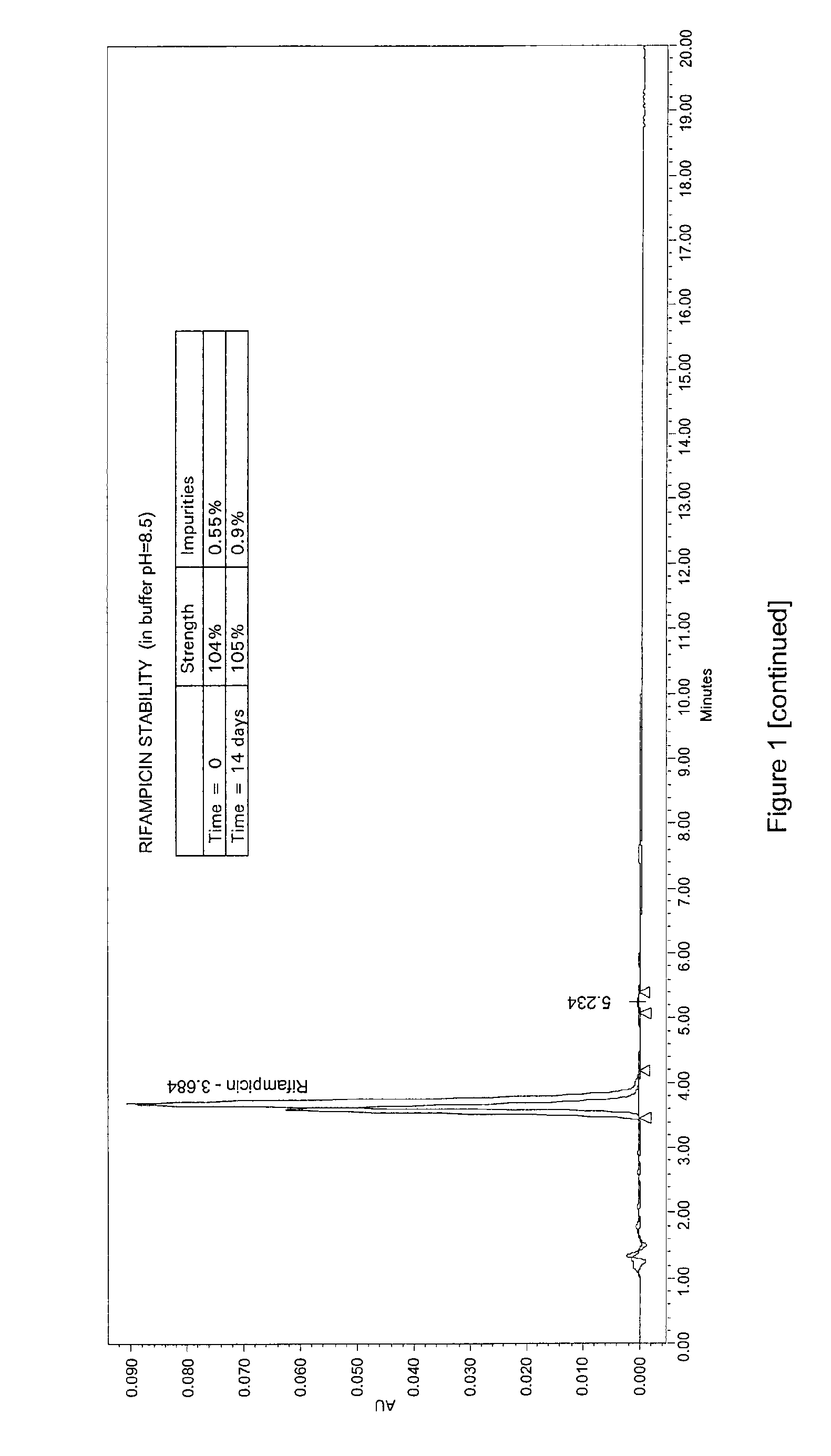
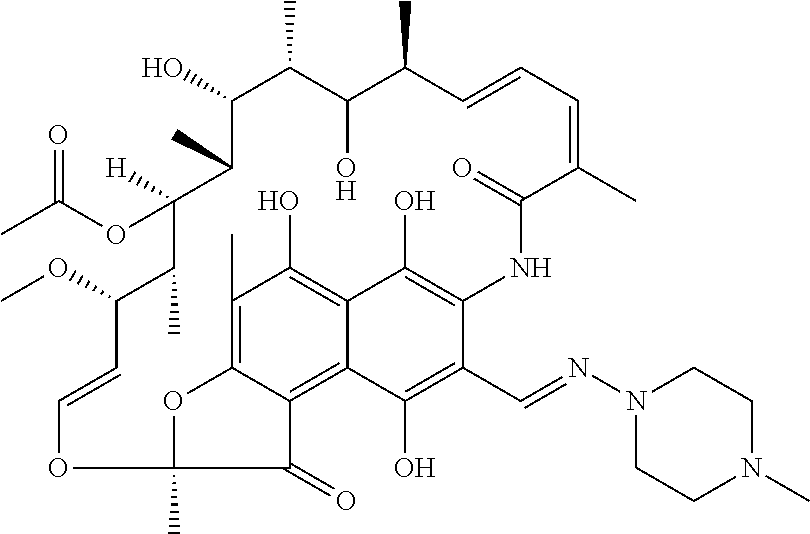
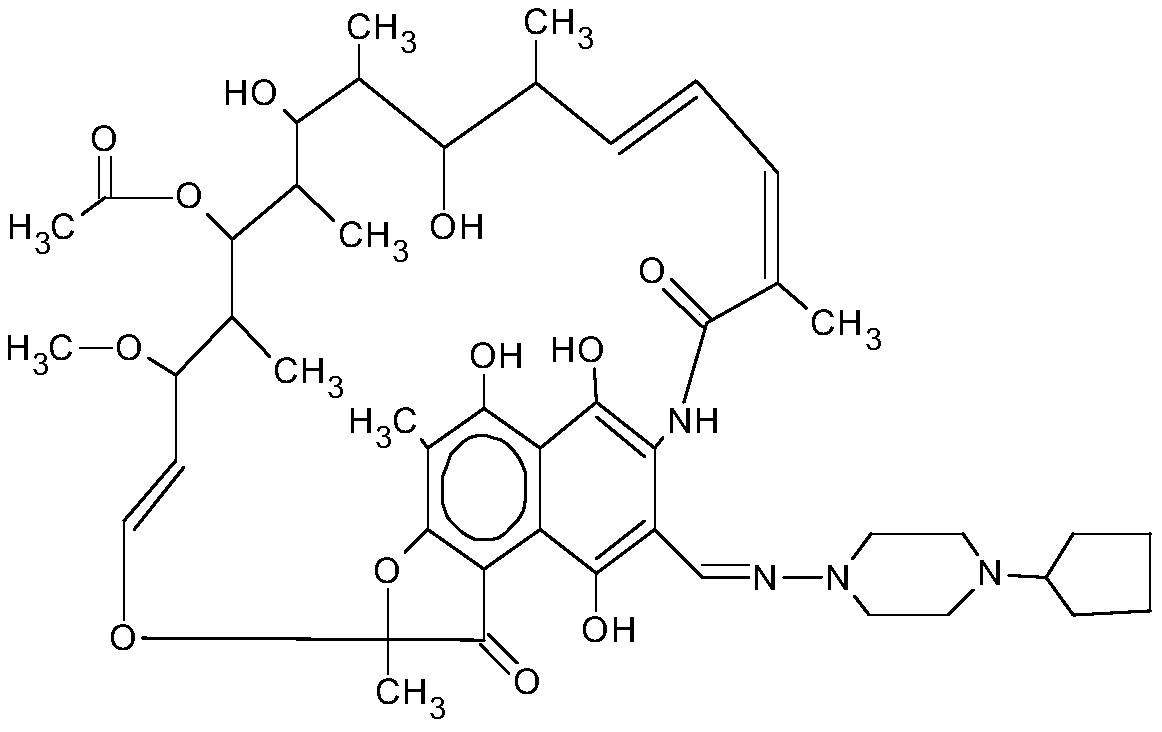
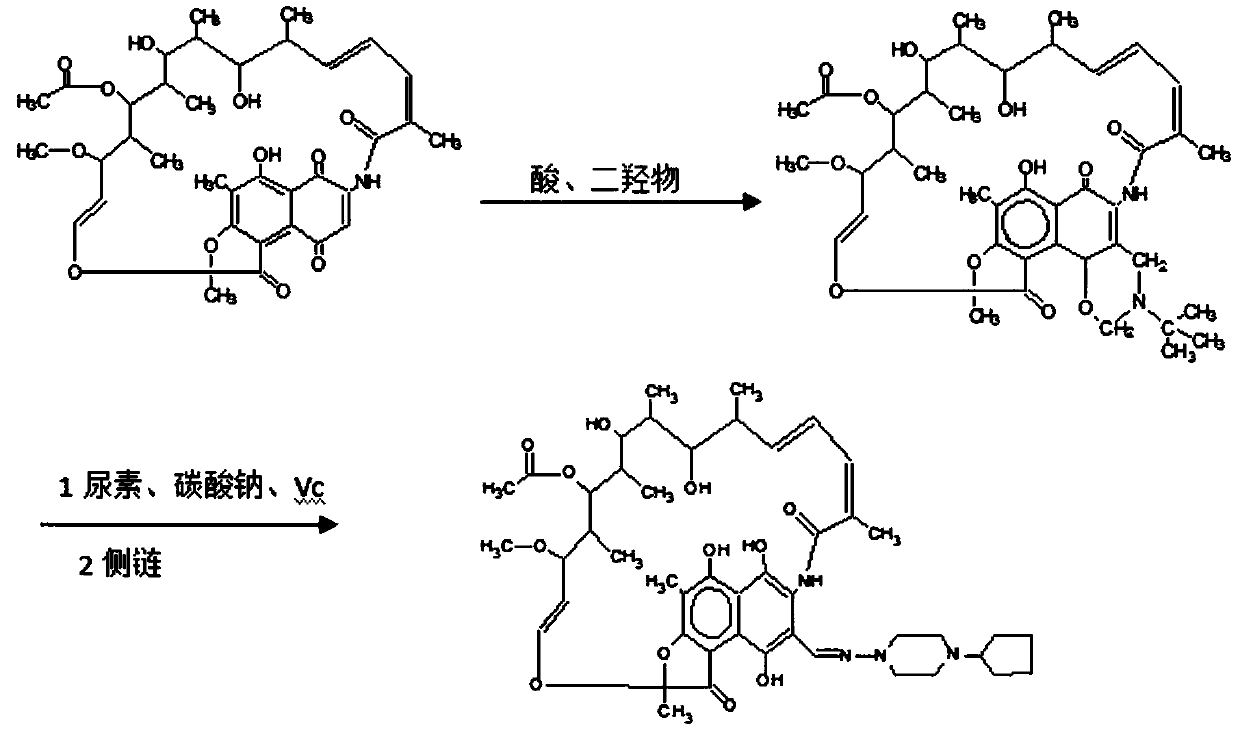
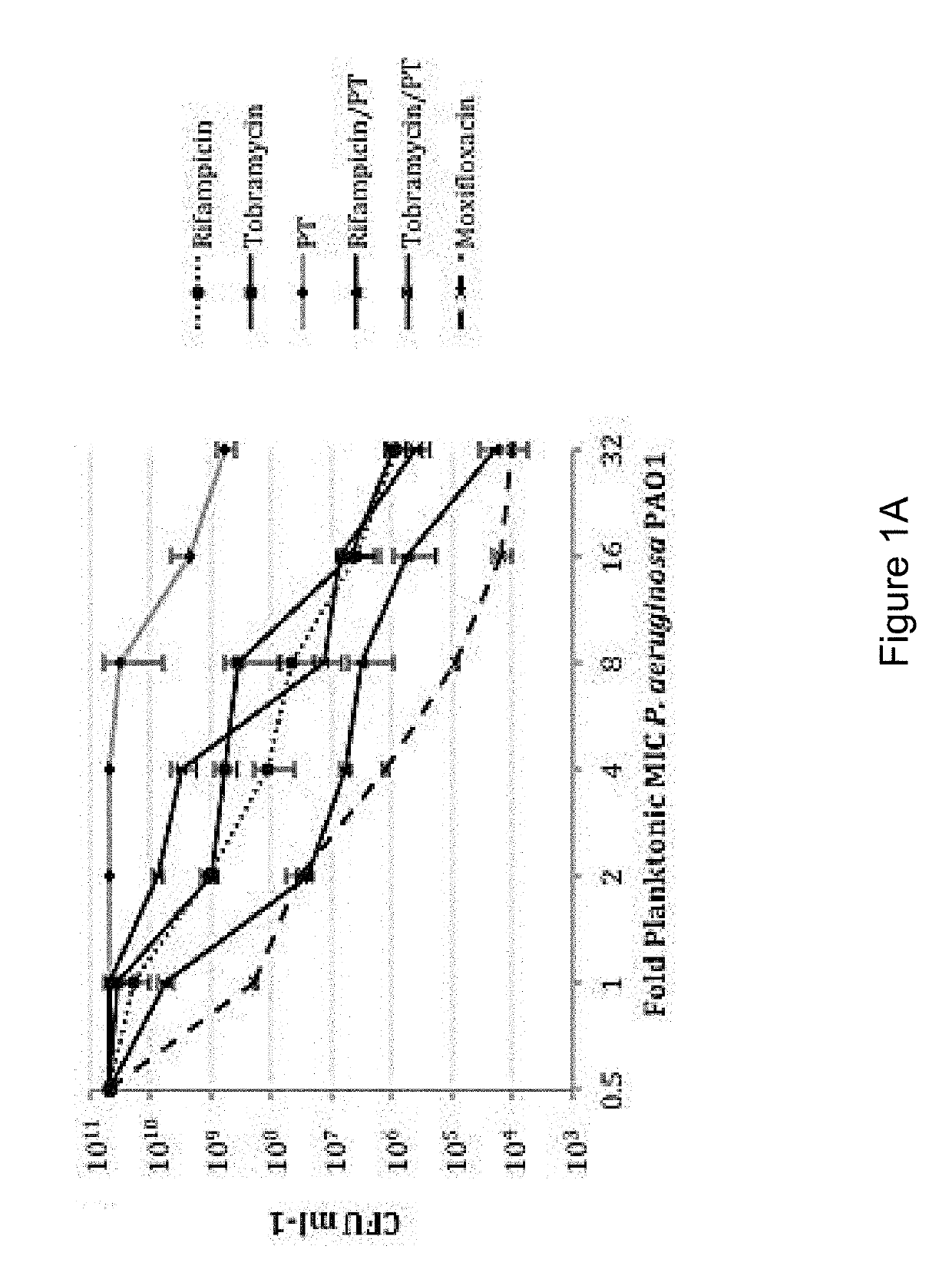
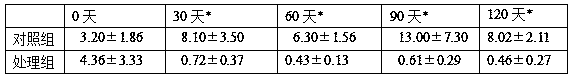
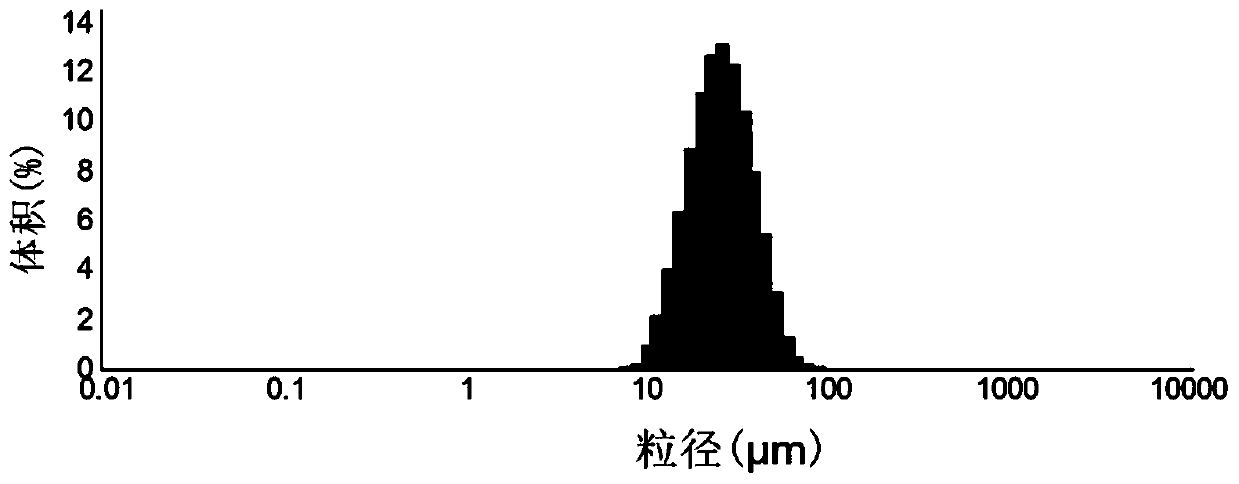
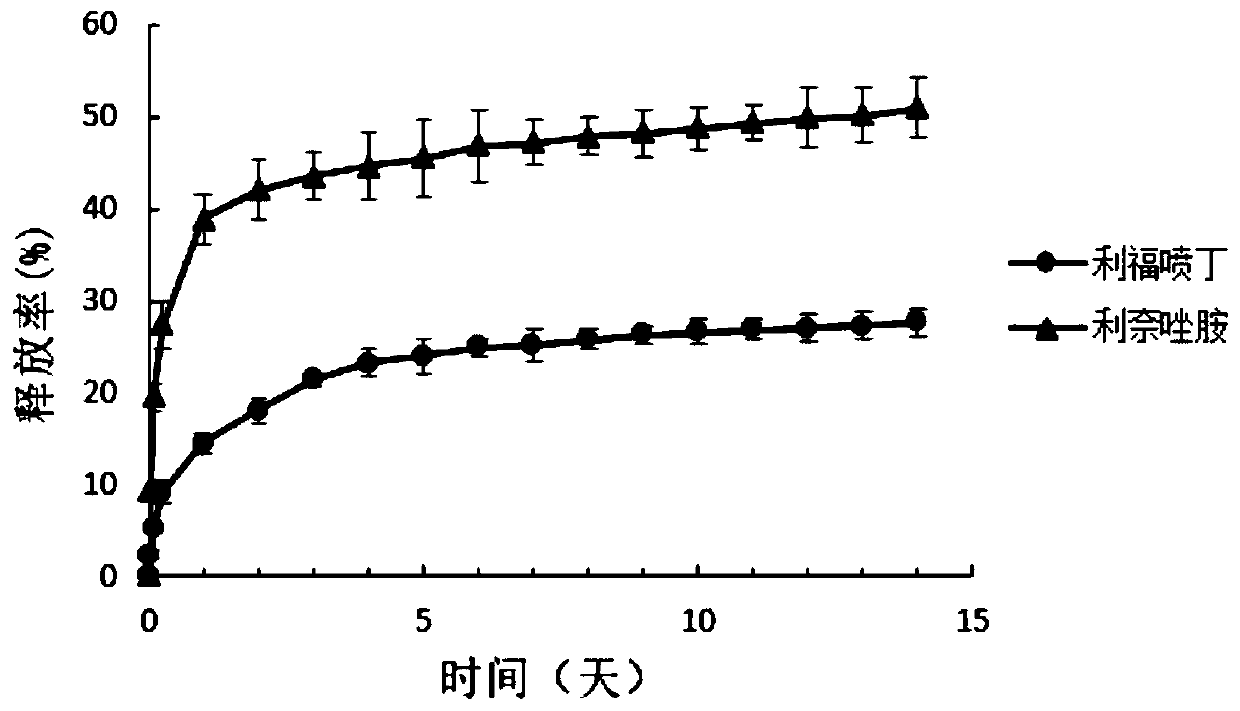
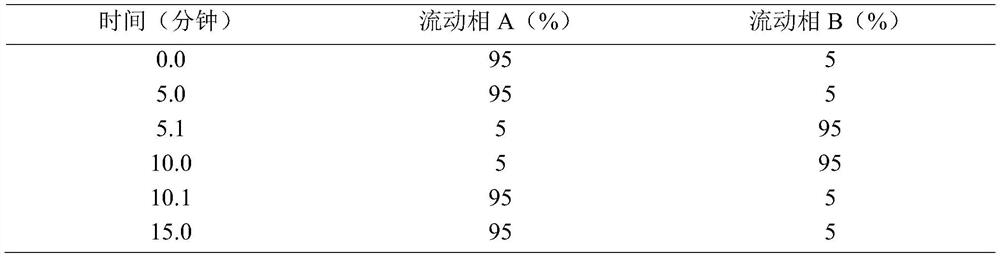
