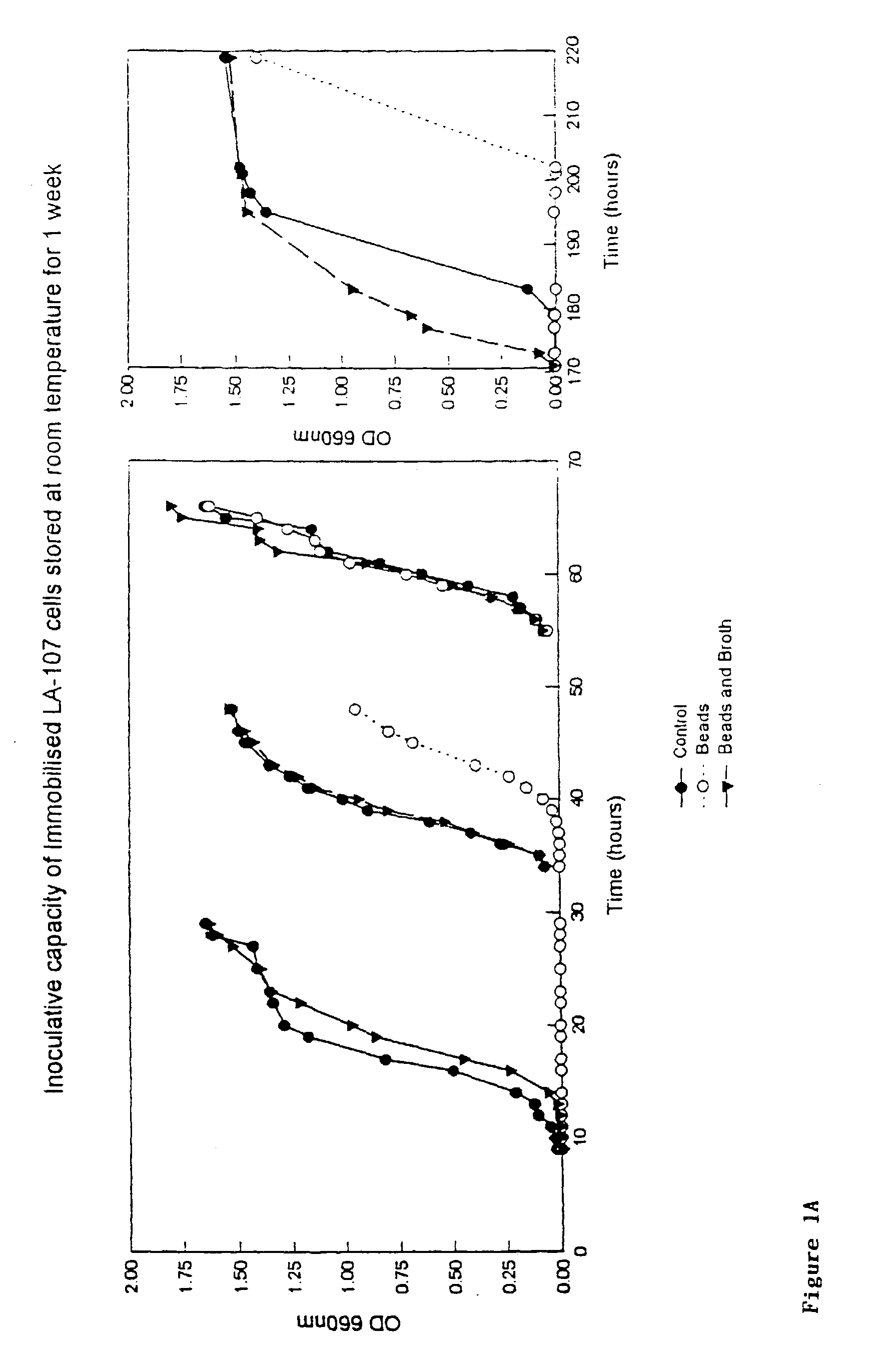
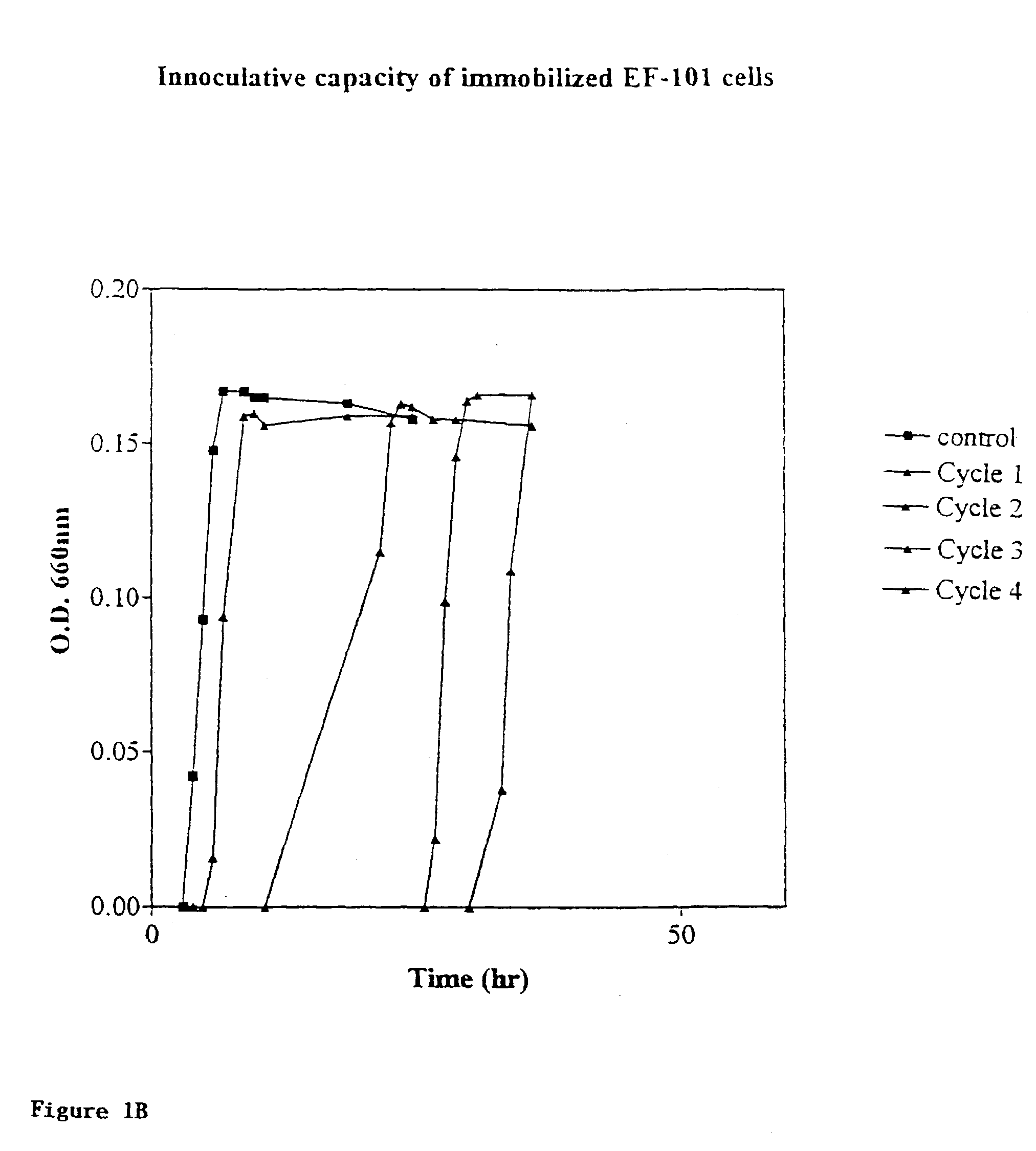
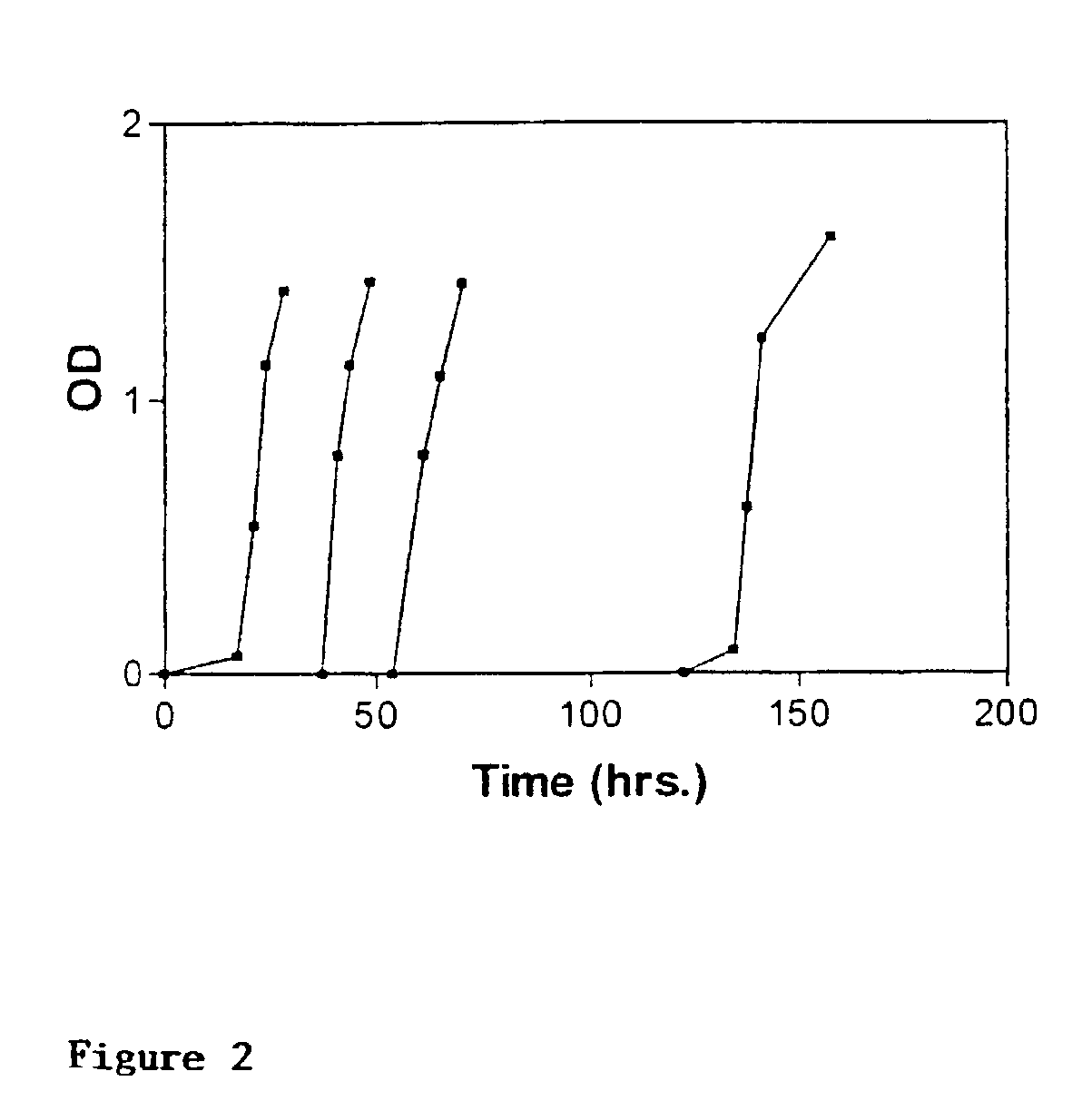
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
2049results about "Microorganism preservation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
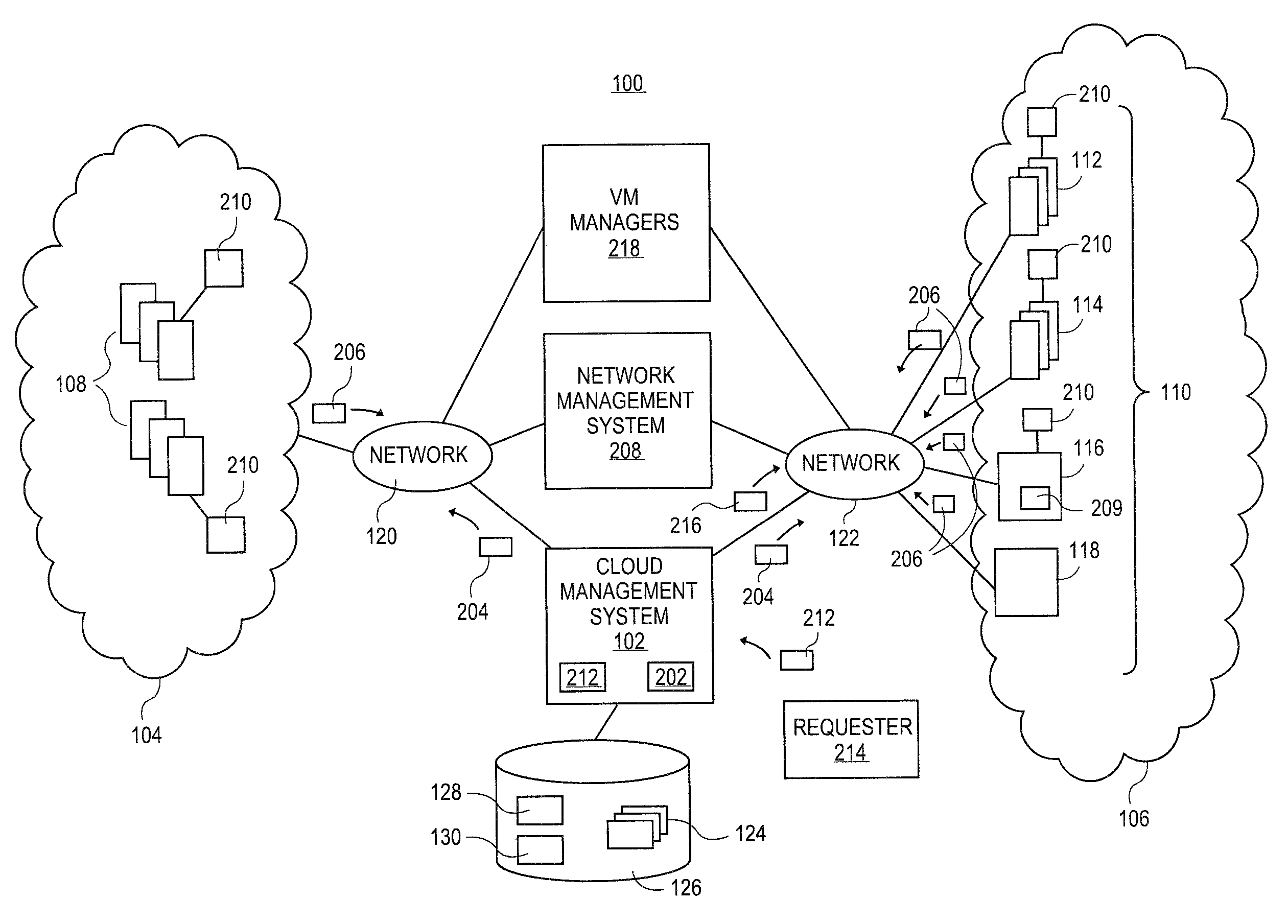
Methods and systems for flexible cloud management with power management support
A cloud management system can determine if the operational state of the virtual machines and / or the computing systems needs to be altered in order to instantiate virtual machines. If the operational state of the computing systems needs to be altered, the cloud management system retrieves an identification of the power management systems supporting the computing systems. The cloud management system can utilize the identification of the power management systems to instruct the power management systems to alter the power state of the computing system in order to instantiate the virtual machines.
