 mL of diethylamine, 1.5 mL to 2.5 mL of formic acid and 1000 mL of methanol; the velocity of the mobile phase is 0.08 mL / min to 0.12 mL / min; a detection wavelength is 220 nm to 230 nm; the resolution of a (1S, 2R)-bedaquiline peak and a (1R, 2S)-bedaquiline peak on a chromatogram is greater than 1.5. The method of the invention can effectively separate (1R, 2S)-bedaquiline and / or (1S, 2R)-bedaquiline, and / or detect the purity and / or the content of (1R, 2S)-bedaquiline and the purity of (1S, 2R)-bedaquiline.
Owner:WUHAN WUYAO SCI & TECH
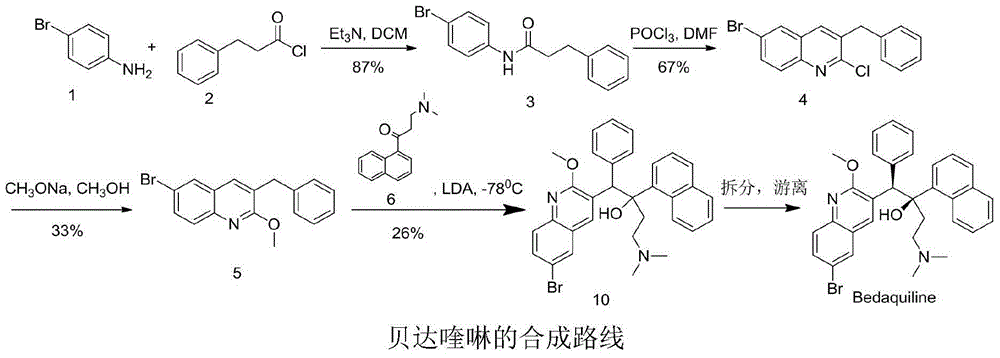
Preparation method of bedaquiline
PendingCN111574444AEasy to synthesizeEfficient synthesisOrganic chemistryPhysical chemistryQuinoline
The invention provides a method for preparing bedaquiline shown as a formula IV (See the specification). The method comprises the step of reacting a compound II with a compound III. According to the method disclosed by the invention, the ultralow-temperature reaction of the original process is changed, and the ultralow-temperature reaction which is difficult to realize industrially in the past iscarried out at the conventional temperature (heating condition), so that large-scale industrial production becomes possible. Besides, the method shortens the reaction route, enhances the conversion rate and reaction yield of the reaction substrate, enables the product to be easier to crystallize and purify, and lowers the production cost.
Owner:ANHUI BIOCHEM BIO PHARMA
A method for reclaiming and utilizing bedaquiline stereochemical isomers
ActiveCN105017147BIncrease profitSolve the reuse problemOptically-active compound separationOrganic racemisationBromineQuinoline
The invention relates to a method for recycling and utilizing Bedaquiline stereochemical isomers. The isomers comprise (alpha R, beta R), (alpha S, beta S), and (alpha R, beta S)-6-bromine-alpha-[2-(dimethyl amidogen) ethyl]-2-methoxy-alpha-1-naphthyl-beta-phenyl-3-quinoline ethanol. The recycled isomers can generate X and Y with the high conversion rate, and X and Y can be used for synthesizing Bedaquiline again. According to the process method, the production cost of the Bedaquiline can be greatly lowered, and the utilization rate of raw materials can be improved. See the formula in the specification.
Owner:INST OF MATERIA MEDICA CHINESE ACAD OF MEDICAL SCI
Method for preparing (1R, 2S)-bedaquiline and (1S, 2R)-bedaquiline
ActiveCN107857727AReduce residualHigh purityOptically-active compound separationAsymmetric synthesesChemical synthesisCarbenium ion
The invention discloses a method for preparing (1R, 2S)-bedaquiline and (1S, 2R)-bedaquiline and belongs to the technical field of chemical synthesis. The method comprises the steps of subjecting (1R,2R)-bedaquiline and (1S, 2S)-bedaquiline to a Lewis acid action to form carbenium ions, then, subjecting the carbenium ions to action by OH<-> in an alkaline solution to form novel chiral tertiary alcohol, and then, carrying out resolution, thereby preparing (1R, 2S)-bedaquiline. According to the method, the number of reaction steps is small, the production efficiency is greatly increased, the (1R, 2S)-bedaquiline of relatively high yield is easy to obtain, and thus, the industrial production is facilitated; and meanwhile, the impurity residual is low, the purity of target products is high, and dangerous steps such as high-pressure hydrogenation are not required to be added, so that the entire production process is high in safety.
Owner:JIANGSU TIANHE PHARMA CO LTD
Long-acting formulations
This invention concerns pharmaceutical compositions for administration via intramuscular or subcutaneous injection, comprising micro- or nanoparticles of the anti-TB compound bedaquiline, suspended in an aqueous pharmaceutically acceptable carrier, and the use of such pharmaceutical compositions in the treatment and prophylaxis of a pathogenic mycobacterial infection.
Owner:JANSSEN PHARMA NV
Bedaquiline medicinal preparation
ActiveCN111888477AEasy to prepareGood dissolution effectAntibacterial agentsPharmaceutical non-active ingredientsQuinolineEngineering
The invention provides a bedaquiline medicinal preparation which comprises a bedaquiline solid composition and accessories. The bedaquiline solid composition comprises bedaquiline or pharmaceuticallyacceptable salt thereof, an acidifier and an adhesive. The bedaquiline medicinal preparation prepared by the invention aims to solve a problem of poor dissolving effect of the bedaquiline and has a good dissolution rate; quality of the obtained product meets standards and requirements; and the bedaquiline medicinal preparation is suitable for industrial large-scale production.