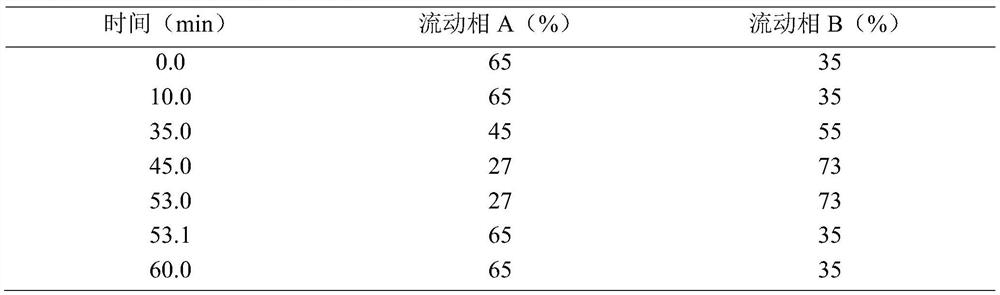
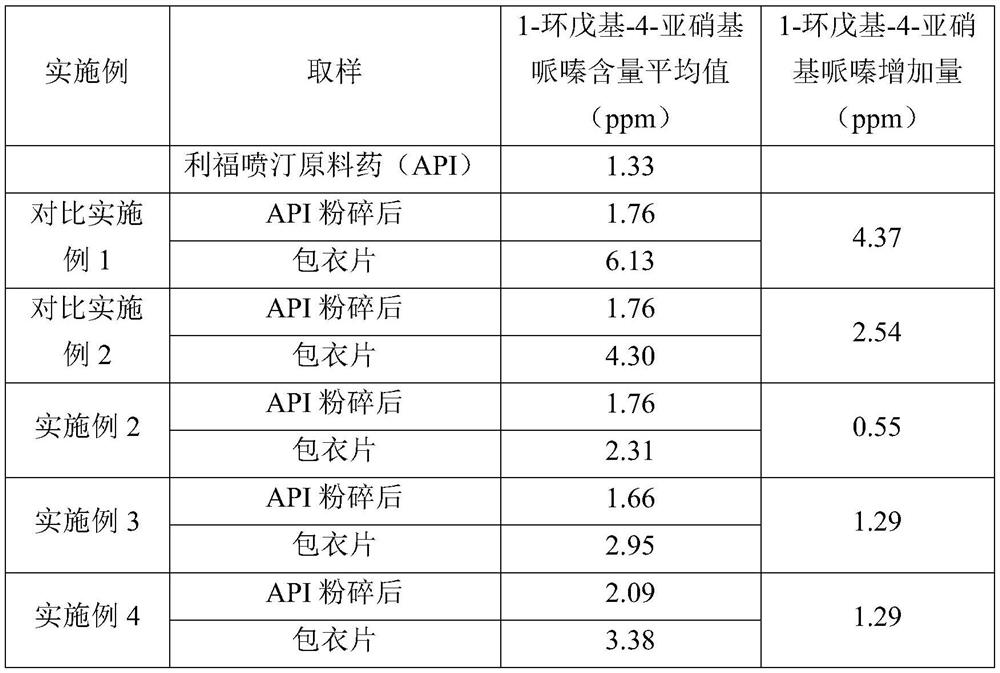
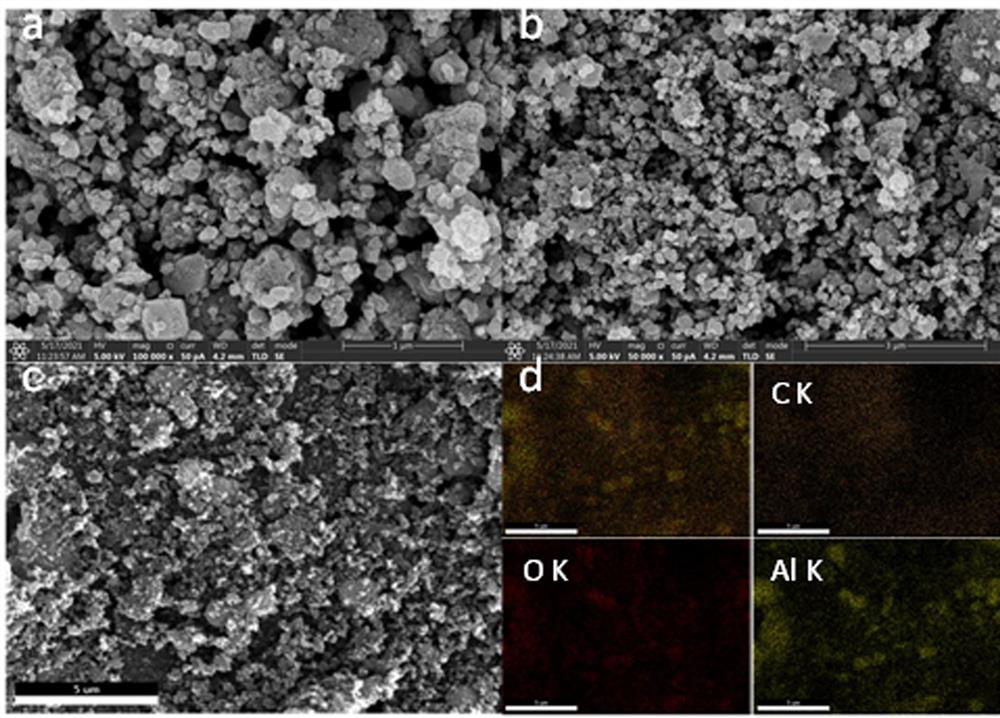
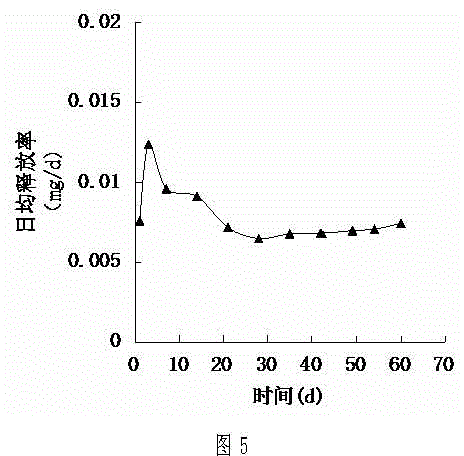
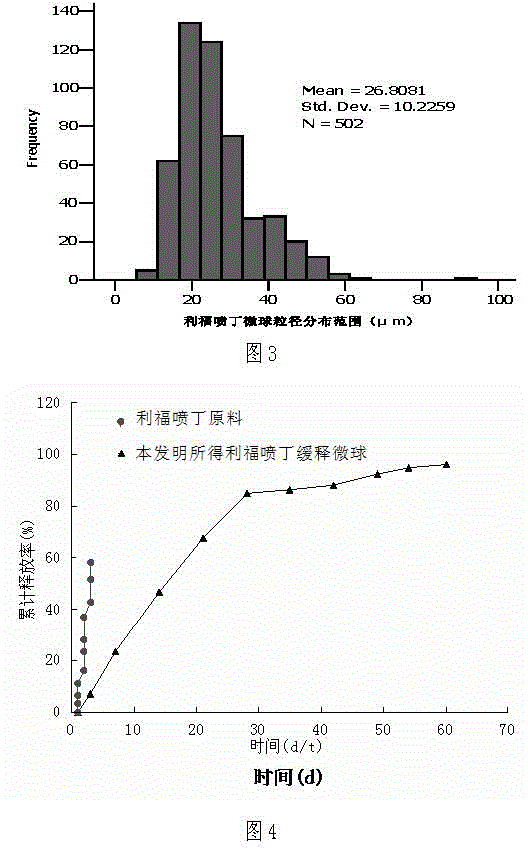
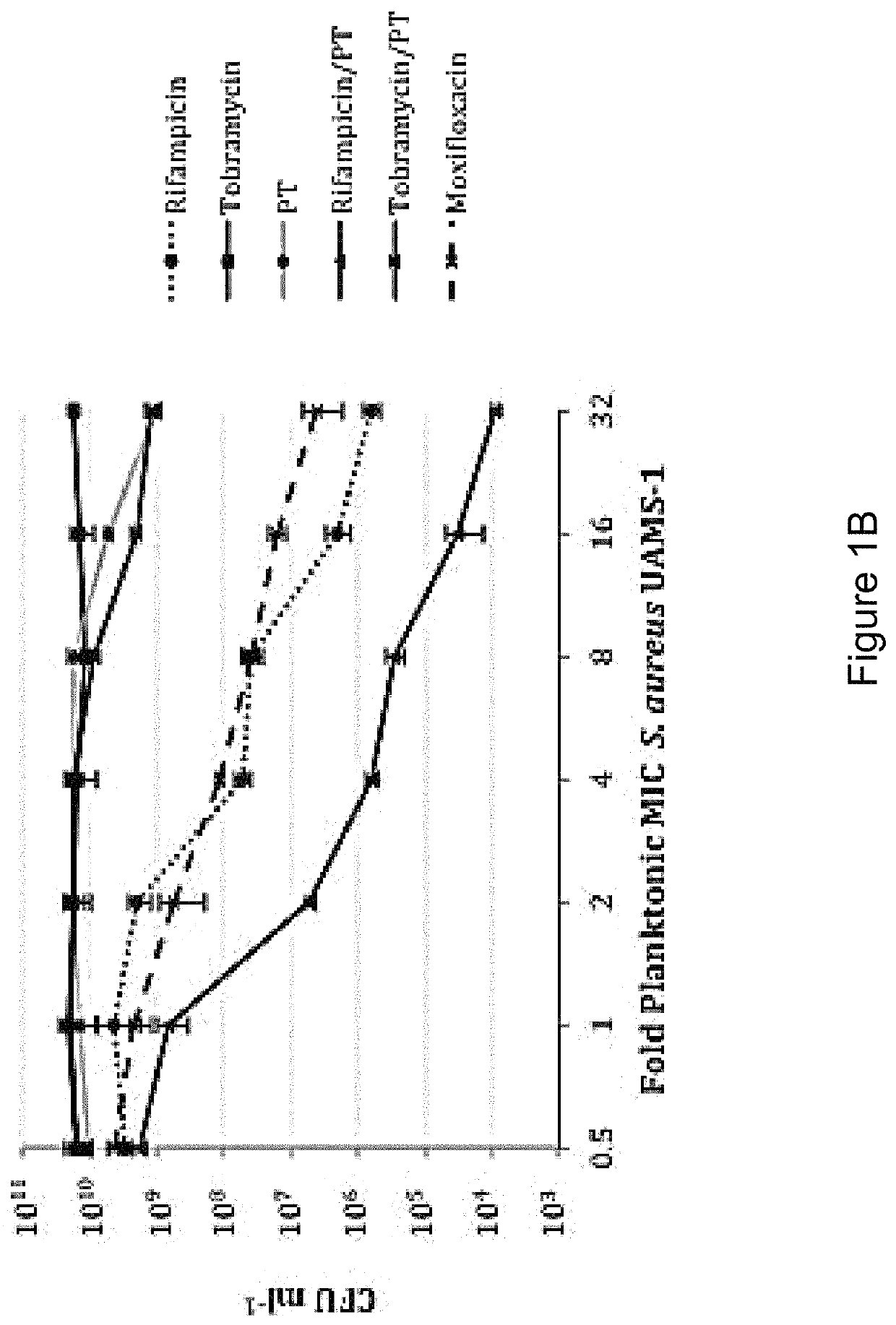
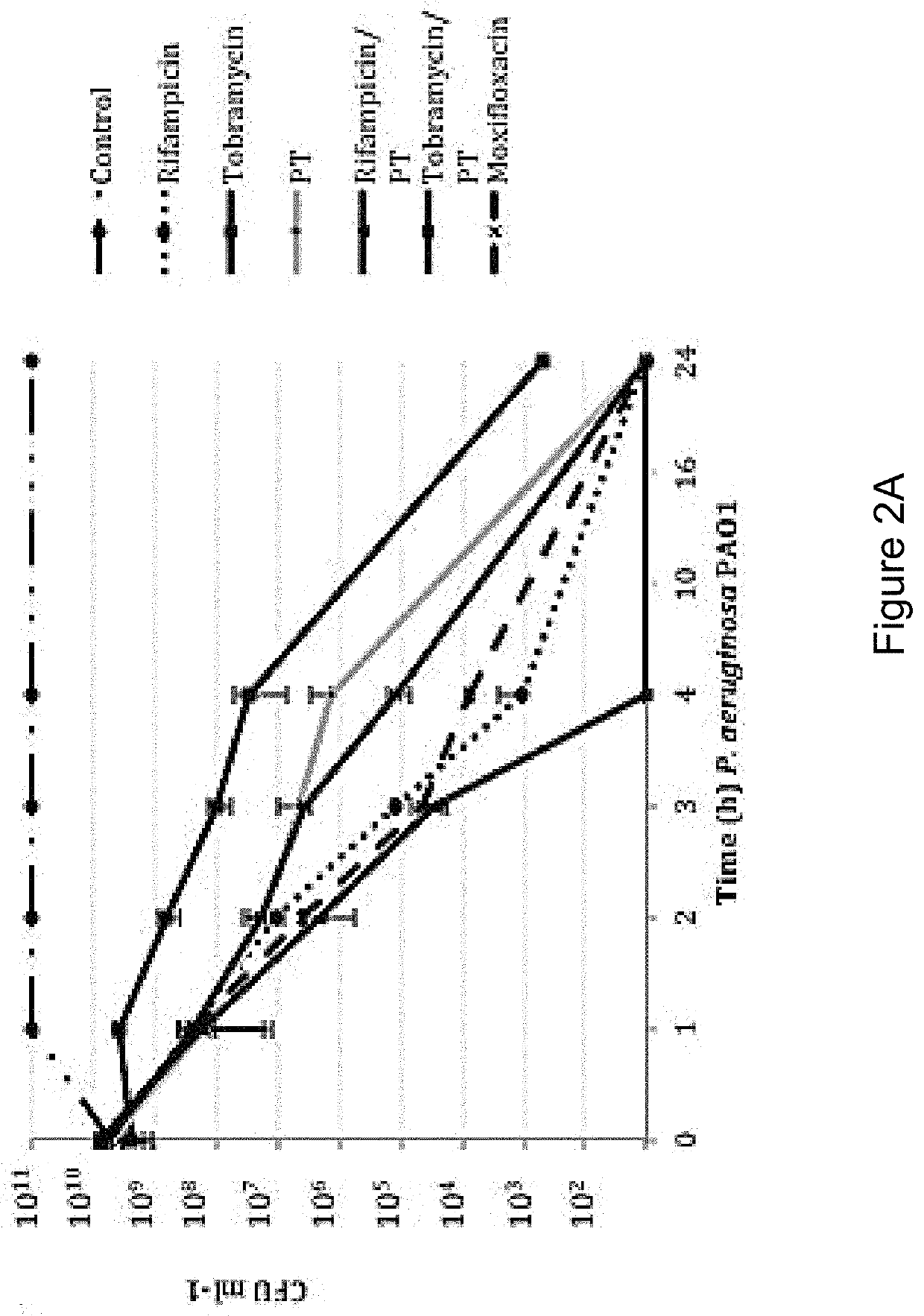
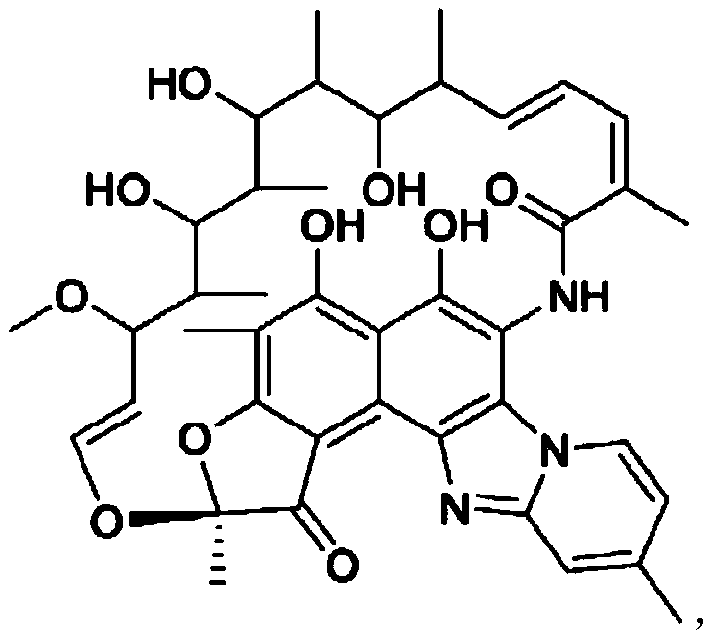
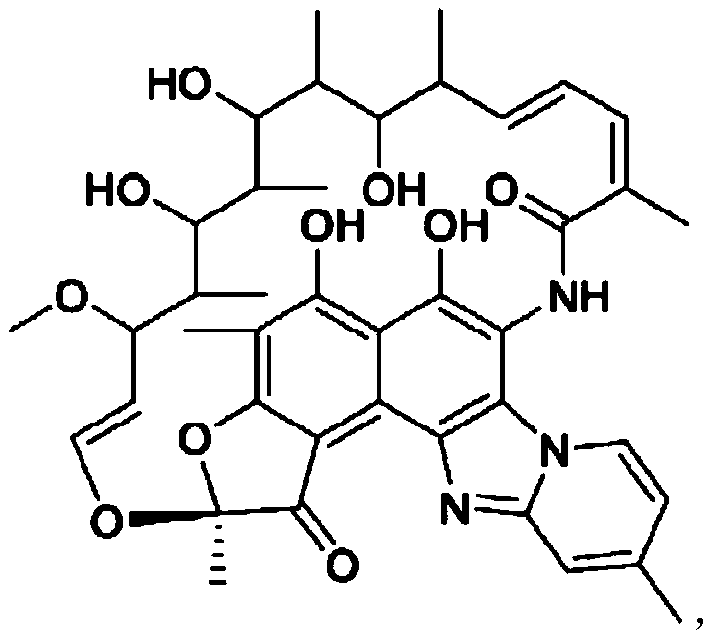
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Rifapentine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
This medication is used with other medications to treat active tuberculosis (TB) of the lungs. It may also be used with another medication (isoniazid) to prevent active TB infections in people who are infected with the bacteria (people with positive TB skin test).
