Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
58 results about "Isonicotinic hydrazide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Isoniazid (Isonicotinic Acid Hydrazide) Pharmacology: Isoniazid is a bactericidal agent active against organisms of the genus Mycobacterium, specifically M. tuberculosis, M. bovis and M. kansasii. It is a highly specific agent, ineffective against other microorganisms.
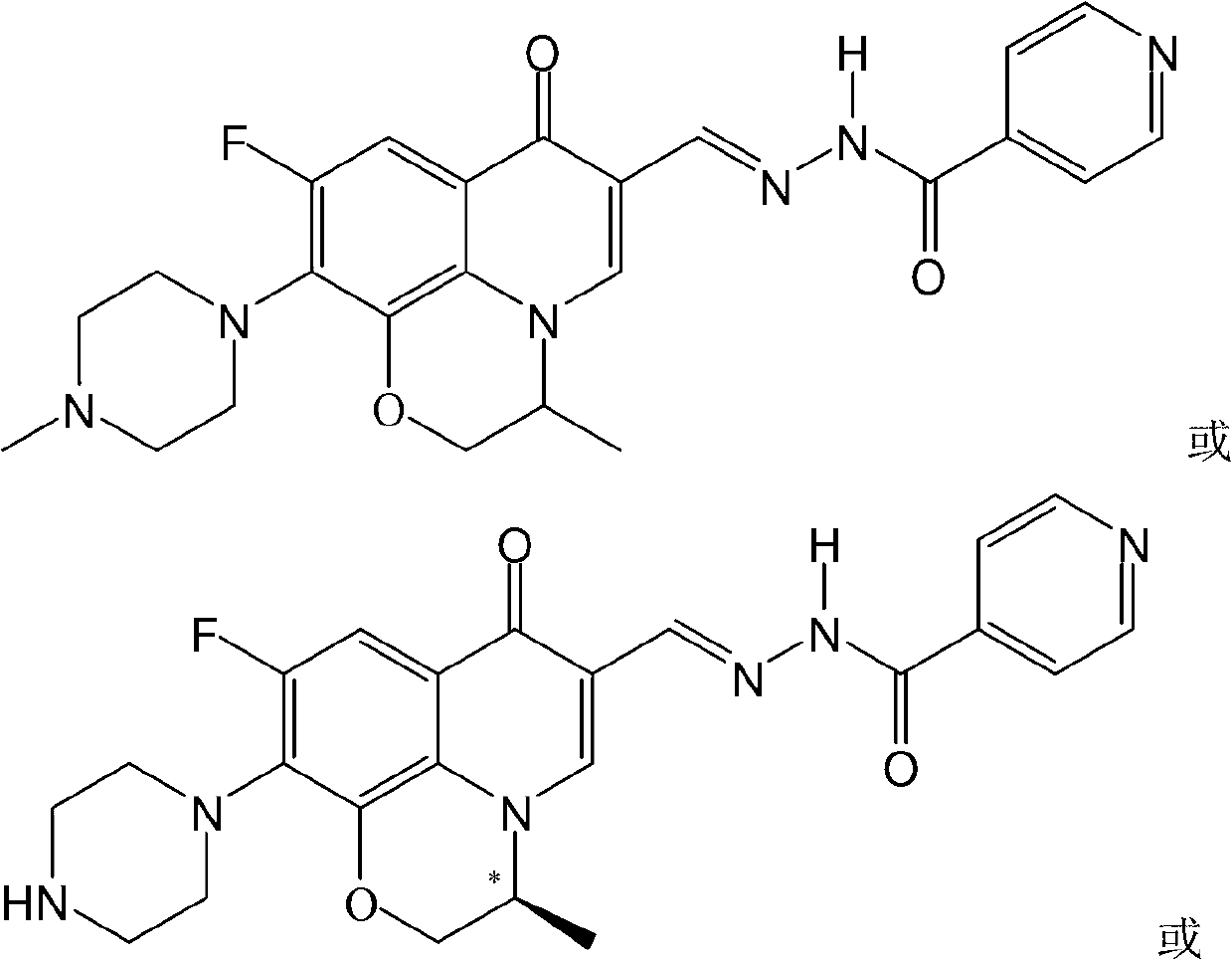
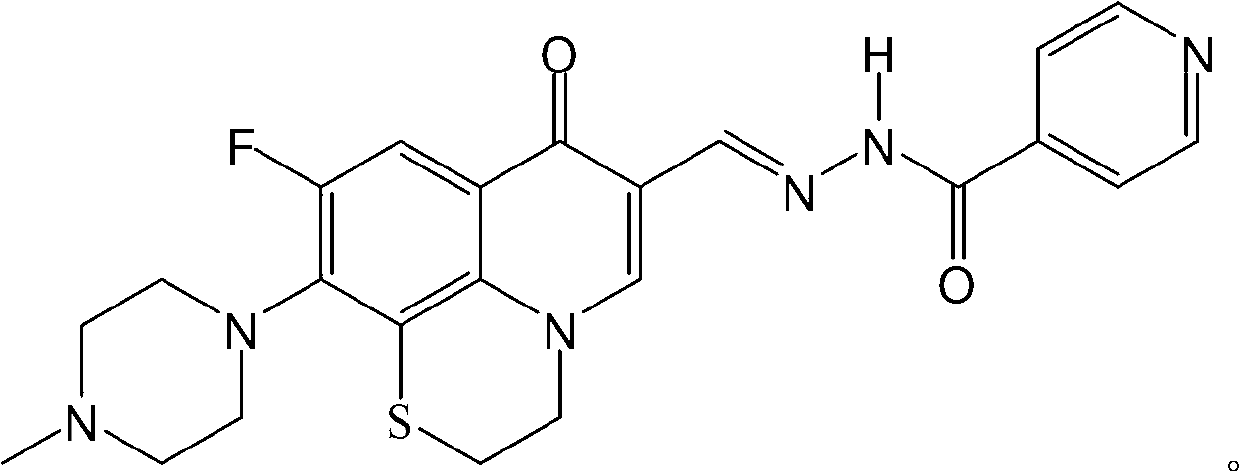
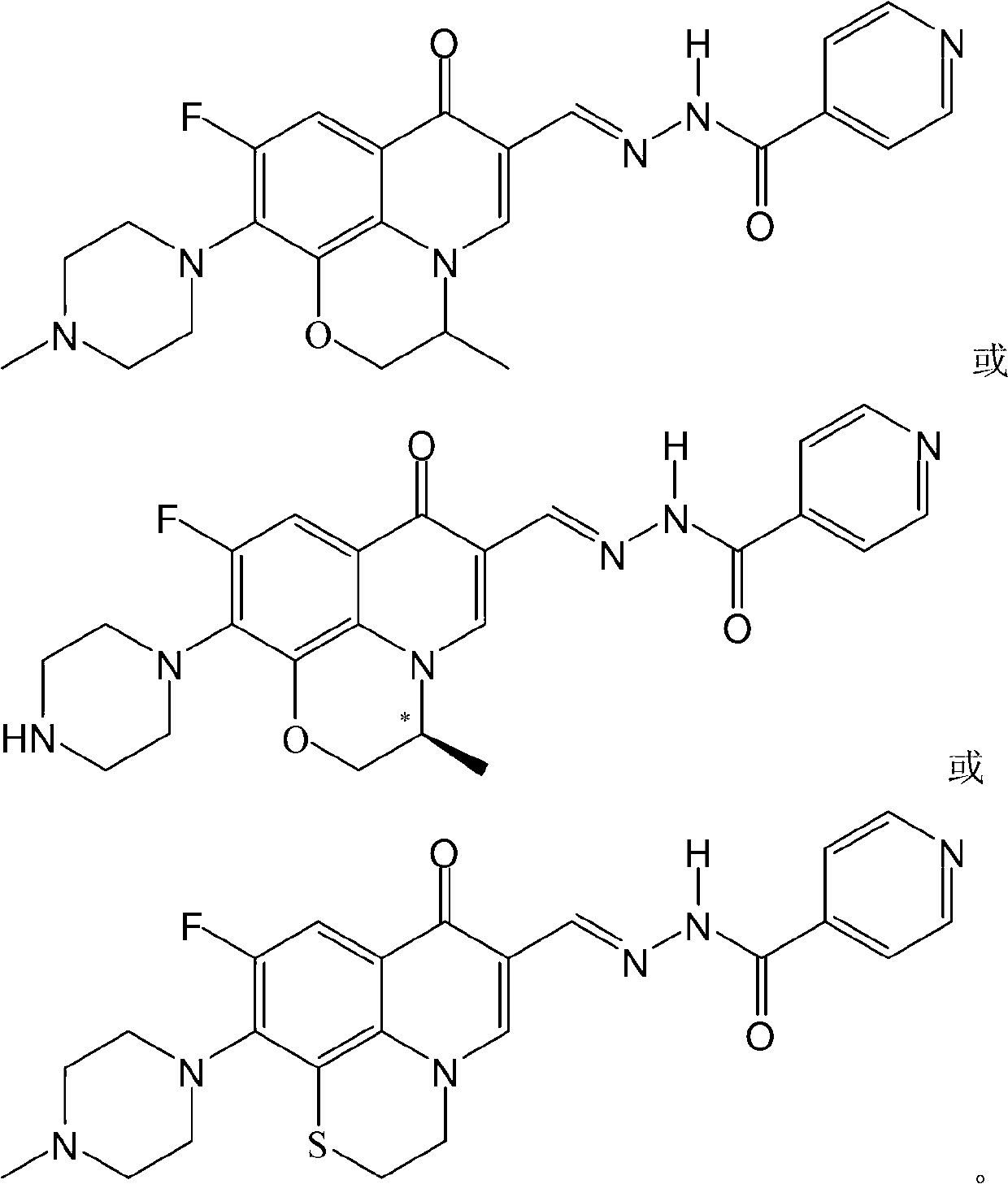
Fluoroquinolone acetal isoniazone, and preparation method and application thereof
InactiveCN102827187ASmall side effectsReduce the chance of developing drug resistanceAntibacterial agentsOrganic active ingredientsOxygen atomSide effect
The invention discloses a fluoroquinolone acetal isoniazone, of which the chemical structure general formula is disclosed as Formula I shown in the description, wherein R1 is hydrogen atom or methyl group; R2 is hydrogen atom or amino group; R3 is hydrogen atom, methyl group, ethyl group, formacyl group, acetyl group, aroyl group or sulfonyl group; R4 is hydrogen atom or methyl group; and X is oxygen atom or sulfur atom. The fluoroquinolone isoniazone disclosed by the invention implements complementarity between the two anti-tuberculosis medicines fluoroquinolone and the isoniazide, lowers the toxic and side effect of the fluoroquinolone and the isoniazide, reduces the generation probability of drug resistance of Mycobacterium tuberculosis for the double-effect antimicrobial agent, and can be used as an anti-Mycobacterium tuberculosis medicine for brand-new structure development of medicinal active substances.