Owner:BEIJING WINSUNNY PHARMA CO LTD
Combination in treatment of nontuberculous mycobacterial diseases
PendingCN113271930AAntibacterial agentsHeterocyclic compound active ingredientsTuberculosis mycobacteriumQuinoline
The present invention relates to a combination of bedaquiline, a macrolide (e.g. clarithromycin) and, optionally, ethambutol for use in the treatment of a disease associated with nontuberculous mycobacteria (NTM).
Owner:JANSSEN PHARMA NV
Combination in the treatment of nontuberculous mycobacterial diseases
PendingUS20220062319A1Antibacterial agentsHeterocyclic compound active ingredientsTuberculosis mycobacteriumQuinoline
The present invention relates to a combination of bedaquiline, a macrolide (e.g. clarithromycin) and, optionally, ethambutol for use in the treatment of a disease associated with nontuberculous mycobacteria (NTM).
Owner:JANSSEN PHARMA NV
Application of compound, antibacterial composition and application of antibacterial composition
ActiveCN113768909AHigh antibacterial activityImprove antibacterial propertiesAntibacterial agentsHeterocyclic compound active ingredientsProtein targetAntituberculosis drug
The invention provides application of a compound, an antibacterial composition and application of the antibacterial composition and relates to the technical field of medicinal chemical industry. The compound is a compound X9, and the antibacterial composition is prepared from the compound X9 and an antituberculosis drug. According to the compound X9 provided by the invention, the problems that in the prior art, a combined medicine for treating tuberculosis cannot achieve a very good antibacterial effect, and