Slow released compound antituberculotic preparation
The slow released compound antituberculotic preparation contains at least one of rifampicin, pyrazinamide, kanamycin, isoniazide, rifapentine, etc. The slow released preparation is slow released injection or slow released implanting agent. The slow released injection consists of slow released microsphere and solvent, the slow released microsphere contains slow releasing supplementary material and antituberculotic, and the solvent is special solvent containing suspending agent carboxymethyl cellulose sodium and of viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is EVAc, PLA, PLGA, sebacic acid copolymer, etc. The slow released compound antituberculotic preparation is set or injected into local tuberulosis focus to treat various kinds of intractable tuberulosis, and has medicine releasing period up to 30-40 days, less systemic toxicity and unique curative effect.
Owner:JINAN SHUAIHUA PHARMA TECH
Rifapentine, refampicin, rifabutin or rifamdin injection and its preparing method
InactiveCN1775214ALower doseEasy to useAntibacterial agentsOrganic active ingredientsRifabutinMedicine
The present invention relates to a kind of rifapentine, rifampicin, rifabutine or rifandin injection and its preparation method. It includes main medicine, medicinal organic cosolvent, alkaline antioxidant, injection water surfactant and sodium hydroxide. Its main medicine can be rifapentine, rifampicin, rifabutine or rifandin. Said invention also provides the concrete steps of its preparation method.
Owner:WUHAN UNIV
Preparation method of rifapentine-carried polycaprolactone microspheres
InactiveCN106420663ARound shapeGood particle size dispersionAntibacterial agentsOrganic active ingredientsDispersityMicrosphere
The invention discloses a preparation method of rifapentine-carried polycaprolactone microspheres. The preparation method comprises steps as follows: rifapentine crude drugs and polycaprolactone 20 are jointly dissolved in dichloromethane, the mixture is subjected to ultrasonic treatment, and an oil phase is formed; the formed oil phase is dropwise added to a 2% polyvinyl alcohol water solution, mechanically stirred at the rotating speed of 3,000 rpm for 30 min, sealed, left to stand for 30 min and continuously stirred at the rotating speed of 100 rpm for 3 h, and an emulsion is formed; centrifugal treatment is performed, obtained solids are washed with distilled water, centrifugal separation and washing are performed again, finally, drying is performed in a freezing vacuum drier, and the rifapentine-carried polycaprolactone microspheres are obtained and put at the temperature of subzero 4 DEG C in the dark place for storage. The prepared rifapentine-carried polycaprolactone microspheres have round and complete shapes, have a micropore structure on the surface, are good in particle size dispersity and high in drug loading capacity, have a good slow release function and can be used for long-acting interventional therapy of tuberculosis of bones and joints.
Owner:SHAANXI YIPINDA PETROCHEM CO LTD
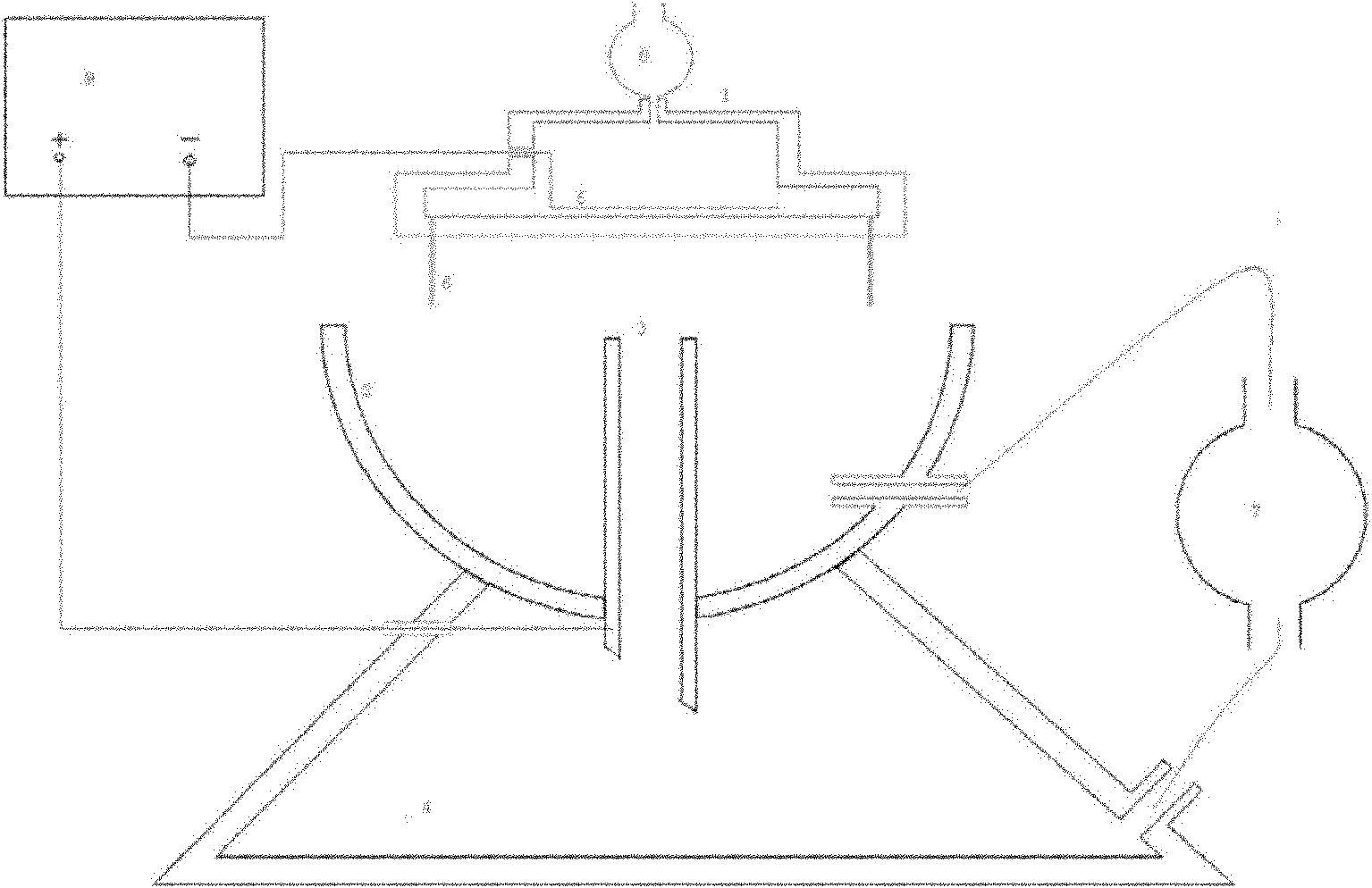
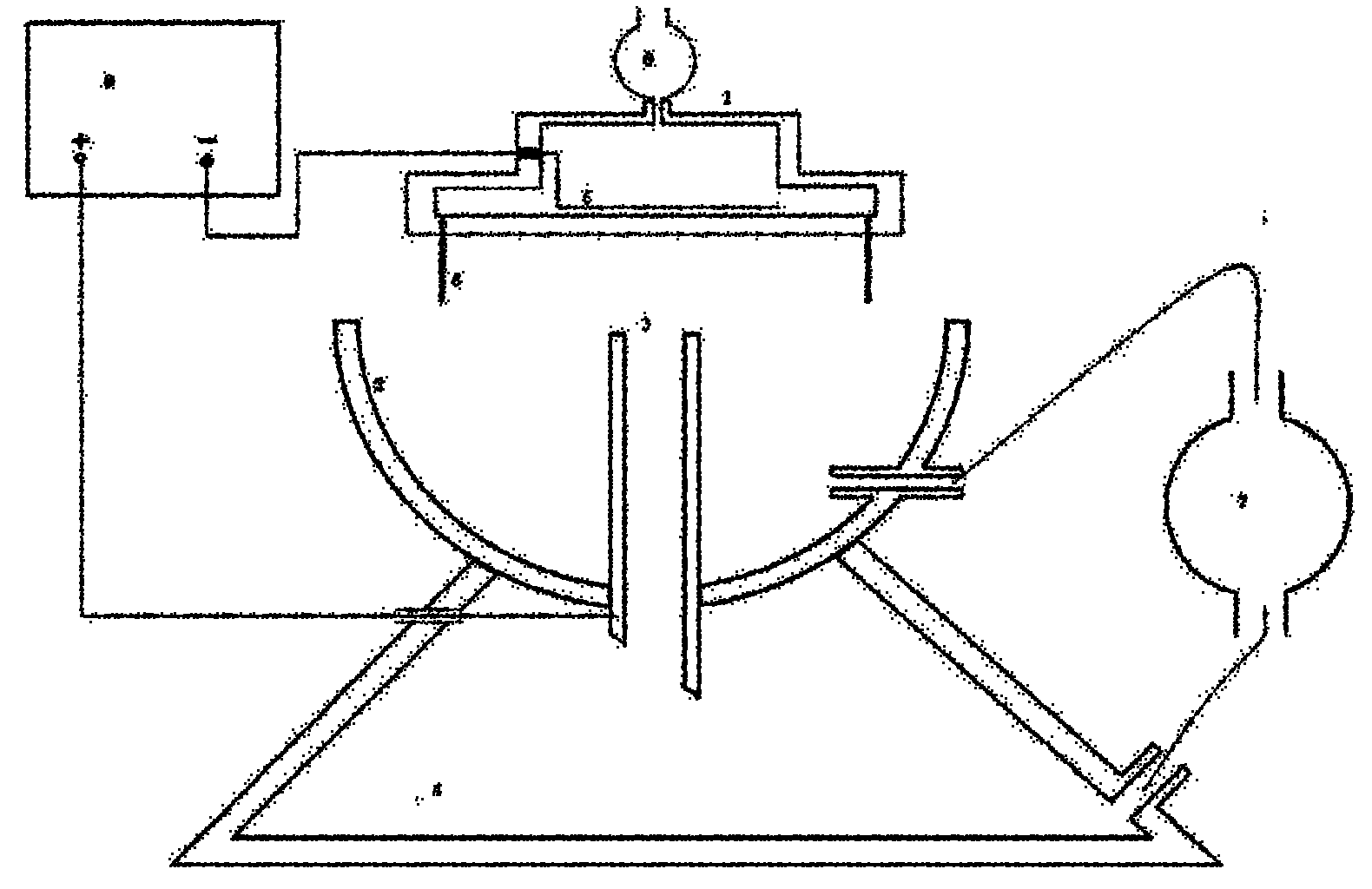
Device and method for preparing gel microsphere and gel microsphere in which antitubercular agents can be injected
InactiveCN102178604AHeight adjustableReduce dwell timeAntibacterial agentsOrganic active ingredientsRifabutinMicrosphere
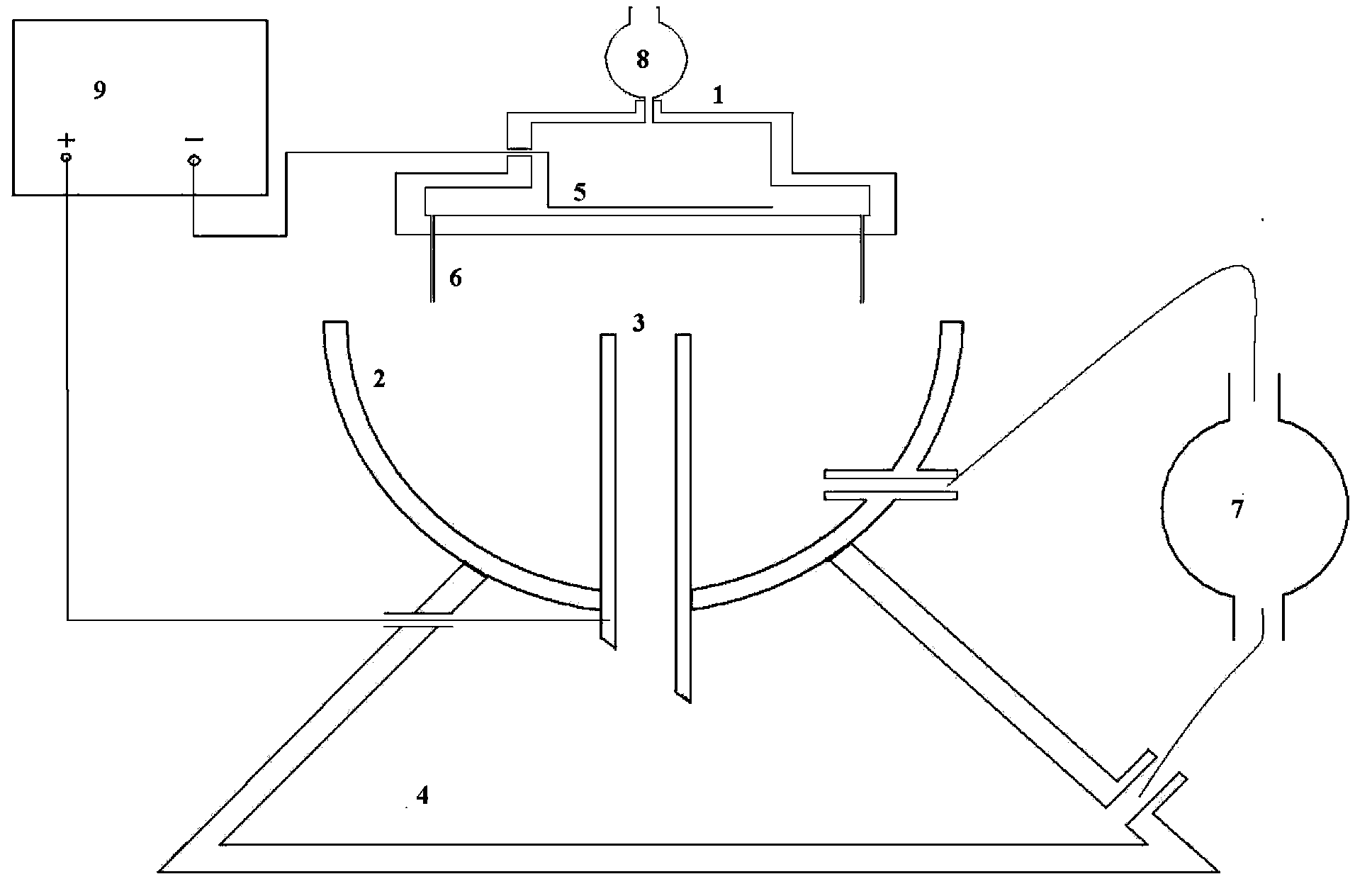
The invention relates to a device and a method for preparing a gel microsphere and a gel microsphere in which antitubercular agents can be injected. The device comprises: a porous shower nozzle, a collecting cup, a collecting tubule, a high pressure static generator, a constant current pump, a settling basin, an injection pump and an infiltration electrode. On the basis of a common static liquid drop generating device, the collecting tubule is arranged to form a liquid level flow regulating system, so that a liquid exchange passage exists between the collecting cup and the settling basin and can collect the low-density gel microspheres floating on the liquid level of the collecting cup and rapidly transfer the microspheres to the settling basin. Meanwhile, the built-in infiltration electrode type porous shower nozzle can enable the drug liquid droplet in drug-carrying sol solution to be fully charged, and form a uniform high electric field with the collecting tubule, thus enhancing the atomizing effect of the liquid column of the nozzle and enabling the drug-carrying sol solution to form monodispersity gel microspheres. The gel microsphere in which antitubercular agents can be injected contains rifapentine or rifabutin, made by the device and the method. The gel microsphere is high in yield, and large in drug-carrying capacity, and does not sink down or adhere after drying.
Owner:中国人民解放军第三0九医院
Agent for yellow shoot prevention and control
InactiveCN106070262AConvenient prevention and controlBiocideDead animal preservationShootSODIUM DODECYL BENZENE SULFONATE
The invention discloses an agent for yellow shoot prevention and control. The agent is applied by a trunk transfusion method. Each 100mL of the agent mainly comprises 0.5-1 gram of penbritin, 0.2-0.5 gram of rifapentine, 0.5-2mL of 1,2-butanediol serving as a stabilizer, 0.1-2mL of glycol serving as an anti-freezing agent, 0.1-2mL of azone serving as a novel permeation promoter, 0.1-2 grams of sodium lignin sulfonate serving as a dispersing agent, 0.1-2 grams of sodium dodecyl benzene sulfonate serving as an emulsifier, 0.1-2mL of DMSO (dimethyl sulfoxide) serving as a co-solvent and the rest deionized water supplemented to reach 100mL. Different bactericides are reasonably mixed by two sterilization mechanisms according to the objective requirement of yellow shoot prevention and control, the agent can achieve complementary advantages and has the advantages of high efficiency, low toxicity, safety, economization and multifunction, and yellow shoot can be more effectively prevented and controlled.