Owner:HENAN UNIVERSITY
Medicine-release system of compound Rifampicin
The present invention relates to a compound rifampin preparation, in particular, it relates to a medicine-releasing system of compound rifampin. Said system comprises rifampin and isoniazid, and further selectively can include pyrazinamide or pyrazinamide and etambol, which is characterized by that the isoniazid, pyrazinamide and etambol are gastric soluble, and the rifampin is enteric soluble orisoniazid is enteric soluble, and others are gastric soluble. Said preparation can be made into capsule, granules, tablet, multi-layer table and suspension preparation. Said invention also provides its preparation method.
Owner:SICHUAN LONG MARCH PHARMA CO LTD
Slow released compound antituberculotic preparation
The slow released compound antituberculotic preparation contains at least one of rifampicin, pyrazinamide, kanamycin, isoniazide, rifapentine, etc. The slow released preparation is slow released injection or slow released implanting agent. The slow released injection consists of slow released microsphere and solvent, the slow released microsphere contains slow releasing supplementary material and antituberculotic, and the solvent is special solvent containing suspending agent carboxymethyl cellulose sodium and of viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is EVAc, PLA, PLGA, sebacic acid copolymer, etc. The slow released compound antituberculotic preparation is set or injected into local tuberulosis focus to treat various kinds of intractable tuberulosis, and has medicine releasing period up to 30-40 days, less systemic toxicity and unique curative effect.
Owner:JINAN SHUAIHUA PHARMA TECH
Benzothiazinone compound, preparation method thereof and application of benzothiazinone compound as antituberculosis drug
ActiveCN112409293AGood antibacterial effectWork around the bug with high cLogP valuesAntibacterial agentsOrganic active ingredientsAntituberculosis drugAntituberculous drugs
The invention discloses a benzothiazinone compound and a preparation method and application of the benzothiazinone compound as an antituberculosis drug, and particularly relates to a novel compound with a benzothiazinone skeleton. The compound has an inhibition effect on tubercle bacillus, especially tubercle bacillus with clinical drug resistance. Results show that the compound shows an obvious antibacterial effect, the antibacterial effect far exceeds that of a positive control isoniazide, and particularly, compared with a positive control pBTZ169, the compound has an obvious and good cLogPvalue.
Owner:SUZHOU UNIV
Preparation method of topiroxostat
PendingCN113666909ASimple and efficient operationHigh reaction yieldOrganic chemistryPtru catalystAcyl group
The invention belongs to the field of pharmaceutical chemicals, and particularly relates to a preparation method of topiroxostat. The preparation method disclosed by the invention comprises the following steps: 2-cyanopyridine and formamide are taken as raw materials to react to prepare 2-cyano-4-carbamoyl-pyridine; the 2-cyano-4-carbamoyl-pyridine continues to react with isoniazide to obtain a key intermediate 4-picolinic acid hydrazide-N'-(2-cyanopyridine-4-carbodiimide), and the key intermediate 4-picolinic acid hydrazide-N'-(2-cyanopyridine-4-carbodiimide) is subjected to ring closing to obtain the topiroxostat. The invention provides the novel method for synthesizing topiroxostat, which avoids the use of highly toxic chemical reagents, replaces a traditional catalyst with a green catalyst, is milder in reaction, is economical and environment-friendly, is higher in yield, and is suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Vitamin-medicine composition and its use
ActiveCN1636573AOvercome the disadvantage of poor stabilityImprove stabilityOrganic active ingredientsPowder deliveryDiseaseFreeze-drying
The present invention relates to vitamin medicine composition, and is especially one kind of medicine composition compounded with vitamin B1, vitamin B6 and vitamin B12 and its use in treating peripheral nerve injury, polyneuritis, trigeminal neuralgia, ischialgia, isonicotinyl hydrazine poisoning and other diseases. These three kinds of vitamins are synergistic in regulating body's metabolism and maintaining body's important physiological function. The medicine preparation is preparation into freeze dried powder for injection with high stability and is intravenous instilled to reduce pain.
Owner:吉林津升制药有限公司
Method for determining isoniazid by using quantum dot fluorescence quenching method
ActiveCN110672576AImprove researchSimple methodFluorescence/phosphorescenceRelative standard deviationFluorescent quenching
The invention belongs to the field of application of nano materials and relates to a method for determining isoniazid by using a quantum dot fluorescence quenching method. According to the method of the invention, cadmium telluride quantum dots are synthesized by means of aqueous-phase synthesis through adopting a two-step method so as to be used for the fluorescence detection of isoniazid content. The influence of a pH value, ion strength, a quantum dot dosage, reaction time and temperature on a reaction system is investigated, results show that when the concentration of the isoniazid rangesfrom 2.92*10<-5> to -1.02*10<-3>mol / L, the luminescence of the quantum dots in the system is obviously inhibited; the concentration of the isoniazid and the intensity of the luminescence presents a good linear relationship; the detection limit of the method is 4.826*10<-7>mol / L; the relative standard deviation of the method is 0.296% (n= 10); and the precision of the method is very good. The method provided by the invention has the advantages of simplicity, rapidity, low cost and good anti-interference capability, and can be directly used for detection and analysis. With the method adopted, asimple method for detecting isoniazid content can be realized; and research on the application of a quantum dot fluorescence detection method is expanded.