Owner:RED HAT
Microbial pesticidal composition
Owner:KUMIAI CHEM IND CO LTD
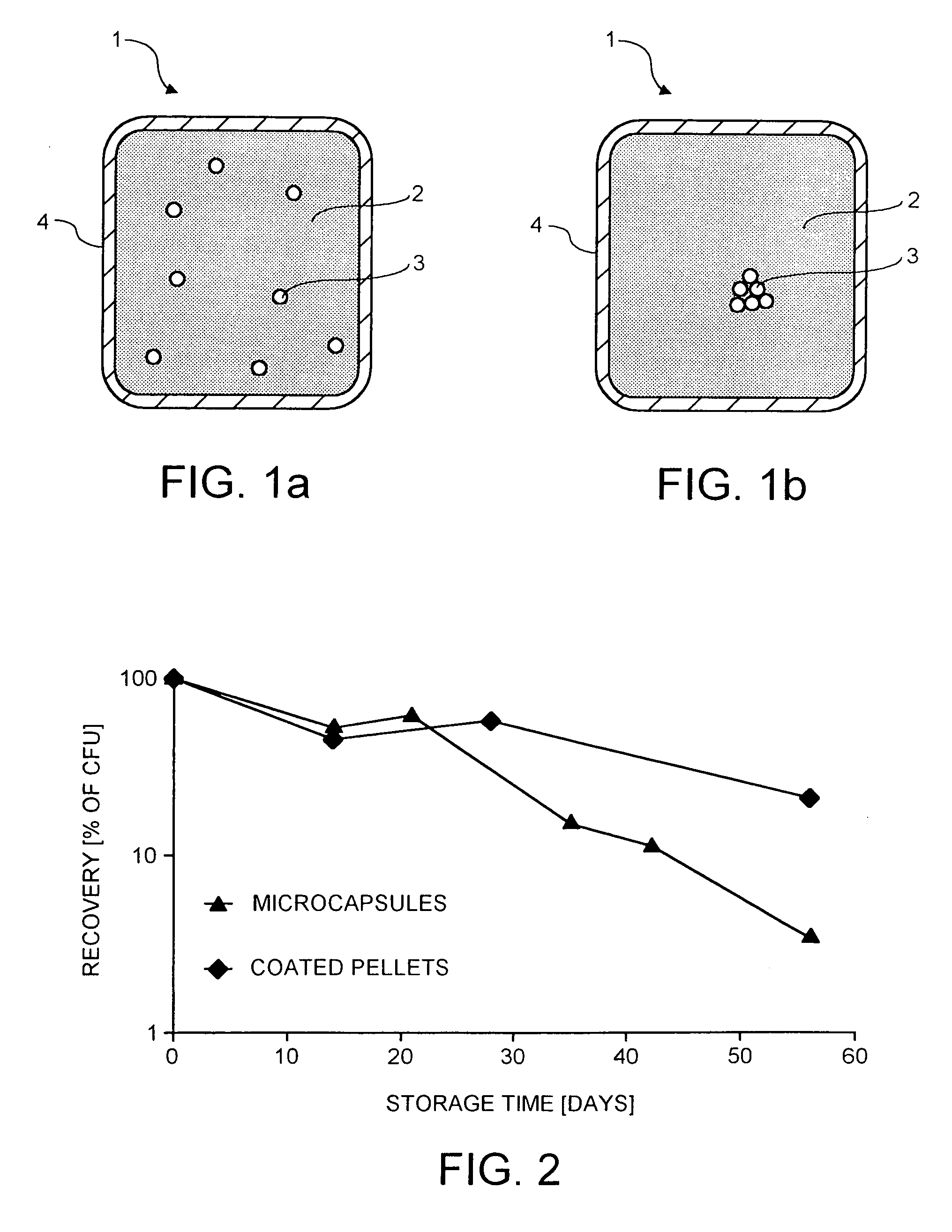
Probiotic delivery system
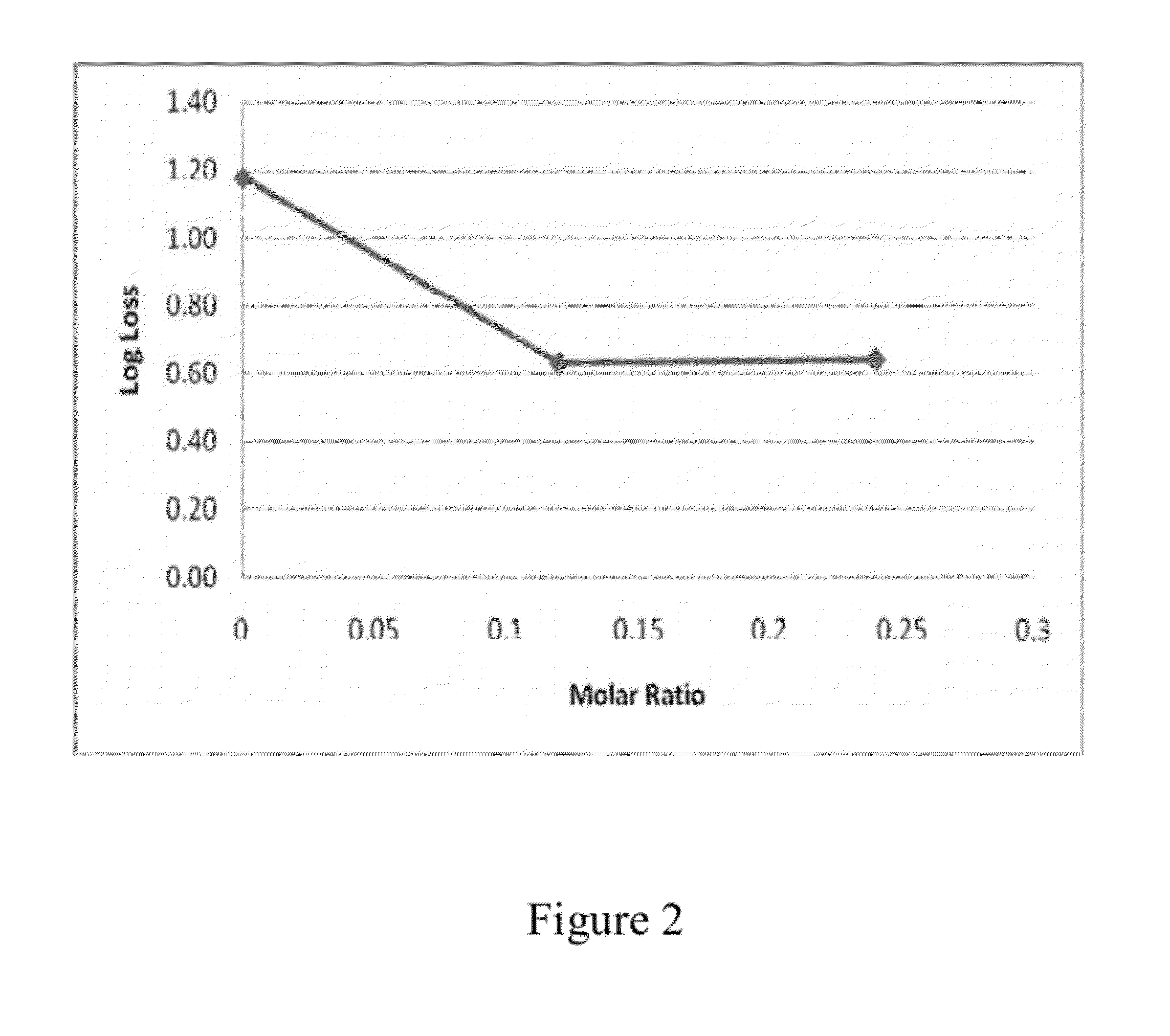
InactiveUS20050153018A1Improve stabilityProcessing is easy and straightforwardFood ingredient as barrier agentMicroorganism preservationMicroorganismBiotechnology
The present invention relates to a probiotic delivery system that is preferably added to a food product. In particular, the invention shows that compacted pellets having a volume of at least 0.02 cm3, that comprise, besides viable micro-organisms, arbitrary or eligible components, such as fillers, binder, plasticizer, other functional ingredients and a coating may be added to semi-moist, moist or semi-dry products. The micro-organisms remain viable for a longer time than commercially obtainable preparations of probiotics.
Owner:NESTEC SA
Integration of sample storage and sample management for life science
InactiveUS20060099567A1Reduce degradationImmobilised enzymesMaterial nanotechnologyEnzymeDry storage
Compositions and methods are disclosed for automated storing, tracking, retrieving and analyzing biological samples, including dry storage at ambient temperatures of nucleic acids, proteins (including enzymes), and cells using a dissolvable dry storage matrix that permits recovery of biologically active materials. RFID-tagged biological sample storage devices featuring dissolvable or dissociable matrices are described for use as supports of biological samples, which matrices can be dried and subsequently rehydrated for sample recovery. Also disclosed are computer-implemented systems and methods for managing sample data.
Owner:BIOMATRICA INC
Method for processing and preserving collagen-based tissues for transplantation
InactiveUS20060210960A1Minimize surface tension damageAugment selective preservationSsRNA viruses positive-senseViral antigen ingredientsOrganismBiology