Owner:NANJING AGRICULTURAL UNIVERSITY
Slow released antituberculotic preparation
The slow released antituberculotic preparation is implanting agent or injection with slow releasing of medicine in the local tuberculosis focus to maintain the local effective medicine concentration while lowing the systemic toxicity. The slow released injection consists of slow released microsphere and solvent. The slow released microsphere consists of slow releasing supplementary material and antituberculotic selected from rifampicin, rifamycin, rifapentine and / or rifabutine. The solvent is special solvent containing suspending agent of carboxymethyl cellulose sodium, etc. and has viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is selected from EVAc, PLA, PLGA, etc. The slow released preparation may be prepared with slow released microsphere. The present invention has obvious and unique treating effect on various kinds of intractable tuberculosis.
Owner:SHANDONG LANJIN PHARMA
Method for detecting anti-tuberculosis drug in serum by ultra-high performance liquid chromatography-tandem mass spectrometry technology
InactiveCN111766311AHigh sensitivityStrong specificityComponent separationAntituberculosis drugPyrazine
The invention discloses a method for detecting an anti-tuberculosis drug in serum by an ultra-high performance liquid chromatography-tandem mass spectrometry technology. The antitubercular drug comprises cycloserine (CYS), pyrazinamide (PZN), isoniazid (INZ), p-aminosalicylic acid (P-ASA), ethiisonicotinamide (ETN), ethambutol (ETB), clofazimine (CFM), bedaquiline (BDQ), rifampicin (RFP), rifbutine (RFB) and rifapentine (RFT). The method includes: detecting the content of the antituberculosis drug in the pretreated serum by adopting an ultra-high performance liquid chromatography-tandem mass spectrometry method, performing quantifying by utilizing a mass spectrometry isotope internal standard method, establishing a calibration curve by taking the concentration ratio of the standard substance to the internal standard substance as an X axis and the peak area ratio of the standard substance to the internal standard substance as a Y axis, and calculating the concentration of the target drug in the serum; the method is high in sensitivity, strong in specificity and simple in pretreatment process, separation and detection of the anti-tuberculosis drugs in serum are completed within 5 min, and a simple and rapid detection method is provided for clinical concentration monitoring of the anti-tuberculosis drugs.
Owner:南京品生医学检验实验室有限公司
Kit and method for detecting antituberculous drugs and metabolites thereof in sample
PendingCN114354804AExpand the types of testingIncrease varietyComponent separationMetaboliteAntituberculous drug
The invention particularly provides a kit and a method for detecting antituberculous drugs and metabolites thereof in a sample. The kit is used for detecting antituberculous drugs and metabolites thereof in a sample, and comprises a calibration product, a quality control product, an instrument quality control product and an isotope internal standard product, both the calibration material and the quality control material contain rifampicin, isoniazide, rifapentine, pyrazinamide, ethambutol, clofazimine, cycloserine, moxifloxacin, levofloxacin, linezolid, acetyl isoniazide, bedaquiline and deacetylrifampicin; the instrument quality control product comprises a methanol solution containing rifampicin, isoniazid, rifapentine, pyrazinamide, ethambutol, clofazimine, cycloserine, moxifloxacin, levofloxacin, linezolid, acetyl isoniazid, bedaquiline and deacetylrifampicin; the isotope internal standard substance contains an internal standard substance corresponding to a substance contained in the calibrator. The kit disclosed by the invention can be used for detecting the concentrations of the antituberculous drugs and metabolites thereof in various sample types.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
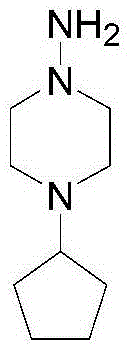
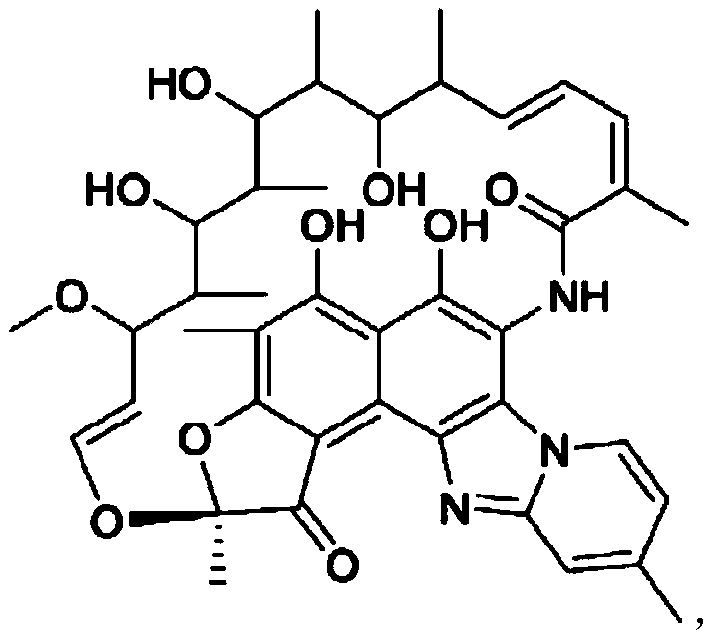
Preparation method of rifapentine
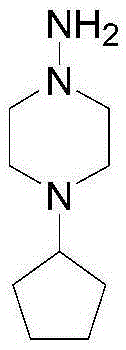
The invention discloses a preparation method of rifapentine, and the steps are as follows: rifamycin S is used as a starting material for reacting with dihydroxy methyl tert-butyl amine in a first organic solvent to convert into intermediate rifaoxazine; a catalyst and a reductant are added into a second organic solvent for hydrolysis ring opening of the intermediate rifaoxazine; a rifapentine solution is obtained by condensation of a rifaoxazine hydrolysis product and 1-amino-4-cyclopentyl piperazine; and a rifapentine finished product is obtained by filtration, segmented crystallization at multistage crystallization temperature, first separation, washing, second separation, drying, soaking, third separation, drip washing, spin drying, sieving, drying, and powder mixing refining processes of the rifapentine solution. The preparation method adopts the reductant which can play a role in anti oxidation; different crystallization temperature is adopted in the segmented crystallization process; and by combination with the adding of a crystallization solvent, the crystalline polymorph is uniform, the particles are uniform, the stability is good, and the defects of high impurity content and nonuniform particles of rifapentine processes in the prior art can be solved.