a new way for treating the tuberculosis is lacked, are solved, the compound X9 is high in antibacterial activity, can achieve an excellent antibacterial effect at a relatively low concentration, and can achieve a synergistic antibacterial effect with rifampin, linezolid and bedaquiline. The compound X9 has relatively high binding power with a germ target protein, and is non-toxic to cells. The compound can be used for providing a new way for treating the tuberculosis, and can be combined with the existing tuberculosis medicines for treatment to achieve a better antibacterial effect.
Owner:深圳市南山区慢性病防治院
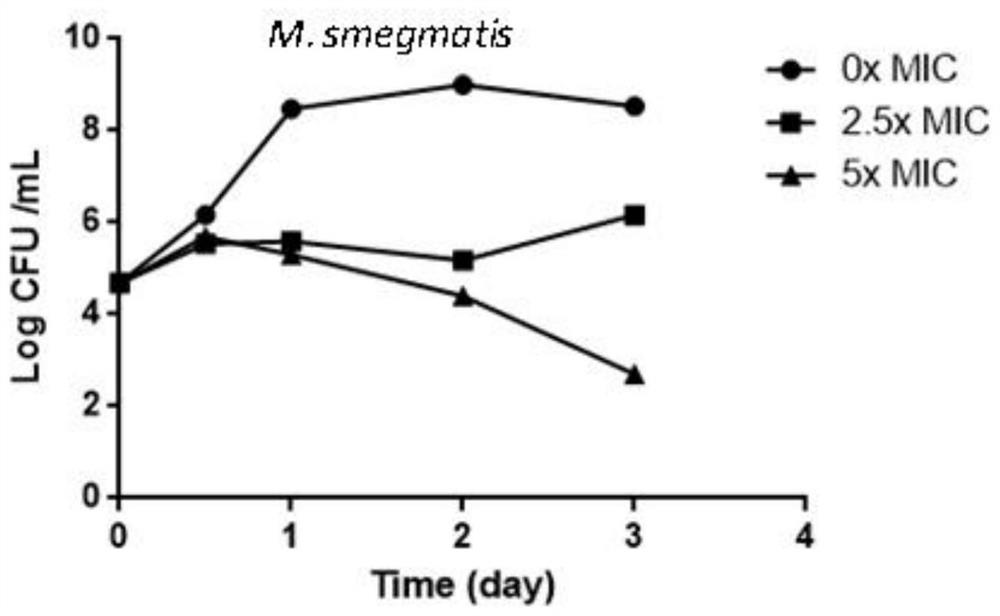
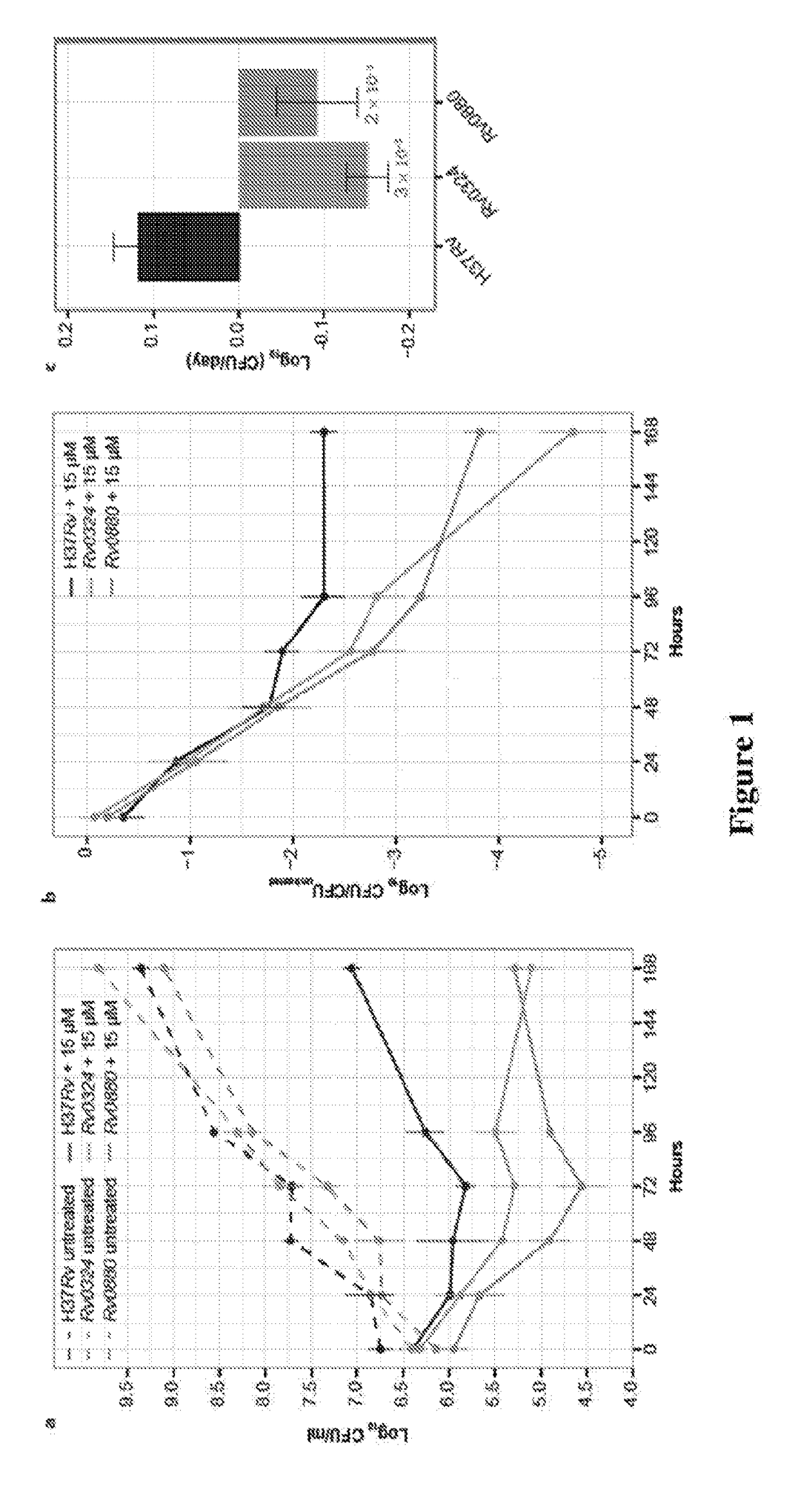
Methods to identify antituberculosis compounds
ActiveUS20190032157A1Reduce decreaseConducive to survivalAntibacterial agentsMicrobiological testing/measurementBiotechnologyQuinoline
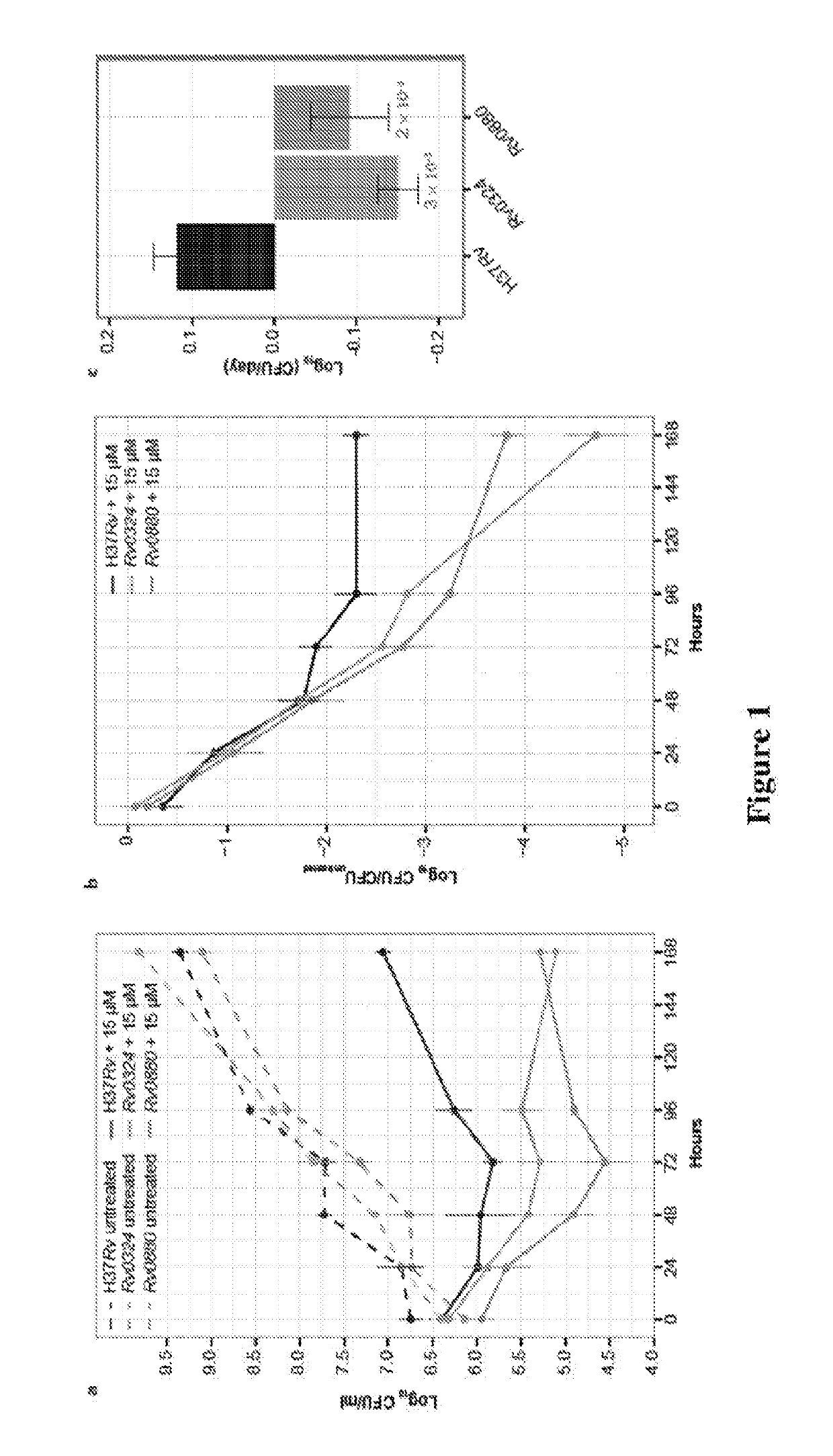
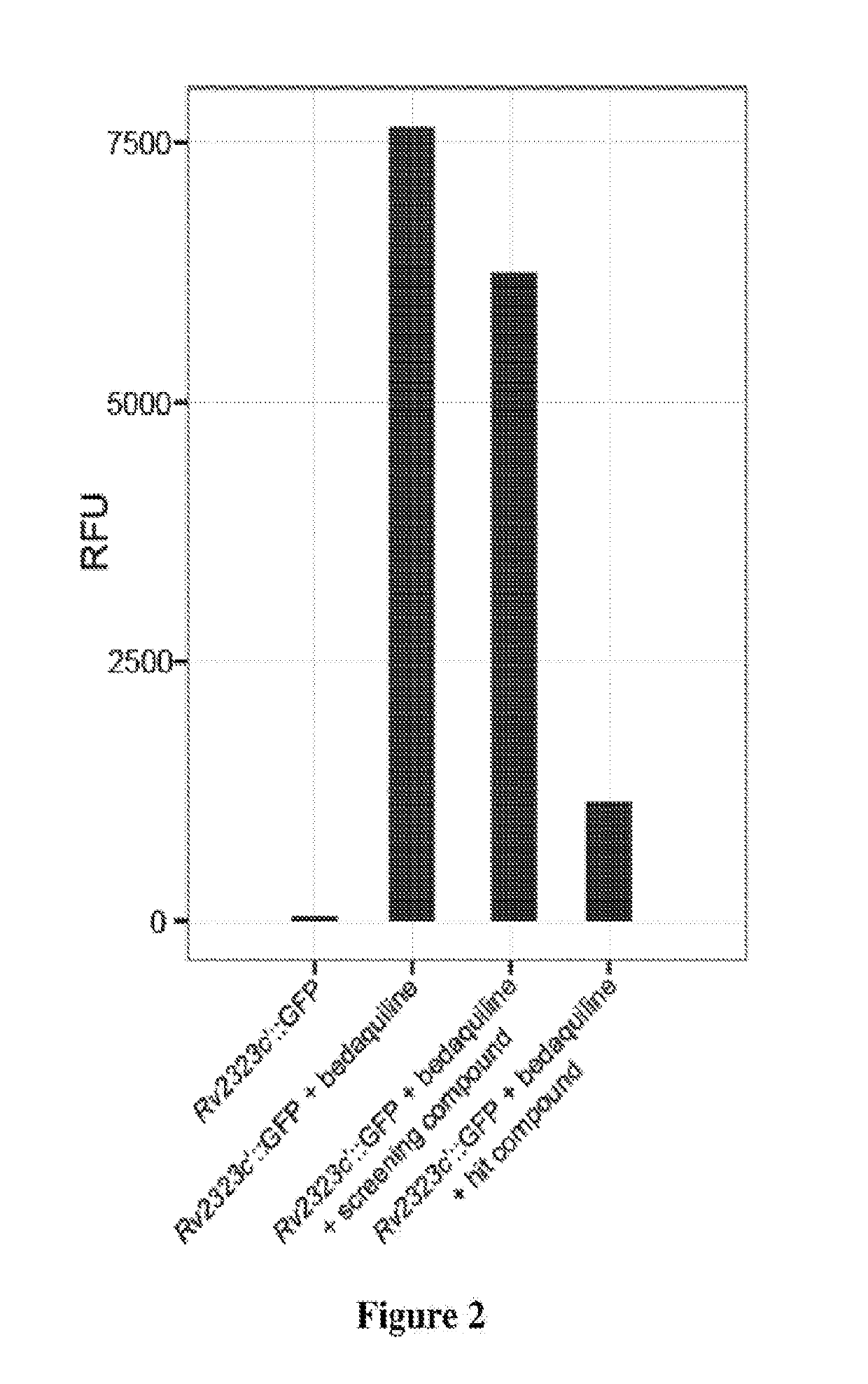
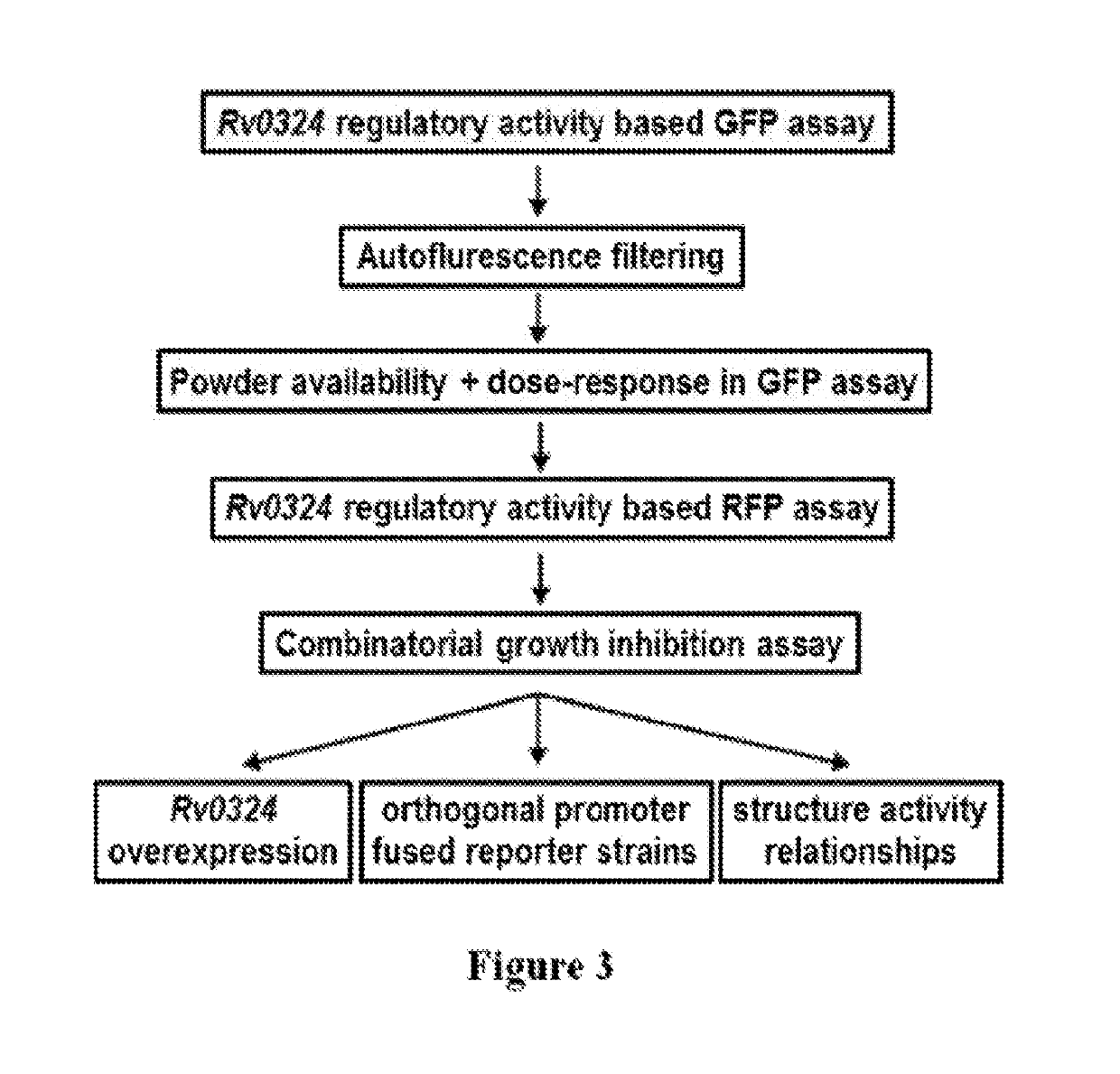
Practical assays to identify compounds that overcome the resistance of M. tuberculosis to bedaquiline are based on transcription factors Rv0324 and Rv0880 shown to mediate this resistance.
Owner:INSTITUTE FOR SYSTEMS BIOLOGY +1
Preparation method of bedaquiline and intermediate thereof
PendingCN111606850ANovel structureEase of industrial productionOrganic chemistry methodsQuinolineEngineering
The invention discloses a preparation method of a bedaquiline racemate and a key intermediate compound used in the preparation method. According to the method for preparing the bedaquiline racemate, the ultra-low temperature reaction in the prior art is changed, and