A method for processing and preserving an acellular collagen-based tissue matrix for transplantation is disclosed. The method includes the steps of processing biological tissues with a stabilizing solution to reduce procurement damage, treatment with a processing solution to remove cells, treatment with a cryoprotectant solution followed by freezing, drying, storage and rehydration under conditions that preclude functionally significant damage and reconstitution with viable cells.
Owner:LIFECELL
Preservation by Vaporization
ActiveUS20080229609A1Easy to controlImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsIndustrial scaleVacuum chamber
Significant research is being done to develop and improve delivery mechanisms for biopharmaceuticals and vaccines, including pulmonary (inhalation), nasal, transdermal, and oral alternatives. Market projections indicate that the delivery of proteins and vaccines by inhalation and oral formulation has become and will continue to be increasingly important. These delivery mechanisms, to be effective, will require better stabilization of the biologicals so that they can maintain potency and effectiveness at ambient temperatures for extended periods of time. The novel Preservation by Vaporization (PBV) Technology described herein provides cost-effective and efficient industrial scale stabilization of proteins, viruses, bacteria, and other sensitive biologicals, thereby allowing a production of products that are not possible to be produced by existing methods. The suggested new PBV process comprises primary drying under vacuum from a partially frozen state (i.e. slush) at near subzero temperatures followed by stability drying at elevated temperatures (i.e., above 40 degrees Celsius). The new suggested method can be performed aseptically in unit doze format (in vials) and / or in bulk format (in trays, bags, or other containers). The drying can be performed as a continuous load process in a manifold vacuum dryer comprising a plurality (e.g., 30) of vacuum chambers attached to a condenser during the drying.