Owner:SICHUAN LONG MARCH PHARMA CO LTD
Oral administration forms for controlled release of rifampicin for the treatment of bacterial infections and inflammatory diseases of the gastrointestinal tract
Oral administration forms are described for the controlled release of an antibiotic selected from the group consisting of rifampicin, rifabutin, rifapentine, rifalazil and mixtures thereof, for treating bacterial infections of the gastrointestinal tract, in particular travellers' diarrhoea, hepatic encephalopathy, ulcerative colitis, irritable bowel syndrome (IBS), Crohn's disease, and IBD (inflammatory bowel disease) in general. Moreover, said oral administration forms allow reduction of the amounts of antibiotic to be taken, with respect to the known administration forms and without reaching blood concentrations such as to select resistant strains of tuberculosis mycobacteria.
Owner:FARM CABER
Preparation method of high-purity rifapentine
The invention provides a preparation method of high-purity rifapentine, which is characterized by comprising the following steps: 1. carrying out a cyclization reaction on rifamycin S acid, acid and dimethyloltert-butylamine in an N, N-dimethylformamide solvent; 2, pouring the reaction solution into acid water, standing, and filtering to obtain a rifaxizine filter cake; 3, adding urea, n-butyl alcohol, Vc and sodium carbonate into the rifaxizine filter cake for a ring-opening reaction; 4, after the reaction is completed, increasing the purity to 1-amino-4-cyclopentylpiperazine for a condensation reaction until the reaction is completed so as to obtain a rifapentine reaction solution; and step 5, dropwise adding acid water into the rifapentine reaction solution, stirring, stopping heating after crystallization, lowering the rotating speed after turning red, cooling after dropwise adding is finished, filtering to obtain the filter cake, and carrying out secondary recrystallization on thefilter cake. According to the preparation method of the high-purity rifapentine, provided by the invention, the maximum single impurity of the rifapentine can be controlled within 0.1%, and the residual n-butyl alcohol and the residual ethanol can be controlled within 0.5%.
Owner:WUXI FORTUNE PHARMA
Pharmaceutical Composition Containing Polymyxin B/Trimethoprim based Therapeutics
ActiveUS20190175550A1Increase virulenceAntibacterial agentsOrganic active ingredientsRifabutinTrimethoprim
The present invention features an antibacterial composition comprising 1) a composition A comprising polymyxin B and trimethoprim; and 2) an antibiotic agent selected from the group consisting of rifampicin, rifabutin, rifapentine, rifaximin, pefloxacin mesylate, sparfloxacin, sarafloxacin HCl, tobramycin, lomefloxacin, besifloxacin, danofloxacin mesylate, enrofloxacin, nadifloxacin and clinafloxacin, a topical pharmaceutical thereof, and a method of treating bacterial infections using mixtures of 1 and 2.
Owner:UNIVERSITY OF ROCHESTER
Compositions, devices and methods for treating autism
The present invention relates to a method for treating, ameliorating, reversing and / or preventing autism or an autism spectrum disorder (ASD) via the administration of a formulation, a pharmaceuticalpreparation or a pharmaceutical composition comprising or consisting of: (a) a rifaximin (optionally a XIFAXAN, XIFAXANTA or NORMIXT), an extended intestinal release (EIR) rifaximin, a rifamycin derivative, a rifampicin (or rifampin) (optionally RIFADIN), a rifabutin (optionally MYCOBUTIN), a rifapentine (optionally PRIFTIN), a rifalazil, a bicozamycin, or a mixture or combination thereof, or (b)a rifaximin (optionally a XIFAXAN, XIFAXANTA or NORMIX) and at least one additional antimicrobial or antibiotic agent, wherein optionally the at least one additional antimicrobial or antibiotic agentcomprises a vancomycin, a metronidazole (optionally FLAGYLTM, METROTM), a tinidazole (optionally FASIGYN, SIMPLOTAN, TINDAMAX) or a combination thereof
Owner:托马斯·朱利叶斯·波洛迪
A medicament for the prevention and control of Huanglongbing
InactiveCN106070262BConvenient prevention and controlBiocideDead animal preservationPenicillinButanediol
Owner:NANJING AGRICULTURAL UNIVERSITY
A kind of sustained-release microsphere loaded with rifapentine and linezolid and its preparation method and application
InactiveCN106580915BGood slow releaseIncrease concentrationAntibacterial agentsOrganic active ingredientsPatient complianceMicrosphere
The invention provides a sustained release microsphere carrying rifapentine and linezolid as well as a preparation method and applications of the sustained release microsphere. The sustained release microsphere contains rifapentine, linezolid and a medicinal carrier, wherein the medicinal carrier wraps rifapentine and linezolid, and the medicinal carrier is poly lactic-co-glycolic acid. The invention further provides the preparation method of the sustained release microsphere. The sustained release microsphere can treat pulmonary tuberculosis, especially, cavity tuberculosis through bronchoscope local administration; the problems that the existing medicines are poor in sustained release effect, the keeping time of the effective medicine concentration is short, and the compliance of patients is poor, are solved.
Owner:THE 309TH HOSPITAL OF CHINESE PEOPLES LIBERATION ARMY
Rifapentine-containing pharmaceutical composition and preparation method thereof
PendingCN114306247ALow impurity contentReduce contentAntibacterial agentsOrganic active ingredientsNitrosoUse medication
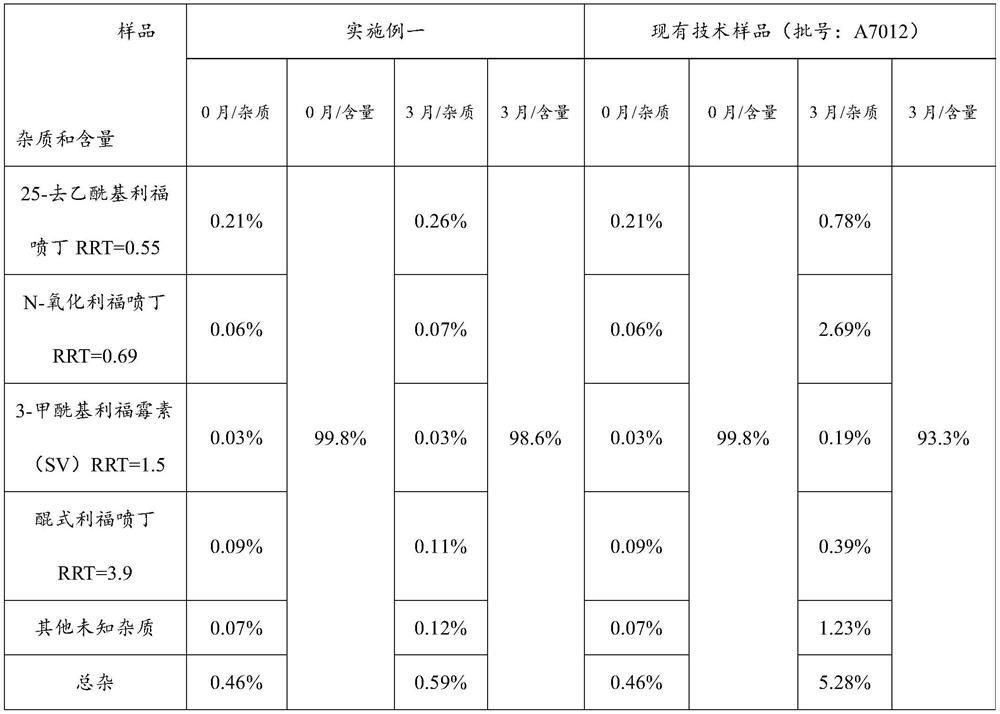
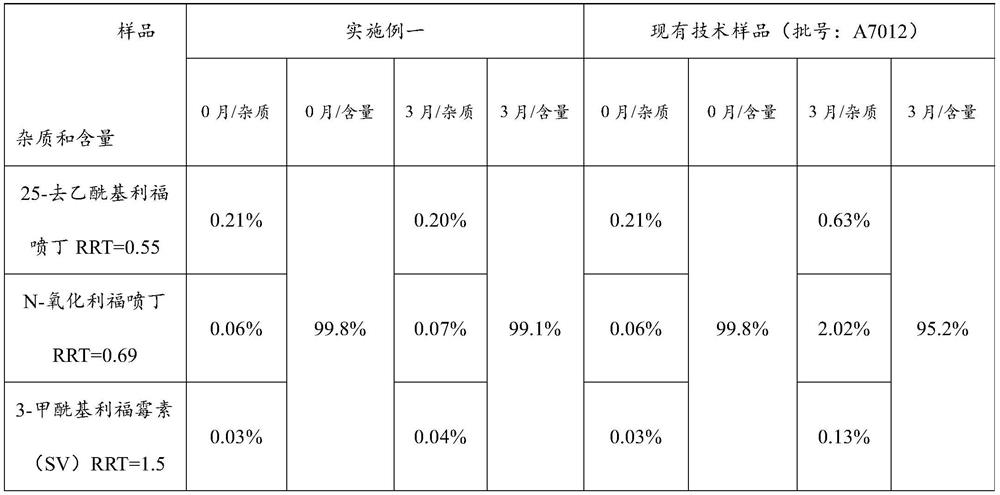
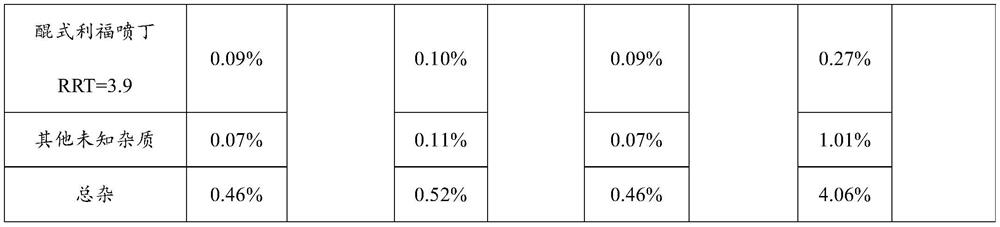
The invention relates to a rifapentine-containing pharmaceutical composition and a preparation method thereof, rifapentine-containing particles are prepared through dry granulation, the content of impurities in the pharmaceutical composition in the prior art is reduced, especially the content of genotoxic impurity 1-cyclopentyl-4-nitrosopiperazine is reduced, and the safety of medication and the curative effect of the medicine are improved.
Owner:JIANGSU SIMCERE PHARMA +1
Refapentine or rifampin liposome perfusate, injection and preparation process thereof
InactiveCN1176655CEasy to prepareWith slow and controlled releaseAntibacterial agentsOrganic active ingredientsEmulsionCholesterol
The present invention is characterized by that it uses (by weight percentage) 1-22% of rifampin or rifapentine, 1-27% of lecithin, 1-18 % of cholesterol and 70-100 % of distilled water as main components, and adds emulsifier and auxiliary medicine so as to respectively obtain the rifapentine or rifampin liposome micro emulsion perfusate and injection and rifupentine or rifampin composite medicine liposome micro emulsion perfusate and injection. Said invention also provides their preparation method.