Owner:SOUTH CENTRAL UNIVERSITY FOR NATIONALITIES
Benzothiazinone compound, preparation method thereof and application of benzothiazinone compound as antituberculosis drug
InactiveCN111269197ALow cLogP valueGood medicineAntibacterial agentsOrganic active ingredientsAntituberculosis drugPharmaceutical drug
The invention discloses a benzothiazinone compound, a preparation method thereof and an application of the benzothiazinone compound as an antituberculosis drug, and particularly relates to a novel compound with a benzothiazinone skeleton. The compound has an inhibition effect on tubercle bacillus, especially tubercle bacillus with clinical drug resistance. Results show that the compound shows an obvious antibacterial effect, the antibacterial effect far exceeds that of a positive control isoniazide, and particularly, compared with a positive control pBTZ169, the compound has an obviously goodcLogP value.
Owner:SUZHOU UNIV
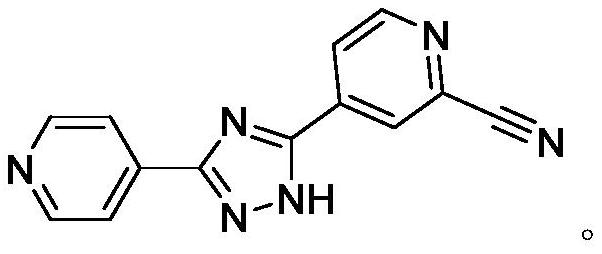
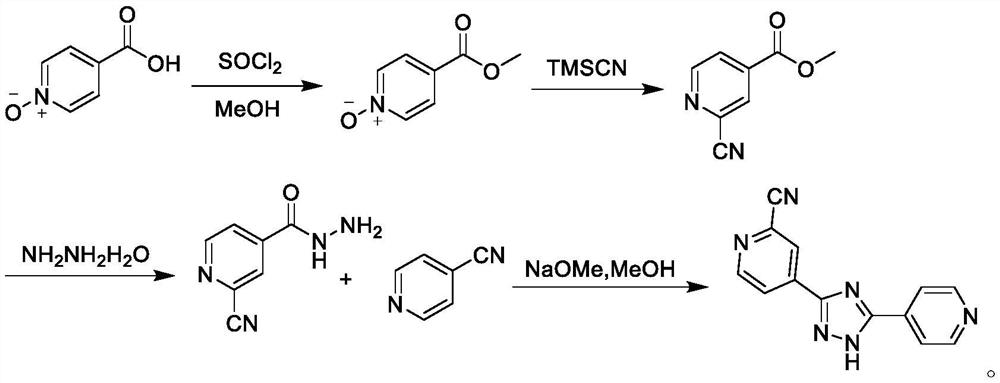
Preparation method of topiroxostat
The invention provides a preparation method of topiroxostat. The method comprises the following steps: subjecting isonicotinic acid as a starting material to oxidation with hydrogen peroxide to obtainisonicotinic acid-nitric oxide (an intermediate 1); then esterifying with methanol to obtain methyl isonicotinate-nitric oxide (intermediate 2); then performing hydrazinolysis with hydrazine hydrateto obtain isoniazide-nitric oxide (intermediate 3); then reacting with 4-cyanopyridine to obtain an intermediate 4; then cyaniding with trimethylsilyl cyanide to obtain an intermediate 5; and finallyperforming dehydration and cyclization to generate topiroxostat. According to the method, initial raw materials and reagents are cheap and easily available; experimental operation is simple and controllable, extreme reaction conditions are avoided, and the method is suitable for laboratory development and even industrial production; the total yield is high, and the production cost is reduced; purity of the finished product can be ensured.
Owner:孙哲
Isoniazide derivative, homogeneous enzyme immunoassay reagent and preparation method
ActiveCN111848507AStrong specificityHigh potencyOvalbuminSerum albuminAntiendomysial antibodiesEnzyme immunoassays
The invention discloses an isoniazid derivative, a detection reagent and a preparation method, and relates to the technical field of biological detection. An isoniazid immunogen prepared from the isoniazid derivative has high immunogenicity, and an obtained antibody has strong specificity and high titer; an isoniazid enzyme-labeled conjugate in a homogeneous enzyme immunoassay reagent prepared from the derivative is connected with recombinant glucose-6-phosphate dehydrogenase modified by gene engineering; the detection sensitivity is remarkably improved, a sample with the concentration as lowas 5 ng / ml or below can be effectively detected, the specificity is high, and cross reaction with 62 common drugs is avoided; the homogeneous enzyme immunoassay reagent can realize high-throughput andrapid detection of isoniazid content on a full-automatic biochemical analyzer, and is stable in detection result, high in accuracy, simple in detection method and easy to realize, popularize and use.