Owner:UNIVERSAL STABILIZATION TECH INC
Methods and compositions for extending the life span and increasing the stress resistance of cells and organisms
ActiveUS20050267023A1Increasing and extending life of cellStress resistantOrganic active ingredientsSenses disorderOrganismNicotinamide
The invention provides methods and compositions for modulating the life span of eukaryotic and prokaryotic cells and for protecting cells against certain stresses, e.g., heatshock. One method comprises modulating the flux of the NAD+ salvage pathway in the cell, e.g., by modulating the level or activity of one or more proteins selected from the group consisting of NPT1, PNC1, NMA1 and NMA2. Another method comprises modulating the level of nicotinamide in the cell.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Formulation of preservation mixtures containing sensitive biologicals to be stabilized for ambient temperature storage by drying
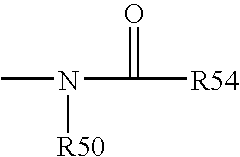
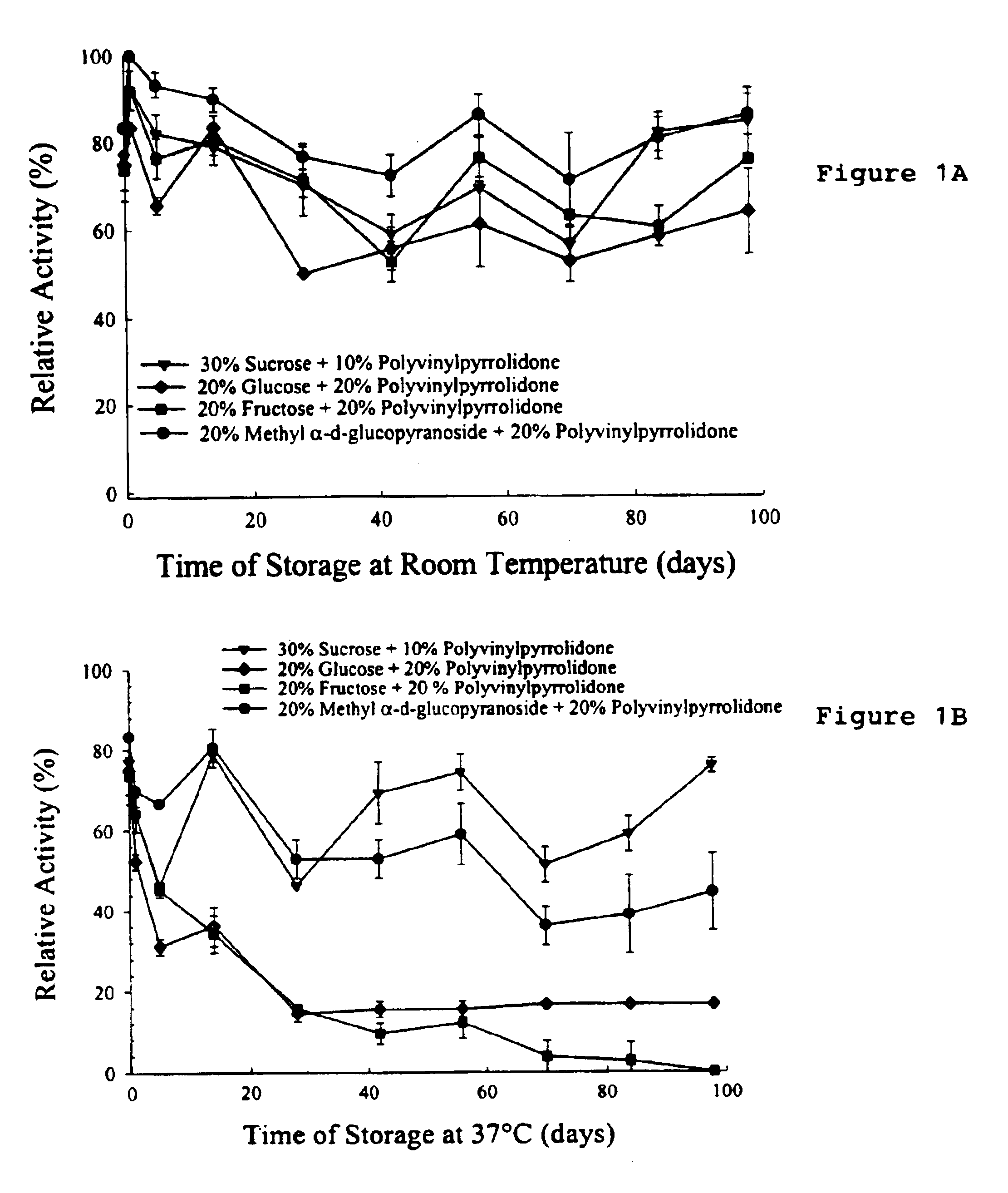
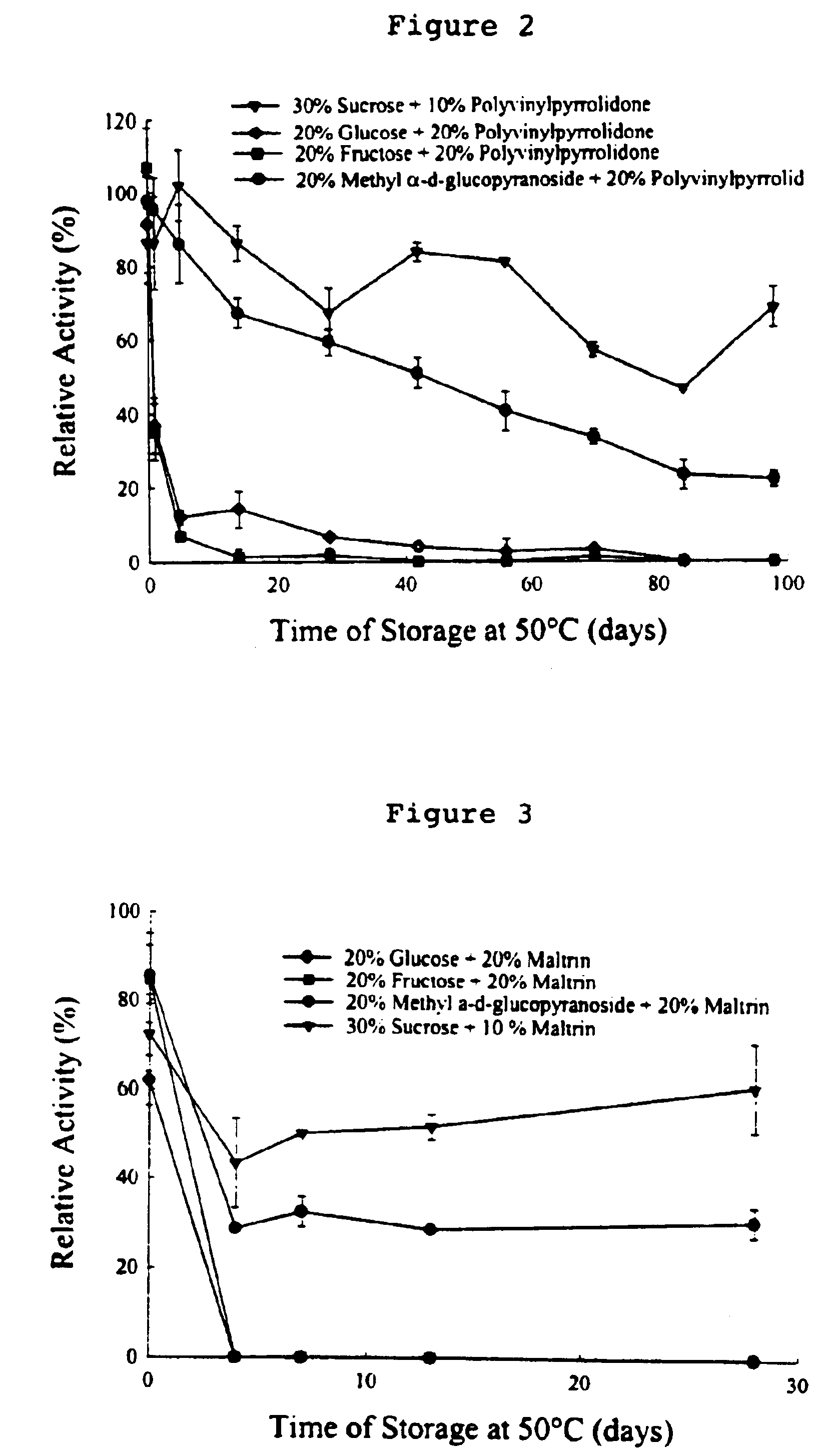
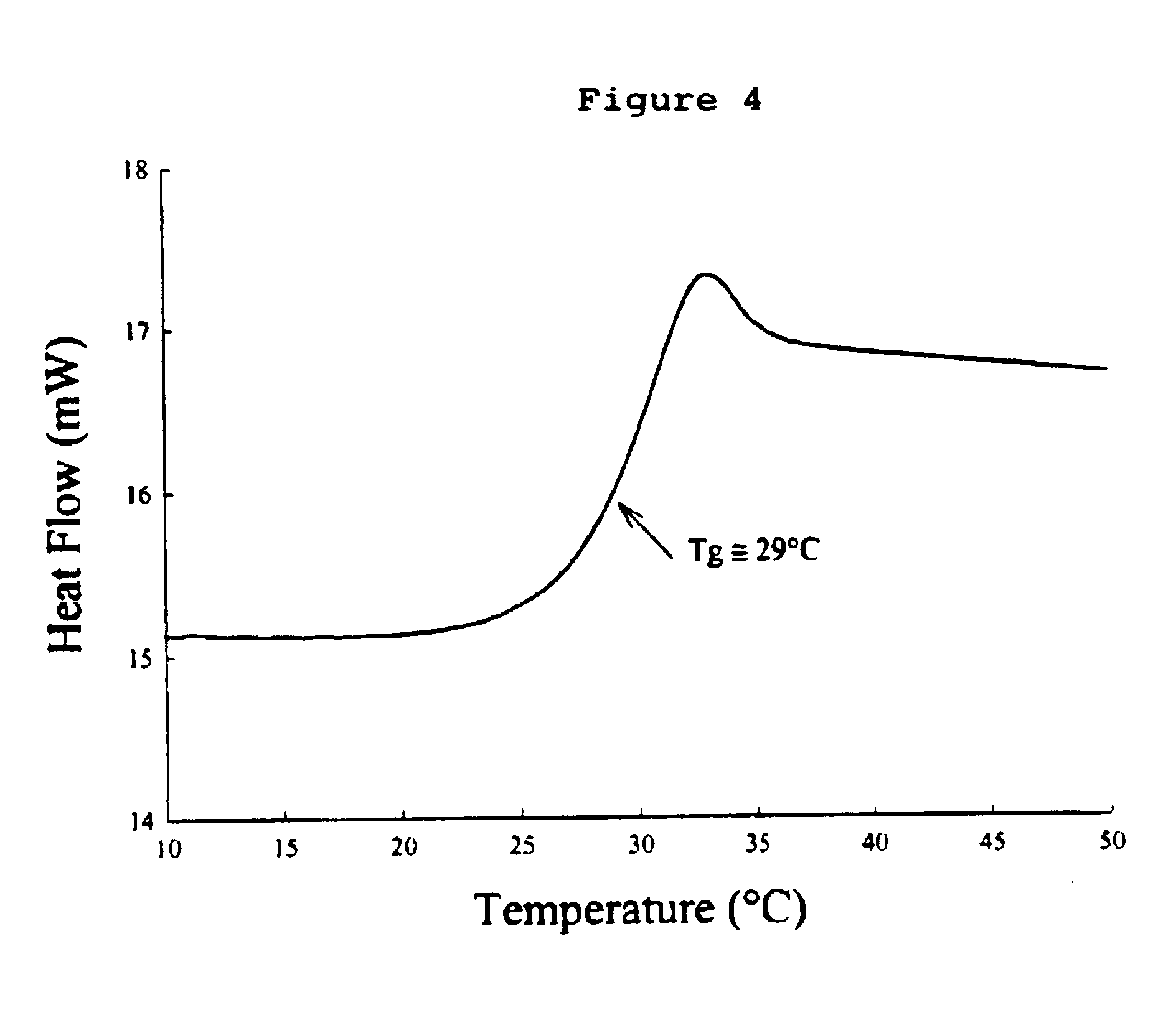
This invention relates to formulations and methods for preserving sensitive biologicals, viruses, bacteria and eukaryotic cells by drying. More particularly, the invention relates to