the ultra-low temperature reaction which is difficult to realize in the prior art is carried out at the conventional temperature, sothat large-scale industrialization becomes possible. Besides, the method provided by the invention greatly improves the conversion rate of the reaction substrate, improves the reaction yield, makes the product more easily crystallized and purified, and reduces the production cost at the same time.
Owner:ANHUI BIOCHEM BIO PHARMA
Preparation method of bedaquiline racemate and intermediates thereof
ActiveCN111747975AEasy to synthesizeIncrease production capacityOrganic chemistry methodsGroup 3/13 element organic compoundsBiochemical engineeringDrugs synthesis
The invention belongs to the technical field of medicine synthesis, and particularly relates to a preparation method of a bedaquiline racemate and intermediates thereof. According to the method disclosed by the invention, convenient, efficient and economical synthesis and industrial production of the bedaquiline and the intermediates thereof are realized. Specifically, the method provided by the invention has the following advantages that: the ultralow-temperature reaction required for preparing bedaquiline from a compound I and a compound II is thoroughly changed, and the original ultralow-temperature reaction which is difficult to realize industrially is changed into the reaction at a conventional temperature, so that large-scale industrial production becomes possible; according to the method disclosed by the invention, the conversion rate of the reaction substrate is greatly improved, the reaction yield is improved, the product is easier to crystallize and purify (recrystallizationof the intermediates can be realized by using a conventional solvent ethyl acetate or methanol), and meanwhile, the production cost is reduced.