Owner:广一平 +1
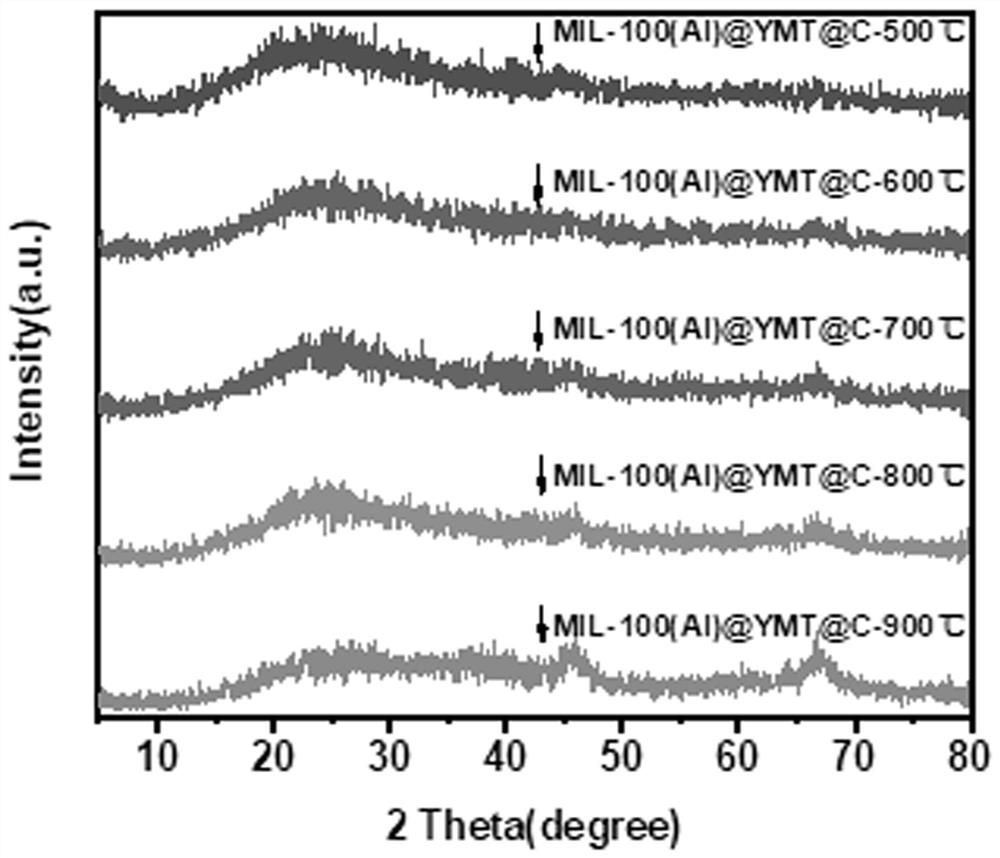
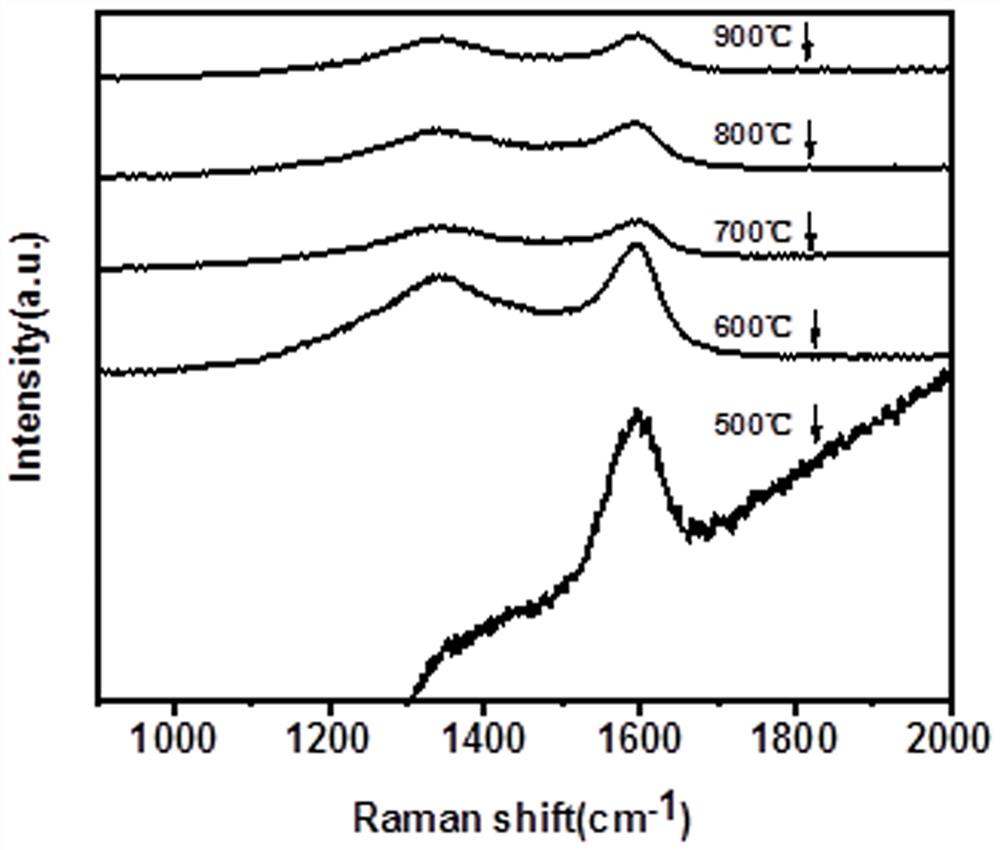
A polyphenol-modified gamma-type alumina-based porous carbon composite material, preparation method and application
ActiveCN113457633BEasy to synthesizeMild reaction conditionsOther chemical processesWater contaminantsPorous carbonNanoparticle
The invention belongs to the field of rifamycin adsorption materials, and in particular relates to a polyphenol-modified gamma-type alumina-based porous carbon composite material, a preparation method and an application. The polyphenol-modified gamma-type alumina-based porous carbon composite material is gamma-(Al 2 o 3 ) 1.333 / C BT‑900 Composite materials are composed of nanoparticles with a length of 100-200 nm and uniform size. Polyphenol-modified gamma-type alumina-based porous carbon composite material gamma-Al provided by the invention 2 o 3 / C BT‑900 As a new removal material for rifapentine molecules, it can exert the synergistic effect of nanoporous carbon and metal oxides, further increase the specific surface area of the material, and increase the contact area and adsorption sites with rifapentine. Using it as a novel adsorbent for rifapentine shows good adsorption performance.
Owner:FUZHOU UNIV
The preparation method of rifapentine
The invention discloses a preparation method of rifapentine, and the steps are as follows: rifamycin S is used as a starting material for reacting with dihydroxy methyl tert-butyl amine in a first organic solvent to convert into intermediate rifaoxazine; a catalyst and a reductant are added into a second organic solvent for hydrolysis ring opening of the intermediate rifaoxazine; a rifapentine solution is obtained by condensation of a rifaoxazine hydrolysis product and 1-amino-4-cyclopentyl piperazine; and a rifapentine finished product is obtained by filtration, segmented crystallization at multistage crystallization temperature, first separation, washing, second separation, drying, soaking, third separation, drip washing, spin drying, sieving, drying, and powder mixing refining processes of the rifapentine solution. The preparation method adopts the reductant which can play a role in anti oxidation; different crystallization temperature is adopted in the segmented crystallization process; and by combination with the adding of a crystallization solvent, the crystalline polymorph is uniform, the particles are uniform, the stability is good, and the defects of high impurity content and nonuniform particles of rifapentine processes in the prior art can be solved.
Owner:SICHUAN LONG MARCH PHARMA CO LTD
Pharmaceutical composition for treating pulmonary tuberculosis and preparation method thereof
PendingCN114129585AReduced rate of abnormal liver functionAntibacterial agentsOrganic active ingredientsPharmaceutical drugPulmonary tuberculosis
The invention provides a pharmaceutical composition for treating pulmonary tuberculosis, which comprises a main drug and an auxiliary drug, and the main drug comprises rifapentine and glucurolactone. The main drugs comprise the following components in parts by weight: 0.5-5 parts of rifapentine and 0.5-10 parts of glucurolactone, and the weight ratio of the rifapentine to the glucurolactone is (0.375-0.75): 1. According to the pharmaceutical composition for treating pulmonary tuberculosis disclosed by the invention, the rifapentine and the glucurolactone are prepared into the pharmaceutical composition according to a reasonable proportion, so that the abnormal liver function rate of a patient is reduced.
Owner:ZHUOHE PHARM GRP CO LTD
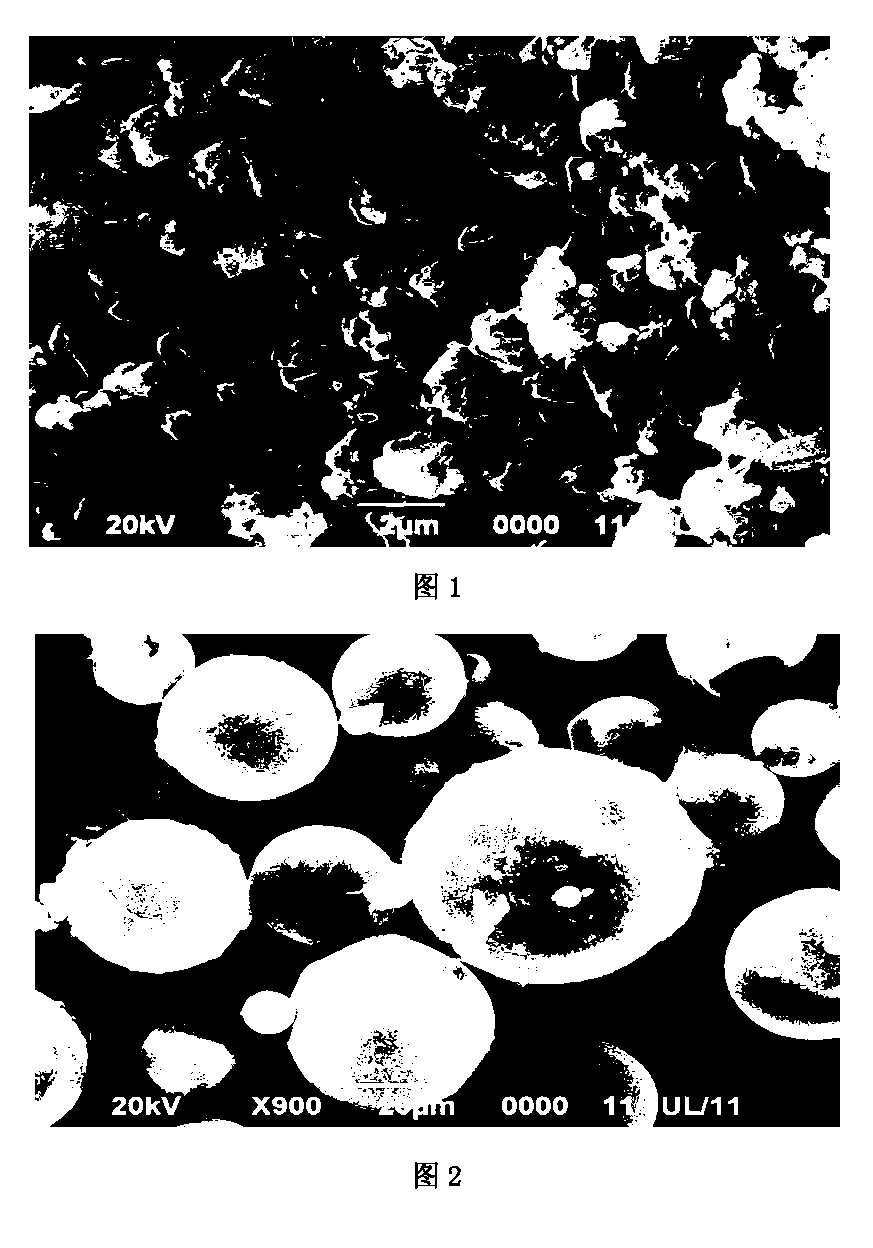
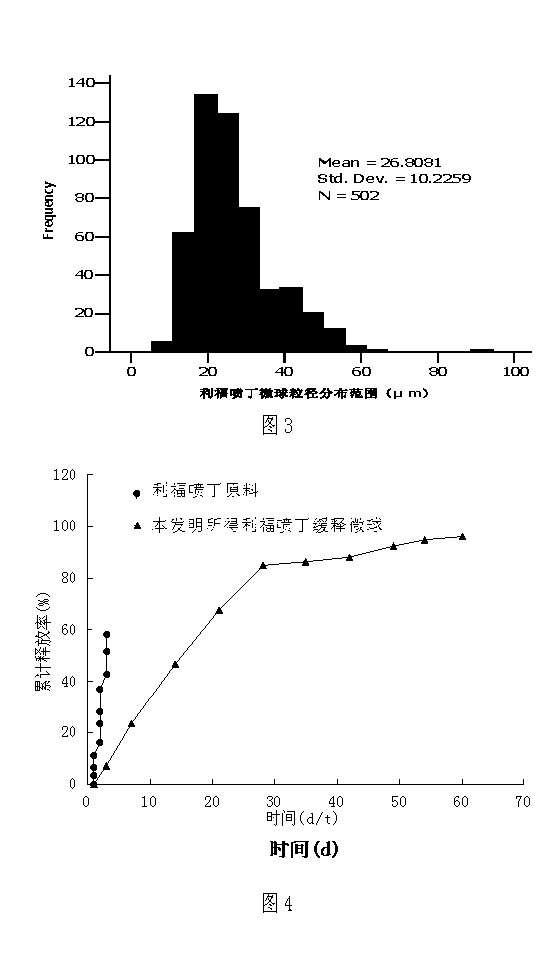
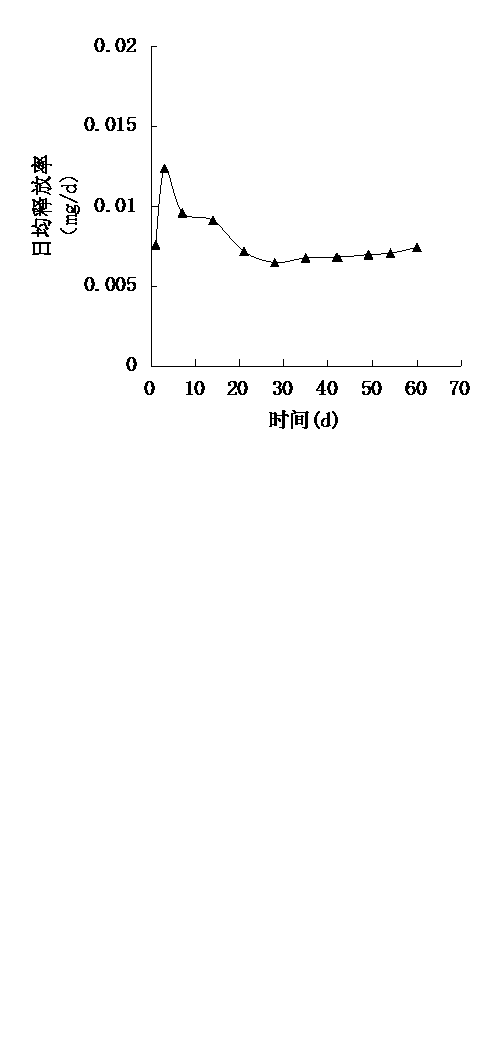
Rifapentine sustained-released microspheres and preparation method thereof
ActiveCN102793675BHigh drug loadingIncrease doseAntibacterial agentsOrganic active ingredientsAntituberculous chemotherapySevere malnutrition
The invention, relating to both the technical field of biology and medicine, discloses rifapentine sustained-released microspheres and a preparation method thereof. The rifapentine sustained-released microspheres disclosed herein have high drug loading capacity, good entrapment efficiency, and good sustained action by in vitro release test. The rifapentine sustained-released microspheres disclosed herein can be directly implanted into tuberculosis local focus to conduct regional continuous chemotherapy to let the tuberculosis focus position have large drug amount, high drug concentration, long duration, large effect, good local curative effect, little amount of the drug distributed to the whole body, and low plasma concentration, thus the curative effect is raised, and the effect of adverse reaction is significantly reduced, so that good treatment of patients (severe liver and kidney dysfunction, combination of a plurality of systemic diseases, severe malnutrition) who can not endure anti-tuberculosis chemotherapy can be achieved. The preparation method of the rifapentine sustained-released microspheres has the advantages of low cost, simple operation, and convenient, simple and repeatable method, and can generate huge economic benefits.
Owner:THE FIRST TEACHING HOSPITAL OF XINJIANG MEDICAL UNIVERCITY
Ophthalmic compositions of rifamycins and uses thereof
Provided herein are ophthalmic pharmaceutical formulations comprising a rifamycin compound. Also provided herein are methods of treating ocular diseases or disorders by administering such ophthalmic formulations. This invention relates generally to pharmaceutical compositions or formulations suitable for administration to an eye. In some aspects, this invention relates to ophthalmic pharmaceutical compositions or formulations comprising one or more rifamycin compounds selected from the group consisting of rifampicin, rifabutin, rifapentine. In one aspect, the invention relates to methods of treating an ocular disease, disorder or condition comprising administering to a patient in need thereof an ophthalmic composition comprising an effective amount of a rifamycin compound selected from the group consisting of rifampicin, rifabutin, rifapentine, and rifaximin.
Owner:SERIZAWA HIROAKI
Slow released antituberculotic preparation
The slow released antituberculotic preparation is implanting agent or injection with slow releasing of medicine in the local tuberculosis focus to maintain the local effective medicine concentration while lowing the systemic toxicity. The slow released injection consists of slow released microsphere and solvent. The slow released microsphere consists of slow releasing supplementary material and antituberculotic selected from rifampicin, rifamycin, rifapentine and / or rifabutine. The solvent is special solvent containing suspending agent of carboxymethyl cellulose sodium, etc. and has viscosityof 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is selected from EVAc, PLA, PLGA, etc. The slow released preparation may be prepared with slow released microsphere. The present invention has obvious and unique treating effect on various kinds of intractable tuberculosis.
Owner:SHANDONG LANJIN PHARMA
