Preparation method of bedaquiline racemate and intermediates thereof
A compound and selected technology, applied in the field of drug synthesis, can solve the problems of many side reactions, low reaction temperature, long time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
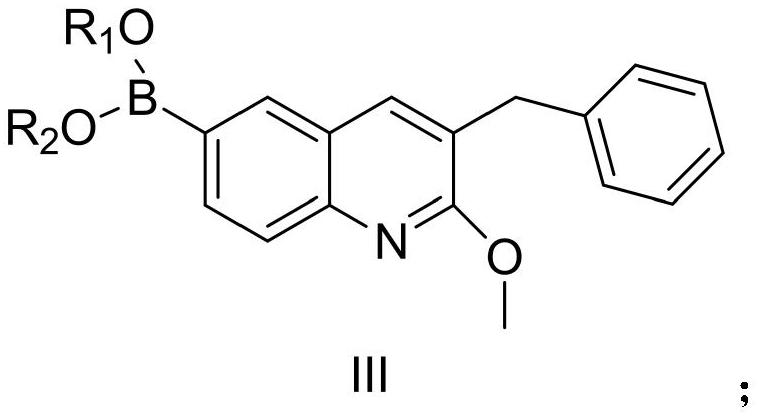
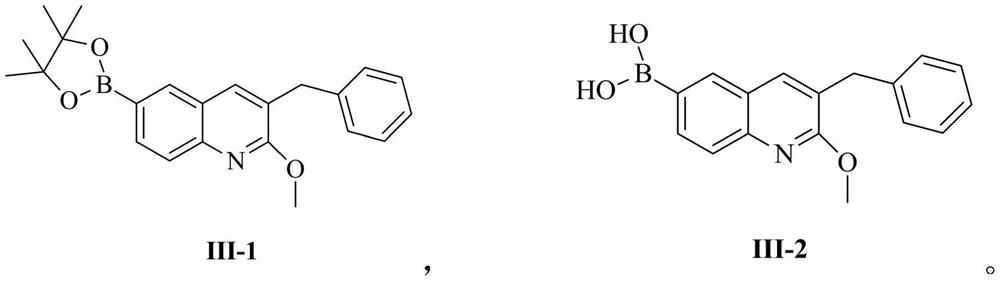
[0093] Example 1: Compound III-1 (3-benzyl-2-methoxy-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) quinoline) preparation
[0094]
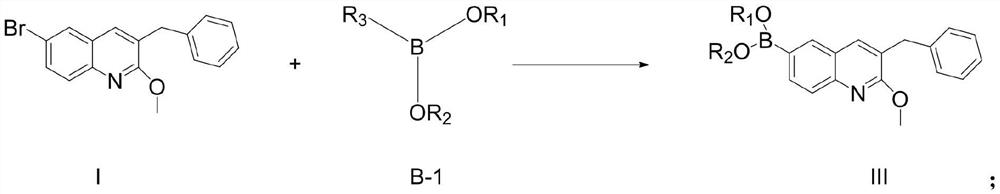
[0095] Compound I (65.6g, 1eq), bis(pinacolate) diboron (53g, 1.05eq), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (7.3 g, 0.05eq), anhydrous sodium acetate (33g, 2eq) was suspended in 500ml of dioxane and replaced with argon. The system was heated up to 100° C. and kept at this temperature for 8 hours. After the reaction was completed, it was extracted with ethyl acetate and the extract was concentrated to about 200ml, stirred and crystallized (ethyl acetate was used as solvent) to obtain compound III-1 (66.8g, yield 89%). 1 H NMR (400MHz, CDCl 3 )δ8.10(s,1H),7.94(dd,J=8.0Hz,J=4Hz,1H),7.81(d,J=4Hz,1H),7.57(s,1H),7.30-7.31(m, 2H), 7.22–7.26(m,3H), 4.11(s,3H), 4.02(s,2H), 1.36(s,12H); Ms(+C,ESI): M=375, measured value: 376( M+1).
Embodiment 2
[0096] Example 2: Compound III-1 (3-benzyl-2-methoxy-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) quinoline) preparation
[0097]
[0098] Mg powder (7.2g, 1.5eq) was added to a 1L three-necked flask, and 50ml of anhydrous THF and 30ml of compound I solution (65.6g of compound I dissolved in 300ml of anhydrous THF, 1eq) were added under argon protection. After heating to reflux temperature, 1,2-dibromoethane (0.5ml) was added dropwise to initiate the reaction. Stir for 2 minutes after the addition, and slowly add the remaining compound I solution (270 ml) dropwise while maintaining slight boiling. After the addition, continue to keep the slight boiling state to react for 1 hour, and cool to room temperature to prepare the Grignard solution. The prepared Grignard solution was slowly added dropwise to methanol pinacol borate (47.4 g, 1.5 eq) and anhydrous THF (50 ml) cooled at 0° C., and the temperature was maintained for 3 hours. After completion of the reaction, extra...
Embodiment 3
[0099] Embodiment 3: the preparation of compound III-2 (3-benzyl-2-methoxy-6-boronic acid quinoline)
[0100]
[0101] Mg powder (7.2g, 1.5eq) was added to a 1L three-necked flask, and 50ml of anhydrous THF and 30ml of compound I solution (65.6g dissolved in 300ml of anhydrous THF, 1eq) were added under argon protection. After heating to reflux temperature, 1,2-dibromoethane (0.5ml) was added dropwise to initiate the reaction. Stir for 2 minutes after the addition, and slowly add the remaining compound I solution (270 ml) dropwise while maintaining slight boiling. After the addition, continue to keep the slight boiling state to react for 1 hour, and cool to room temperature to prepare the Grignard solution. The prepared Grignard solution was slowly added dropwise to trimethyl borate (31.2g, 1.5eq) and anhydrous THF (50ml) cooled at 0°C, and the temperature was maintained for 3 hours. After completion of the reaction, extract with ethyl acetate and concentrate the extract ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com