Owner:ANHUI BIOCHEM UNITED PHARMA CO LTD
Process for preparation of bedaquiline and pharmaceutically acceptable salts thereof
PendingCN114085185AAntibacterial agentsOptically-active compound separationQuinolineCombinatorial chemistry
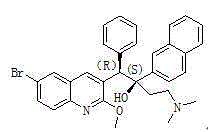
The present invention relates to a process for the preparation of bedaquiline ((1R, 2S)-1-(6-bromo-2-methoxyquinolin-3-yl)-4-dimethylamino-2-(1-naphthyl)-1-phenyl-butan-2-ol) or a pharmaceutically acceptable salt thereof, the process comprising: isolating (1R, 2S)-1-(6-bromo-2-methoxyquinolin-3-yl)-4-dimethylamino-2-(1-naphthyl)-1-phenyl-butan-2-ol. The method can achieve large-scale preparation, so that the preparation method is an economical preparation method and has high yield.
Owner:东亚ST 株式会社
Intermediate for preparing bedaquiline and its preparation method and application
The invention discloses an intermediate for preparing bedaquiline and a preparation method therefor. The intermediate disclosed by the invention has the advantages that the intermediate avoids hydrogenation and enolization of an alpha-site in the intermediate, reduces occurrence of side reactions, and increases the conversion rate of raw materials and the total yield of reaction, and is suitable for large-scale industrial production. The intermediate for preparing bedaquiline is characterized by being a compound with a structural formula (9) or an optical isomer thereof: FORMULA is shown in the description.