Anti-tuberculosis stable pharmaceutical composition in a form of a dispersible tablet comprising granules of isoniazid and granules of rifapentine and its process of preparation
InactiveUS9814680B2Improve availabilityKeep dryAntibacterial agentsHydroxy compound active ingredientsExcipientPharmaceutical preservatives
Owner:SANOFI SA
Rifapentine sustained-released microspheres and preparation method thereof
ActiveCN102793675AHigh drug loadingIncrease doseAntibacterial agentsOrganic active ingredientsLiver and kidneyMicrosphere
The invention, relating to both the technical field of biology and medicine, discloses rifapentine sustained-released microspheres and a preparation method thereof. The rifapentine sustained-released microspheres disclosed herein have high drug loading capacity, good entrapment efficiency, and good sustained action by in vitro release test. The rifapentine sustained-released microspheres disclosed herein can be directly implanted into tuberculosis local focus to conduct regional continuous chemotherapy to let the tuberculosis focus position have large drug amount, high drug concentration, long duration, large effect, good local curative effect, little amount of the drug distributed to the whole body, and low plasma concentration, thus the curative effect is raised, and the effect of adverse reaction is significantly reduced, so that good treatment of patients (severe liver and kidney dysfunction, combination of a plurality of systemic diseases, severe malnutrition) who can not endure anti-tuberculosis chemotherapy can be achieved. The preparation method of the rifapentine sustained-released microspheres has the advantages of low cost, simple operation, and convenient, simple and repeatable method, and can generate huge economic benefits.
Owner:THE FIRST TEACHING HOSPITAL OF XINJIANG MEDICAL UNIVERCITY
Pharmaceutical composition containing polymyxin B/trimethoprim based therapeutics
ActiveUS11096923B2Increase virulenceAntibacterial agentsOrganic active ingredientsRifabutinTrimethoprim
Owner:UNIVERSITY OF ROCHESTER
A kind of rifapentine capsule and preparation method thereof
ActiveCN106913556BSolve the problem of powder fluidityHigh dissolution rateOrganic active ingredientsPharmaceutical non-active ingredientsVitamin CVitamin
The invention discloses a rifapentine capsule and a preparation method thereof. Tween 80, HPMC and K 12 Add pure ethanol to dissolve into a binder, use the binder to spray rifapentine to obtain rifapentine granules; then add HPMC, BHA and vitamin C to dissolve into pure ethanol to form a coating solution, and use The coating solution sprays and coats the rifapentine granules to obtain rifapentine coated granules; finally mixes the rifapentine coated granules with pregelatinized starch for total mixing and then fills , to prepare rifapentine capsules. Adopting the method of the present invention to prepare rifapentine capsules solves the problem of fluidity of the intermediate powder of the original preparation of rifapentine capsules, so as to solve the problems of related substances exceeding the standard and large differences in loading, and at the same time, the dissolution rate is also relatively large improvement.
Owner:SHANGHAI SINE WANXIANG PHARMA +1
Compositions, devices and methods for treating obsessive-compulsive disorder
The present invention relates to a method for treating, ameliorating, reversing and / or preventing an obsessive-compulsive disorder (OCD) via the administration of a formulation, a pharmaceutical preparation or a pharmaceutical composition comprising or consisting of: (a) a rifaximin (optionally a XIFAXAN, XIFAXANTA or NORMIXT), an extended intestinal release (EIR) rifaximin, a rifamycin derivative, a rifampicin (or rifampin) (optionally RIFADIN), a rifabutin (optionally MYCOBUTIN), a rifapentine (optionally PRIFTIN), a rifalazil, a bicozamycin, a pyrido-imidazo rifamycin, or a mixture or combination thereof, or (b) a rifaximin, a polymorphic form of a rifaximin, or rifaximin equivalent thereof (optionally a XIFAXAN, XIFAXANTA or NORMIX) and at least one additional antimicrobial or antibiotic agent.
Owner:CENT FOR DIGESTIVE DISEASES PTY LTD
Pharmaceutical composition for treating pulmonary tuberculosis and preparation method thereof
InactiveCN112472702AGood curative effectImprove securityAntibacterial agentsOrganic active ingredientsAdjuvantPharmaceutical drug
The invention discloses a pharmaceutical composition for treating pulmonary tuberculosis, comprising: a main drug comprising rifapentine or rifampicin; an adjuvant which comprises a stable material, afilling agent, a disintegrating agent, an adhesive and a lubricating agent. The preparation method of the pharmaceutical composition comprises the following specific steps: S1, preparing a first stable material solution; S2, preparing a second stable material solution; S3, preparing a first mixture; S4, preparing a second mixture; S5, mixing a filler, a disintegrating agent, an adhesive and the second mixture, and granulating by using a dry granulator to obtain a third mixture; S6, adding a lubricant into the third mixture, and pressing into tablets or filling into capsules. According to thepharmaceutical composition for treating pulmonary tuberculosis and the preparation method of the pharmaceutical composition, provided by the invention, the preparation is simple, the curative effect and the safety of the pharmaceutical composition are fundamentally improved, and great convenience can be brought to the use and the storage of clinical medicines.
Owner:ZHUOHE PHARM GRP CO LTD
Device and method for preparing gel microsphere and gel microsphere in which antitubercular agents can be injected
InactiveCN102178604BHeight adjustableReduce dwell timeAntibacterial agentsOrganic active ingredientsRifabutinMicrosphere
The invention relates to a device and a method for preparing a gel microsphere and a gel microsphere in which antitubercular agents can be injected. The device comprises: a porous shower nozzle, a collecting cup, a collecting tubule, a high pressure static generator, a constant current pump, a settling basin, an injection pump and an infiltration electrode. On the basis of a common static liquid drop generating device, the collecting tubule is arranged to form a liquid level flow regulating system, so that a liquid exchange passage exists between the collecting cup and the settling basin and can collect the low-density gel microspheres floating on the liquid level of the collecting cup and rapidly transfer the microspheres to the settling basin. Meanwhile, the built-in infiltration electrode type porous shower nozzle can enable the drug liquid droplet in drug-carrying sol solution to be fully charged, and form a uniform high electric field with the collecting tubule, thus enhancing the atomizing effect of the liquid column of the nozzle and enabling the drug-carrying sol solution to form monodispersity gel microspheres. The gel microsphere in which antitubercular agents can be injected contains rifapentine or rifabutin, made by the device and the method. The gel microsphere is high in yield, and large in drug-carrying capacity, and does not sink down or adhere after drying.
Owner:中国人民解放军第三0九医院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com