Owner:长沙博源医疗科技有限公司
S-triazolo-thiadiazole and thiadiazine derivatives, preparation method and application thereof
InactiveCN105001241AInhibitory Activity EquivalentAntibacterial agentsOrganic active ingredientsShikimate dehydrogenaseThiadiazoles
The invention relates to S-triazolo-thiadiazole and thiadiazine derivatives and an application thereof. Research results show that, the minimal inhibitory concentration (MIC) of the derivatives to mycobacterium tuberculosis standard strains H37Rv is 0.25 [mu]g / mL, and the inhibitory activities of the derivatives to isoniazide / rifampicin-resistant mycobacterium tuberculosis (MDRTB) and rifampicin-resistant mycobacterium tuberculosis (RDRTB) are the same and the MIC of the derivatives to MDRTB and RDRTB is 0.25-4 [mu]g / mL. Meanwhile, the resistance of some derivatives to MDRTB or RDRTB is better than the resistance of the derivatives to reference drugs RIF and INH. At the same time, most derivatives are equivalent in inhibitory activity to Shikimate dehydrogenase, thus being good in development prospect.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Substituted N-((1', 3'-azole-4'-yl)-methyl)-4-benzoyl-hexahydropyridine compound and applications thereof
The invention discloses a substituted N-((1', 3'-azole-4'-yl)-methyl)-4-benzoyl-hexahydropyridine compound represented by formula I, a preparation method thereof, and applications of the substituted N-((1', 3'-oxazole-4'-yl)-methyl)-4-benzoyl-hexahydropyridine compound in preparation of antituberculous drugs. The substituted N-((1', 3'-azole-4'-yl)-methyl)-4-benzoyl-hexahydropyridine compound possesses activity on mycobacterium tuberculosis-susceptible strains, and also possesses activity on strains with tolerance on traditional first-line antituberculous drugs such as isoniazide and rifampicin, and is a novel mycobacterium tuberculosis resistant compound with a promising application prospect.
Owner:MEDICINE & BIOENG INST OF CHINESE ACAD OF MEDICAL SCI
Slow released compound antituberculotic preparation containing synergist
The slow released compound antituberculotic preparation containing synergist is implanting agent or slow released injection comprising slow released microsphere and solvent. The slow released microsphere consists of slow releasing supplementary material, at least one antituberculotic selected from rifampicin, isoniazid and pyrazinamide and at least one antituberculotic synergist selected from cycloserine, ofloxacin, ciprofloxacin, sparfloxacin and capreomycin. The solvent is special solvent containing suspending agent carboxymethyl cellulose, etc. and with viscosity of 100-3000 cp at 20-30 deg.c. The slow releasing supplementary material is selected from EVAc, PLA, PLGA, etc. The slow released compound antituberculotic preparation can release antituberculotic in the local tubercolosis part for 30-40 days so as to maintain the local effective medicine concentration while lowering systemic toxicity. The present invention has obvious unique treating effect on various kinds of intractable tuberulosis.
Owner:JINAN SHUAIHUA PHARMA TECH
Method for preparing medicine-carrying hydroxyapatite/poly glycolide-co-lactide (PLGA)/chitosan demixing microspheres
ActiveCN102579361AHigh encapsulation efficiencyLong release timePharmaceutical non-active ingredientsGranular deliveryAcetic acidPolyvinyl alcohol
The invention discloses a method for preparing medicine-carrying hydroxyapatite / poly glycolide-co-lactide (PLGA) / chitosan demixing microspheres. The method comprises the following steps of: dissolving isoniazide in deionized water, adding hydroxyapatite powder, stirring in a dark place, and freeze-drying to obtain powder; mixing PLGA and the powder uniformly to obtain a hydroxyapatite / PLGA commixed solution containing the isoniazide; dissolving chitosan in an acetic acid aqueous solution to obtain a chitosan solution; mixing the chitosan solution and a polyvinyl alcohol aqueous solution to obtain a chitosan / polyvinyl alcohol solution; and pouring the hydroxyapatite / PLGA commixed solution containing the isoniazide into the chitosan / polyvinyl alcohol solution, stirring under vacuum, washingby using water, and freeze-drying to obtain the medicine-carrying hydroxyapatite / PLGA / chitosan demixing microspheres. The prepared medicine-carrying composite microspheres are regular in spherical shapes, uniform in particle size distribution, high in envelop rate of medicines, long in in-vitro medicine release time and small in burst release; and a preparation process is simple, raw materials are readily available, and industrialization is easy to realize.
Owner:广州智园生物科技有限公司
Kit and method for detecting antituberculous drugs and metabolites thereof in sample
PendingCN114354804AExpand the types of testingIncrease varietyComponent separationMetaboliteAntituberculous drug
The invention particularly provides a kit and a method for detecting antituberculous drugs and metabolites thereof in a sample. The kit is used for detecting antituberculous drugs and metabolites thereof in a sample, and comprises a calibration product, a quality control product, an instrument quality control product and an isotope internal standard product, both the calibration material and the quality control material contain rifampicin, isoniazide, rifapentine, pyrazinamide, ethambutol, clofazimine, cycloserine, moxifloxacin, levofloxacin, linezolid, acetyl isoniazide, bedaquiline and deacetylrifampicin; the instrument quality control product comprises a methanol solution containing rifampicin, isoniazid, rifapentine, pyrazinamide, ethambutol, clofazimine, cycloserine, moxifloxacin, levofloxacin, linezolid, acetyl isoniazid, bedaquiline and deacetylrifampicin; the isotope internal standard substance contains an internal standard substance corresponding to a substance contained in the calibrator. The kit disclosed by the invention can be used for detecting the concentrations of the antituberculous drugs and metabolites thereof in various sample types.