Owner:CHINA NAT MEDICINES GUORUI PHARMA +1
Compounds for treating tuberculosis
ActiveUS20200231550A1Address is challengeAntibacterial agentsOrganic chemistryEthyl groupTreating tuberculosis
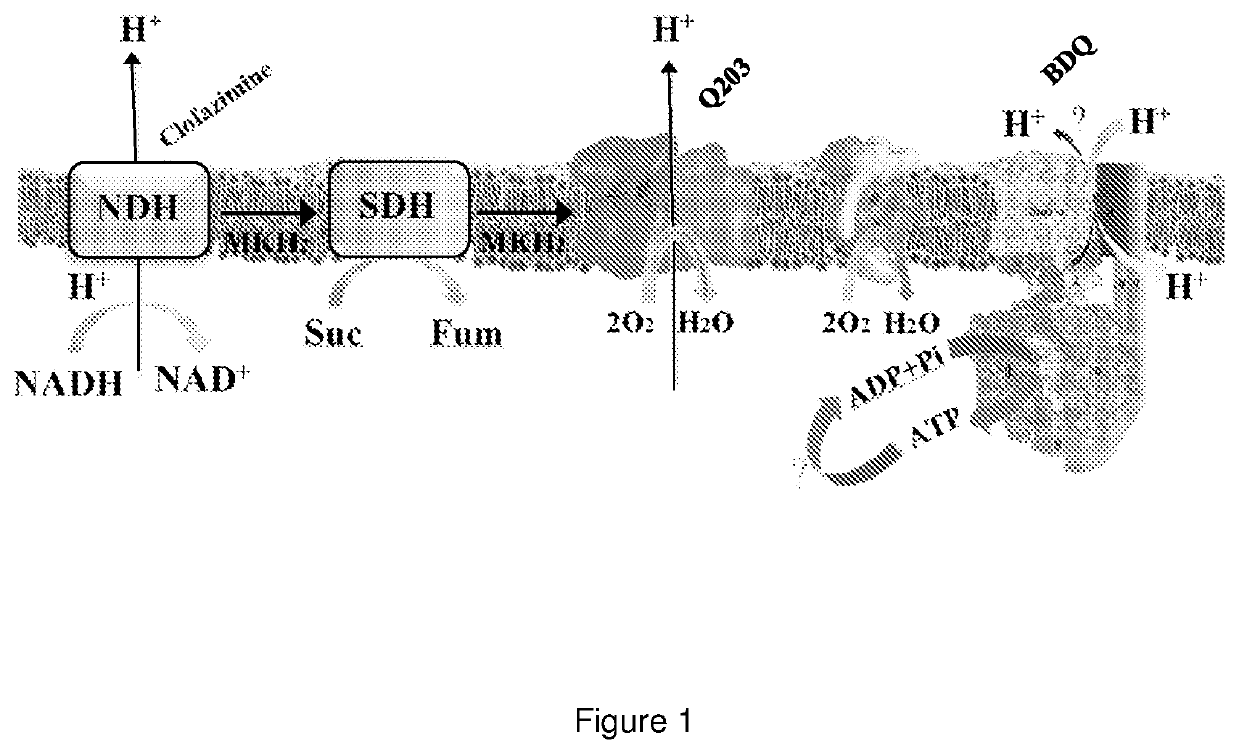
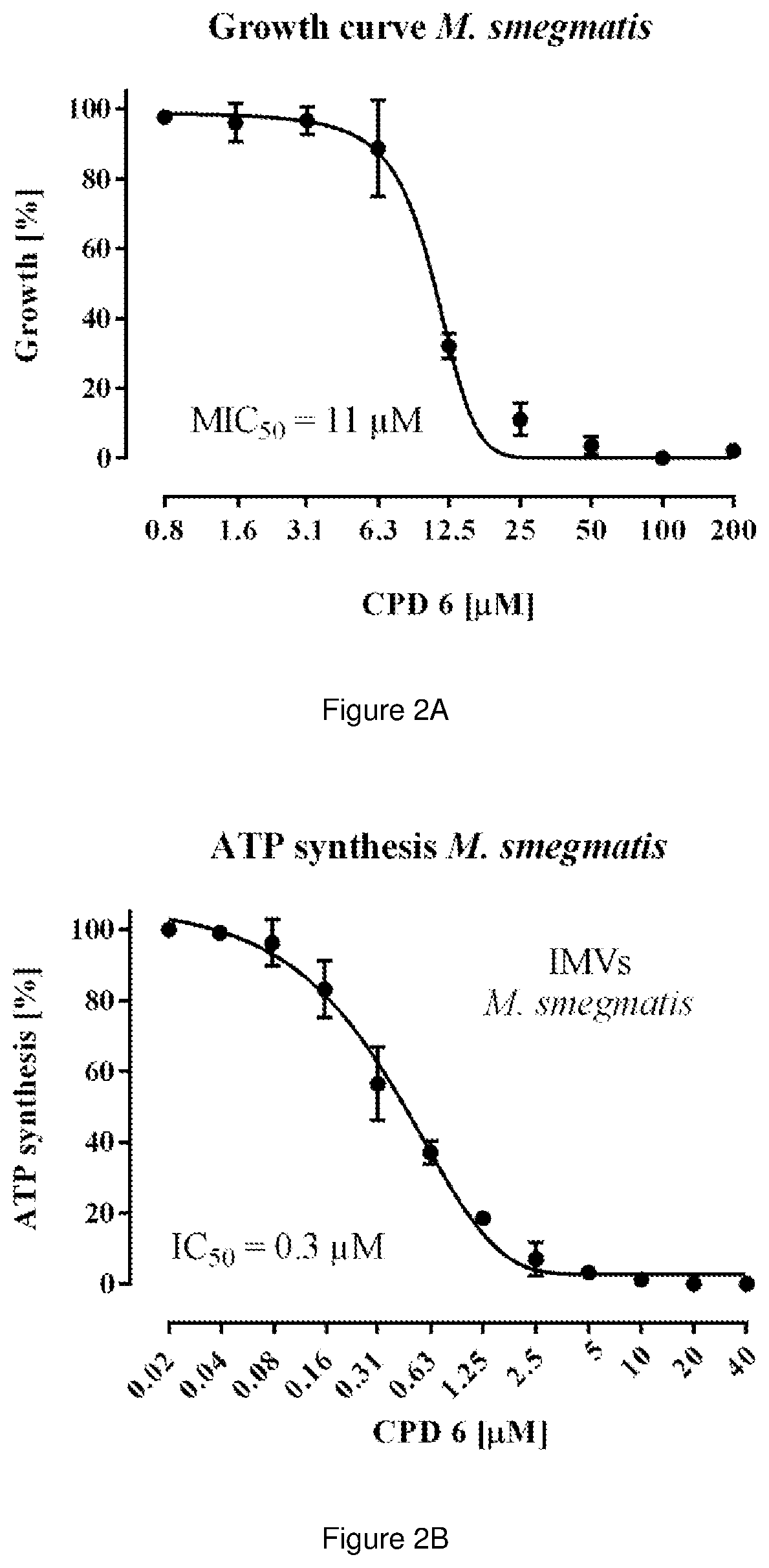
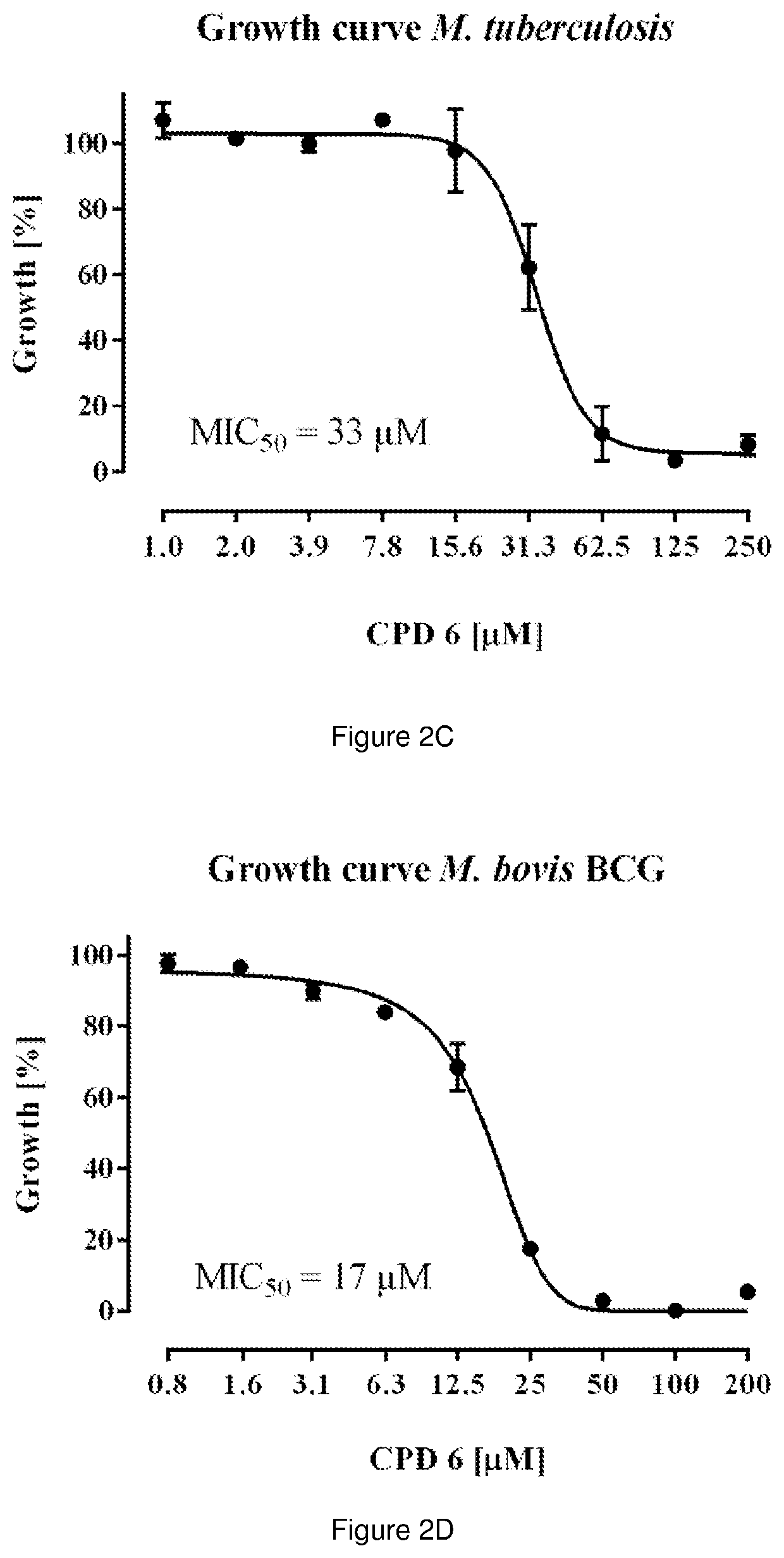
The present invention relates to pyrimidine compounds and compositions for treating tuberculosis. These compounds may be used to target the F1 domain of F-ATP synthase and may be used with bedaquiline or 6-chloro-2-ethyl-N-[[4-[4-[4-(trifluoromethoxy)phenyl]piperidin-1-yl]phenyl]methyl]imidazo[1,2-a]pyridine-3-carboxamide (Q203) or a combination thereof.
Owner:NANYANG TECH UNIV +1
Long-acting formulations
This invention concerns pharmaceutical compositions for administration via intramuscular or subcutaneous injection, comprising micro- or nanoparticles of the anti-TB compound bedaquiline, suspended in an aqueous pharmaceutically acceptable carrier, and the use of such pharmaceutical compositions in the treatment and prophylaxis of a pathogenic mycobacterial infection.
Owner:JANSSEN PHARMA NV
A method for separating bedaquiline diastereomer a
Disclosed is a method for separating a diastereoisomer A of Bedaquiline. The method comprises the following steps: (1) adding a reversed-phase solvent into a Bedaquiline reaction liquid comprising diastereoisomers A and B, so as to precipitate out the diastereoisomer B; and (2) removing the diastereoisomer B precipitated out in step (1), so as to obtain the diastereoisomer A. The separation method of the present invention is easy to operate and is stable, and has higher industrialization value compared with separation and purification in a conventional column chromatography method, and can resolve the problems of difficulty in purifying and separating Bedaquiline due to the small amount of a product caused by an excessively low conversion rate because preparation condition of Bedaquiline is harsh and the conversion rate is difficult to ensure; raw material residuals can be easily removed, the yield is high, and the purity of the diastereoisomer A is high, which facilitates the split; split can be further carried out to obtain a qualified Bedaquiline product with a purity greater than or equal to 99.0%, wherein the impurities of the diastereoisomers are lower than or equal to 0.1%.
Owner:ZHEJIANG HISUN PHARMA CO LTD +1
A kind of preparation method of purification and stable crystal form of bedaquiline
ActiveCN109422679BHigh purityImprove stabilityOrganic chemistry methodsPhosphoric Acid EstersPhosphate
The invention relates to a method for purifying bedaquiline and preparing a stable crystal form. After dissociating the bedaquiline-binaphthol phosphate salt obtained by the resolution with alkaline water to remove the resolution reagent, extract and concentrate with toluene to obtain an oily substance, add ketone and stir to dissolve; then add alcohol and purified water dropwise, and stir evenly , Precipitating powdery crystals of bedaquiline free base. The method of the invention refines and purifies the bedaquiline free base by using a mixed solvent, so that the impurity content is significantly reduced, the quality requirement of the finished product is met, and a stable crystal form is obtained, which is convenient for storage and a subsequent salt-forming process. The process of the invention has the advantages of simple and convenient operation, high product yield and good quality, and is suitable for industrialized production.
Owner:WUHAN WUYAO SCI & TECH
A method for separating and analyzing bedaquiline optical isomers
The invention relates to a method for separating and analyzing optical isomers of bedaquiline, belonging to the field of liquid chromatography. The method is reversed-phase high-performance liquid chromatography; a selected chromatographic column is an amphoteric ion exchange chiral column; the column temperature of the chromatographic column is 9-20 DEG C; a mobile phase is a mixed solution of 2mL to 3 mL of diethylamine, 1.5 mL to 2.5 mL of formic acid and 1000 mL of methanol; the velocity of the mobile phase is 0.08 mL / min to 0.12 mL / min; a detection wavelength is 220 nm to 230 nm; the resolution of a (1S, 2R)-bedaquiline peak and a (1R, 2S)-bedaquiline peak on a chromatogram is greater than 1.5. The method of the invention can effectively separate (1R, 2S)-bedaquiline and / or (1S, 2R)-bedaquiline, and / or detect the purity and / or the content of (1R, 2S)-bedaquiline and the purity of (1S, 2R)-bedaquiline.
Owner:WUHAN WUYAO SCI & TECH
A kind of preparation method of (1r, 2s) and (1s, 2r)-bedaquiline
ActiveCN107857727BReduce residualHigh purityOptically-active compound separationAsymmetric synthesesChemical synthesisCarbenium ion
The invention discloses a method for preparing (1R, 2S)-bedaquiline and (1S, 2R)-bedaquiline and belongs to the technical field of chemical synthesis. The method comprises the steps of subjecting (1R,2R)-bedaquiline and (1S, 2S)-bedaquiline to a Lewis acid action to form carbenium ions, then, subjecting the carbenium ions to action by OH<-> in an alkaline solution to form novel chiral tertiary alcohol, and then, carrying out resolution, thereby preparing (1R, 2S)-bedaquiline. According to the method, the number of reaction steps is small, the production efficiency is greatly increased, the (1R, 2S)-bedaquiline of relatively high yield is easy to obtain, and thus, the industrial production is facilitated; and meanwhile, the impurity residual is low, the purity of target products is high, and dangerous steps such as high-pressure hydrogenation are not required to be added, so that the entire production process is high in safety.
Owner:JIANGSU TIANHE PHARMA CO LTD
The preparation method of bedaquiline
The invention discloses a preparation method for bedaquiline. The preparation method comprises the following steps: enabling a compound (9) to be reacted with a reducing agent in a solvent; and then collecting racemate of bedaquiline from a reaction product. The preparation method has the advantages that the compound (9) is a novel compound which has not been reported in literature; the racemate of bedaquiline is prepared from a compound (8) and the compound (9); the obtained product is greatly increased in yield (greater than 47%) which is remarkably greater than the yield (26%) in the original patent; and the obtained racemate of bedaquiline is high in purity, stable and controllable in quality, and beneficial for subsequent resolution reaction, and has relatively great positive effects and relatively high practical application value. The reaction formula is shown as follows: a FORMULA as shown in the description.
Owner:CHINA NAT MEDICINES GUORUI PHARMA +1
Methods to identify antituberculosis compounds
Practical assays to identify compounds that overcome the resistance of M. tuberculosis to bedaquiline are based on transcription factors Rv0324 and Rv0880 shown to mediate this resistance.
Owner:INSTITUTE FOR SYSTEMS BIOLOGY +1
Method for purifying bedaquiline and preparing stable crystal form of bedaquiline
The invention relates to a method for purifying bedaquiline and preparing a stable crystal form of bedaquiline. The method comprises the steps: adopting alkaline water to dissociate a resolving agentfrom a bedaquiline-binaphthol phosphate salt obtained after resolution, performing extraction with toluene, performing concentration so as to obtain an oily substance, adding ketone, performing stirring so as to achieve dissolution, then adding alcohol and purified water dropwise, performing uniform stirring so as to precipitate powdery crystal of bedaquiline free base. According to the method, refined purification of the bedaquiline free base is achieved through the mixed solvents, so that the impurity content is reduced significantly, the quality of the finished product is achieved, and a stable crystal form is obtained, so that storage and a subsequent salt forming process are facilitated; and the method has simple and convenient operation, high product yield and good quality, and is suitable for industrial production.
Owner:WUHAN WUYAO SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com