Owner:THE THIRD PEOPLES HOSPITAL OF SHENZHEN
Benzothiazinone derivative, preparation method thereof and application of benzothiazinone derivative as antituberculosis drug
InactiveCN111303075ALow cLogP valueGood medicineAntibacterial agentsOrganic active ingredientsAntituberculosis drugAntituberculous drugs
The invention discloses a benzothiazinone derivative, a preparation method thereof and application of the benzothiazinone derivative as an antituberculosis drug, which relate to a novel compound witha benzothiazinone skeleton. The compound has an inhibition effect on tubercle bacillus, particularly tubercle bacillus with clinical drug resistance. The benzothiazinone skeleton benzene ring is creatively changed, especially the substituent group is creatively changed to obtain a series of compounds, and an unexpected technical effect is achieved; the compound disclosed by the invention has an excellent inhibition effect on tubercle bacillus; compared with the existing clinical first-line drug isoniazide (MIC 0.5 muM), the reported compound activity has very great advantages, and more importantly, compared with the benzothiazinone antituberculosis drug pBTZ 169 in the existing research stage, the compound provided by the invention has a lower cLogP value and better druggability.
Owner:SUZHOU UNIV
Preparation method of isoniazid
PendingCN111138354AShorten the hydrazinolysis reaction timeImprove production efficiencyOrganic chemistryNicotinuric acidIsonicotinic acid
The invention discloses a preparation method of isoniazide, comprising the following steps: carrying out an esterification reaction among isonicotinic acid, alcohol and an acylation reagent to generate isonicotinate, carrying out a reaction between isonicotinate and an ether reagent in an alcoholic solution of hydrogen chloride to generate isonicotinate hydrochloride, condensing with hydrazine hydrate to generate an isoniazide crude product, and refining to obtain the finished product. According to the method, by adding the step of reacting isonicotinate to generate isonicotinate hydrochloride, on one hand, the subsequent condensation hydrazinolysis reaction time can be greatly shortened and preparation efficiency is improved; on the other hand, isonicotinic acid which is not fully reacted, isonicotinamide introduced by an isonicotinic acid raw material and potential 2-picolinic acid impurities can be removed, the purity of the finally obtained isoniazide is as high as 99.99%, and thesingle impurity content is smaller than 0.10%. The preparation method is mild in reaction condition, easy to operate, good in product quality, high in yield, high in preparation speed and suitable forindustrial production.
Owner:沈阳双鼎制药有限公司
Primer, probe and kit for detecting gene mutation sites related to drug resistance of mycobacterium tuberculosis and treatment drugs
InactiveCN113388690AThe detection process is fastEasy to detectMicrobiological testing/measurementDNA/RNA fragmentationGenes mutationAntituberculous drug
The invention relates to the technical field of medical detection, in particular to a primer, a probe and a kit for detecting mycobacterium tuberculosis and drug-resistant gene mutation sites. The detection primer and the probe comprise DNA (deoxyribonucleic acid) sequences as shown in SEQ ID NO: 1 to SEQ ID NO: 68. The drug resistance type of mycobacterium tuberculosis is detected based on a Luminex liquid chip, whether the mycobacterium tuberculosis exists in a sample or not can be detected, four first-line antituberculous drugs including isoniazide, rifampicin, pyrazinamide and streptomycin and a second-line antituberculous drug, namely fluoroquinolone, are covered, mutation sites are comprehensive, the detection speed is high, only 3.5 hours are needed to complete one-time detection, and as many as 96 samples can be detected at the same time at one time. Therefore, the detection process is simplified, the experimental period is shortened, the detection accuracy and sensitivity are improved, and clinical application is facilitated.
Owner:HAINAN MEDICAL COLLEGE
Isoniazid lipid derivate and compositions thereof
The invention discloses an isoniazid lipid derivate and compositions thereof. The isoniazid lipid derivate is characterized by having an INH-THTT-R structure, wherein INH is isoniazid, THTT is thiadiazine thione, and R is a long fatly chain having 6-20 carbon atoms. The isoniazid lipid derivate can be used for preparing tablets, capsules, injections, aerosols, dry powder inhalers and strong dispersant excipients, wherein the strong dispersant excipients comprise selected liposome, nonionic surface active agent vesicles, particles, nanometer grains, emulsions, self-emulsification systems, micelles and nanometer gel.
Owner:INST OF RADIATION MEDICINE ACAD OF MILITARY MEDICAL SCI OF THE PLA
Method for adopting isoniazide for preparing nanometer silver sol at room temperature
The invention discloses a method for adopting isoniazide for preparing nanometer silver sol at room temperature. Silver nitrate is used as a silver source, the isoniazide is adopted to serve as a complexing agent and a reducing agent, a water-soluble surface active agent is used as a protecting agent, the nanometer silver sol with the average grain diameter within 100 nm is prepared at the normal temperature, and particle granularity does not change obviously after the silver sol is placed at the room temperature for 30 days. According to the method, operation at normal temperature and normal pressure can be achieved, the environment is protected, the technological process is simple, and the nanometer silver sol which is wide in silver concentration range, controllable in particle diameter and good in dispersibility is obtained. The adopted isoniazide can have the complexing effect while serving as the reducing agent, the grain size of particles can be effectively controlled, and the stability of the sol is improved.
Owner:TIANJIN UNIV
Cyanoguanidine derivative, preparation method thereof and anti-tubercle bacillus medicine
PendingCN114315685AExcellent anti-tuberculosis effectLower resistanceAntibacterial agentsOrganic active ingredientsGuanidine derivativesMycolic acid
The invention belongs to the technical field of materials, and particularly relates to a cyanoguanidine derivative, a preparation method thereof and an anti-tubercle bacillus medicine. Wherein the general formula of the chemical structure of the cyano guanidine derivative is shown as a formula I, R1 is selected from one of aryl and heteroaryl, and R2 is selected from one of alkyl, aryl and heteroaryl; r3-R7 are respectively and independently selected from one of hydrogen, halogen, cyano, alkyl, aryl and heteroaryl. The cyanoguanidine derivative provided by the invention has dual mechanisms for inhibiting mycobacterium tuberculosis in a normal growth state, can inhibit the synthesis of mycobacterium tuberculosis protein, and also can inhibit the synthesis of cell wall mycolic acid. The cyanguanidine derivative has an excellent anti-tubercle bacillus effect which is even superior to that of commercially available isoniazide, and the cyanguanidine derivative is not easy to generate drug resistance to most tubercle bacillus.
Owner:SHENZHEN BAY LAB +1
Isoniazide hapten derivative, isoniazide artificial antigen, hybridoma cell strain, isoniazide monoclonal antibody and application
The invention provides an isoniazide hapten derivative, an isoniazide artificial antigen, a hybridoma cell strain, an isoniazide monoclonal antibody and application, and relates to the technical field of drug monitoring. The isoniazide hapten derivative provided by the invention can realize specific recognition of isoniazide, and the isoniazide antigen obtained by coupling protein by taking-CH2-CH2-CH2-COOH as a coupling arm can accurately obtain an antibody with specific binding capacity to isoniazide. The hybridoma cell strain provided by the invention can generate a high-sensitivity isoniazide monoclonal antibody for equivalent identification of isoniazide, and the antibody has no obvious cross reaction with main metabolites (acetyl isoniazide, isonicotinic acid, nicotinamide and aminosalicylic acid) of isoniazide, and can realize high-sensitivity equivalent identification of isoniazide. Therefore, the compound can be applied to preparation of detection reagents (an isoniazide homogeneous enzyme immunoassay reagent and an isoniazide magnetic particle chemiluminescence detection reagent) for detecting isoniazide, and the clinical detection requirements of isoniazide are met.
Owner:BEIJING DIAGREAT BIOTECH CO LTD
Benzothiazinone derivative, preparation method thereof and application of benzothiazinone derivative as antituberculosis drug
ActiveCN112409294AGood antibacterial effectWork around the bug with high cLogP valuesAntibacterial agentsOrganic active ingredientsBenzeneAntituberculosis drug
The invention discloses a benzothiazinone derivative as well as a preparation method and application thereof as an antituberculosis drug, and particularly relates to a novel compound with a benzothiazinone skeleton, and the compound has an inhibition effect on tubercle bacillus, particularly tubercle bacillus with clinical drug resistance. The benzothiazinone skeleton benzene ring is creatively changed, especially the substituent group is creatively changed to obtain a series of compounds, and an unexpected technical effect is achieved; the compound has an excellent tubercle bacillus inhibition effect, the reported compound activity has great advantages compared with the existing clinical first-line drug isoniazide (MIC 0.5[mu]M), and more importantly, compared with the benzothiazinone antitubercular drug pBTZ 169 in the existing research stage, the compound has a lower cLogP value and better druggability.
Owner:SUZHOU UNIV
Bacterium preservation culture medium and preparation method thereof
PendingCN113684152AGuaranteed survivalGrowth inhibitionBacteriaMicroorganism preservationBiotechnologyWarfarin Sodium
The invention relates to the technical field of strain preservation, in particular to a bacterium preservation culture medium and a preparation method thereof. The culture medium comprises the following components: based on 1000ml of the culture medium, 8 to 13 g of beef extract, 2 to 6 g of peptone, 0.2 to 0.5 g of cholate, 0.2 to 0.3 g of aluminum potassium sulfate, 2 to 5 g of malt extract powder, 3 to 7 g of yeast extract powder, 0.3 to 0.6 g of sodium chloride, 5 to 10 g of agar, 0.2 to 0.5 g of soluble ferric salt, 0.01 to 0.05 g of a calcium supplement, 0.02 to 0.05 g of D-cycloserine, 0.001 to 0.004 g of argentein, 0.01 to 0.02 g of vitamin K1, 0.1 to 0.3 g of casein phosphopeptides, 3 to 5 g of glucose, 1 to 2.5 g of lanthanum nitrate, 3 to 5 g of erythritol, 18 to 23 ml of glycerin, 0.1 to 0.2 mg of warfarin sodium tablets, 8 to 12 mg of isoniazide, and 75 to 85 ml of sterile defiberized sheep blood. The formula of the culture medium is rich and comprehensive in nutrient substances, the survival of the strain can be ensured, and the culture medium can still ensure the effectiveness of the strain even if the preservation environment temperature is greatly changed.
Owner:贵州安康医学检验中心有限公司
Preparation and application of novel chromatic fiber IAHF-PAR
ActiveCN107233868AExtensive sources of raw materialsLow priceOther chemical processesMaterial analysis by observing effect on chemical indicatorRefluxSynthesis methods
The invention discloses a synthesis method of a chelate fiber IAHF and a synthesis method and application of an IAHF-PAR chromatic fiber. The synthesis method of the chelate fiber IAHF comprises the following step: the polyacrylonitrile chelate fiber IAHF is synthesized by taking a polyacrylonitrile fiber as a matrix and isoniazide as a ligand under the condition of a nitrogen protective atmosphere. The synthesis method of the chromatic fiber IAHF-PAR comprises the following steps: putting the polyacrylonitrile chelate fiber IAHF and PAR into a formaldehyde water solution, performing stirring and heating reflux under the condition of a nitrogen protective atmosphere for 2-6 hours, and ending the stirring reaction at 70 DEG C; and performing flushing with warm water, and performing drying to a constant weight to obtain the chromatic fiber IAHF-PAR. The polyacrylonitrile chelate fiber IAHF synthesized by the invention has a heavy metal ion adsorption capability, wherein the selective adsorbability on mercury ions is high, the adsorption capacity is high, and the adsorption rate is high; and the chromatic fiber IAHF-PAR is applicable to detection under different environments, and can be prepared into chromatic materials of different forms.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Cut flower preservative and application thereof in lily cut flower preservation
InactiveCN112120015AImprove liquidityNo change in preservation propertiesDead plant preservationChlorophyll degradationChlorophyllin
The invention discloses a cut flower preservative. The preservative comprises the following components in parts by weight: 100-400 parts of inorganic salt, 10-20 parts of a bacteriostatic agent, 10-20parts of isoniazid, 500-1000 parts of sulfamic acid, 100-200 parts of a nutritional supplement, 0-200 parts of anhydrous magnesium sulfate and 10000 parts of a nutritional substrate. The preservativecan effectively delay the occurrence time of the maximum flower diameter of cut flowers through mutual cooperation of the components, prolong the ornamental time of the cut flowers, relieve chlorophyll degradation of the leaves of thecut flowers and prolong the vase life of the cut flowers. On the other hand, the invention discloses a cut flower preservative bottling liquid which is obtained by mixing and dissolving the cut flower preservative and water according to the mass-volume ratio of 1: 50-200kg / L.
Owner:四川润尔科技有限公司
Method for determining content of 4-cyanopyridine and impurities of 4-cyanopyridine in isoniazide starting material
InactiveCN108226323AEfficient separationDo not interfere with detectionComponent separationProduct gasVaporization
The invention belongs to the field of analytical chemistry, and particularly relates to a method for determining the content of 4-cyanopyridine and impurities of 4-cyanopyridine in an isoniazide starting material. The measuring method is a gas chromatographic method, and comprises the following specific steps: vaporizing a sample in a vaporization chamber, and introducing the vaporized sample intoa chromatographic column through flowing gas; separating the 4-cyanopyridine SM1 and the impurities of 4-cyanopyridine by means of programmed heating; calculating the content of the impurities by using a principal component self-contrasted method with a correction factor. At present, no reports of simultaneous separation and determination of a plurality of impurities in the SM1 exist. The methodfor measuring the content of the plurality of impurities in the SM1 provided by the invention is simple and feasible, and has high sensitivity and high specificity, so that established quality controlitems of SM1 relevant substances can improve the quality controllability and safety of a product when being applied to control of relevant substances of the product.
Owner:CHONGQING HUABANGSHENGKAI PHARM
A kind of preparation method containing rifampicin compound preparation
ActiveCN105769868BReduce mutual contactQuality assuranceAntibacterial agentsOrganic active ingredientsCaplet Dosage FormPharmaceutical formulation
The invention belongs to the field of medicinal preparations, and particularly relates to a preparing method for a compound preparation containing rifampicin.The preparing method includes the steps that rifampicin, other active components and a hot melting film-forming material are prepared into solid coated particles, the solid coated particles are further prepared in various dosage forms, and for example, the solid coated particles are loaded into capsules to prepare a capsule preparation or tabletted and coated to prepare film-coated tablets.According to the purpose, all the active components in the prescription are isolated, it is avoided that medicine makes close contact in a preparation environment to be degraded, and stability of the compound rifampicin preparation is ensured.The preparing method is simple and stable in process and high in reproducibility, polymers generated by rifampicin and other active components like isoniazide are greatly reduced, and the product curative effect is ensured; it is indicated by analyzing test results of preparation stability that the preparation is stable in quality.
Owner:CHONGQING HUAPONT PHARMA
Isoniazid-indole heptamethine coupling compound and its preparation method and application
ActiveCN111072635BEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryTumor targetTumor targeting
The invention belongs to the technical field of medicine, and relates to a class of isoniazid-indole heptamethine coupling compound and its preparation and application. The invention adopts the combination principle to combine isoniazid with excellent in vivo fluorescence imaging characteristics and tumor targeting Functional indole heptamethine dyes are connected by hydrazone bonds to design a novel tumor-targeting isoniazid-indole heptamethine-isoniazid coupling compound, and its structural formula is shown in formula (I)-(III) , where R 1 , R 2 , R 3 , R 4 , m, n as described in the claims and specification. In vitro tumor cell growth inhibition test proves that the compound has good inhibitory effect on prostate tumor cells. The monoamine oxidase inhibitory activity test test shows that this type of compound has a moderate inhibitory effect on monoamine oxidase A, which is obviously stronger than isoniazid.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of isoniazid
ActiveCN112724081AHigh yieldLow impurity contentOrganic chemistryPhysical chemistryReaction temperature
The invention discloses a preparation method of isoniazide, which adopts temperature control and proper increase of hydrazine hydrate feeding proportion for reaction, solves the problems of narrow temperature range and difficult operability and controllability of the traditional condensation reaction, widens the reaction temperature to 129-138 DEG C, and has high yield of isoniazide. Meanwhile, refining steps such as decoloration, washing and recrystallization are combined, so that the content of impurities in isoniazid is remarkably reduced, and the requirements of related evaluation work of crude drugs are met. The reaction temperature range is wide, the yield and purity of isoniazide are improved, and a larger selection range is provided for operability and controllability of industrial production of isoniazide.
Owner:GUANGZHOU BAIYUNSHAN MINGXING PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com