preservation mixtures containing viruses or cells and protectants, including a combination of a methylated monosaccharide and a disaccharide, or oligosaccharide, wherein the mixtures are adapted to stabilize these during dehydration and subsequent storage at ambient and higher temperature.
Owner:AVANT IMMUNOTHERAPEUTICS
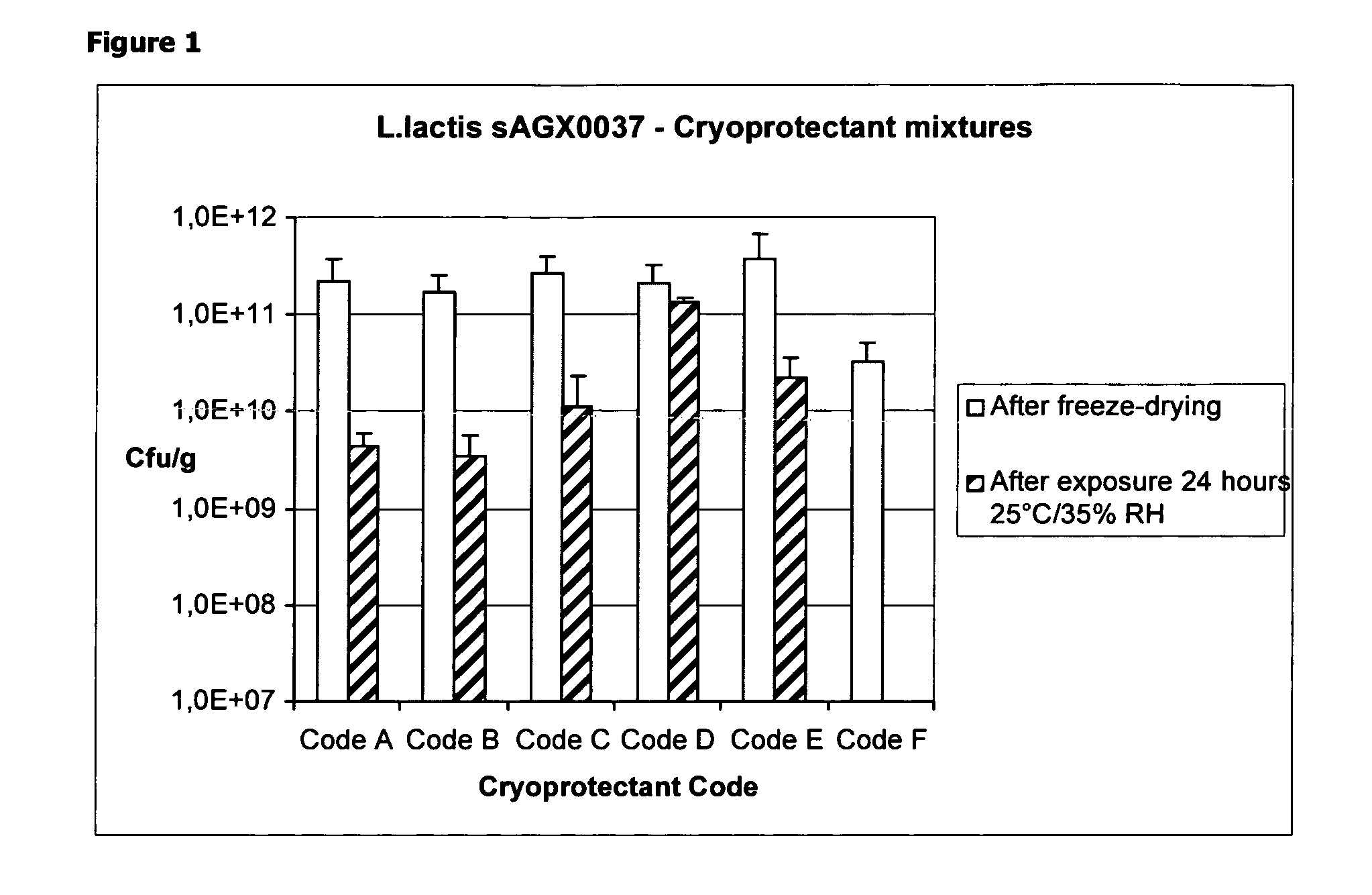
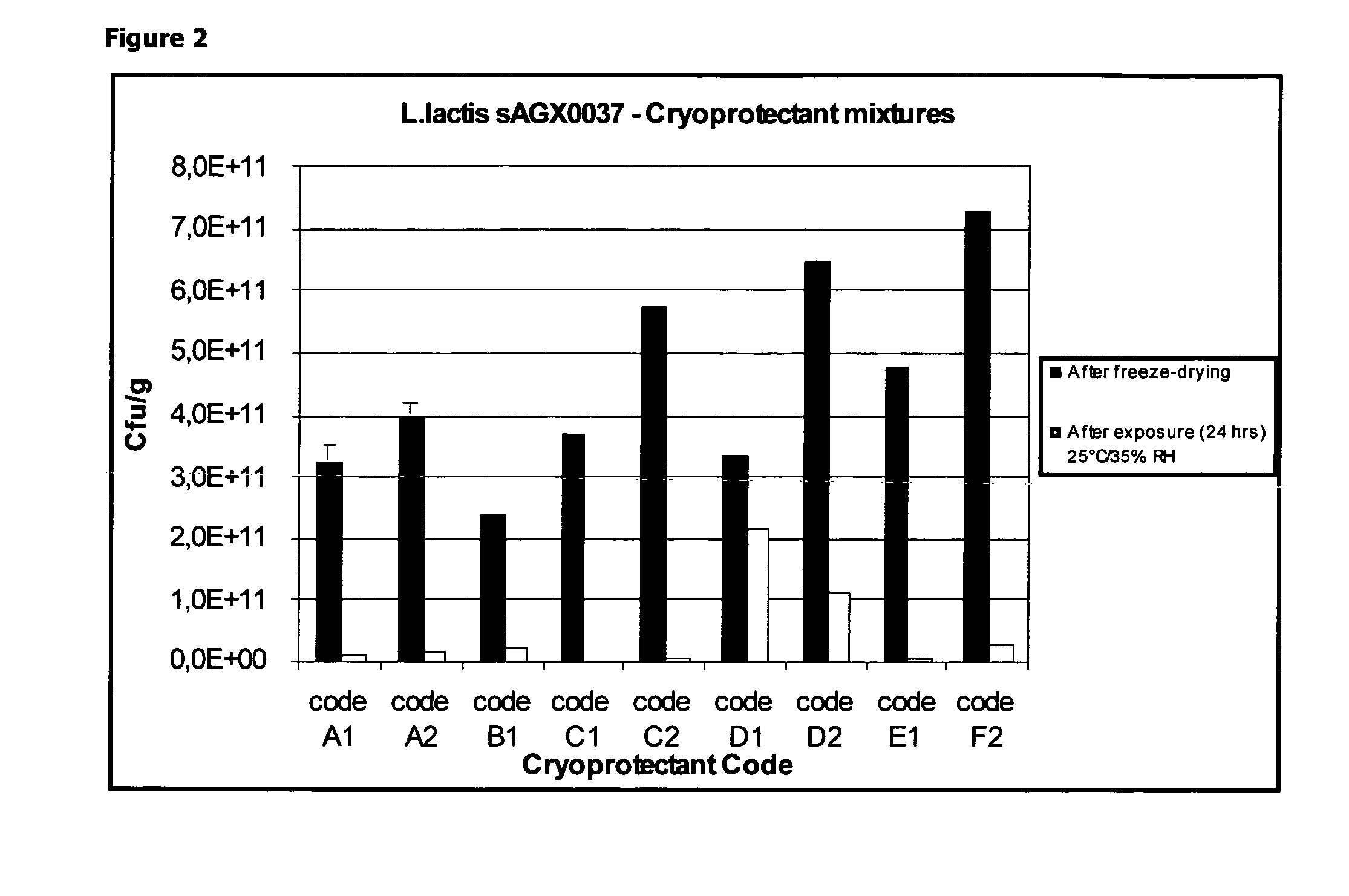
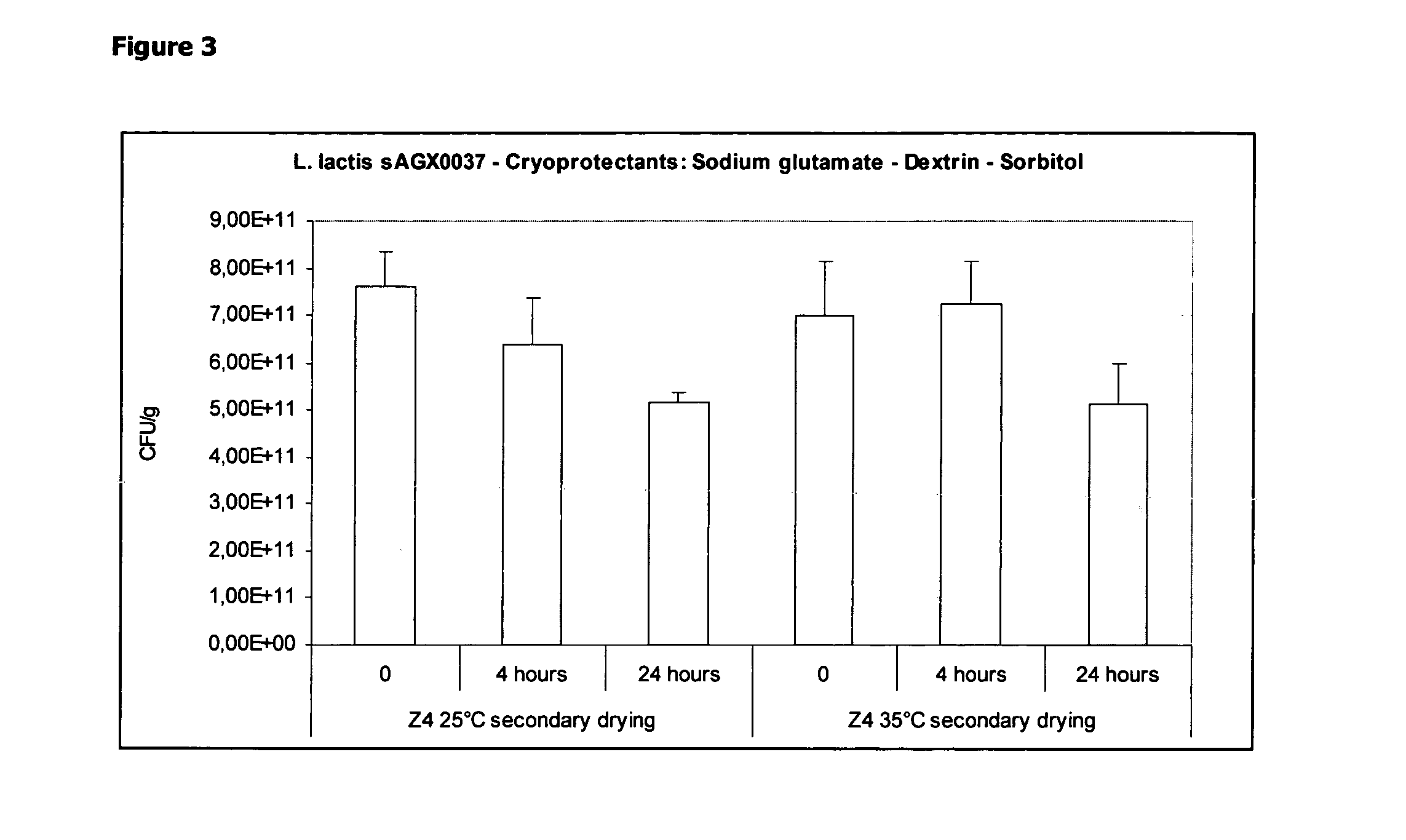
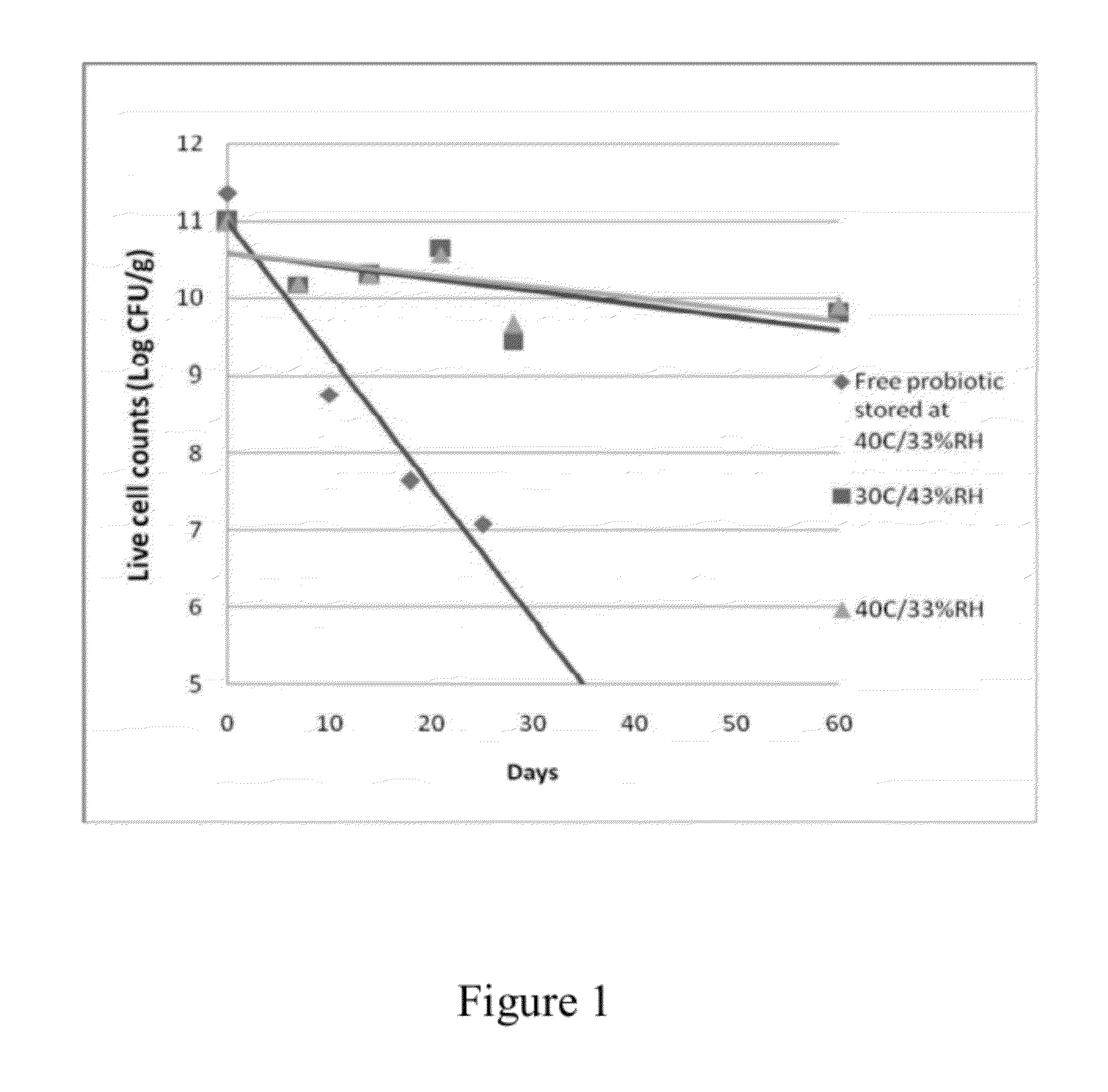
Cryoprotectants for freeze drying of lactic acid bacteria
ActiveUS20120039853A1Improve survivabilityImprove textureBiocideMilk preparationBacteroidesVaccination
The present invention comprises the discovery and development of an effective cryoprotectant composition, without containing skim milk or any other animal-derived compounds, to achieve long-term stability of freeze-dried lactic acid bacteria (LAB), at different temperatures, whereby the retention of viability of the freeze-dried LAB after 6 months of storage, preferably after 9 months of storage, more preferably after 12 months of storage is more than 50%. The invention is in the field of producing freeze dried bacteria, in particular Lactic acid bacteria. More in particular, the invention relates to the use of a novel combination of cryoprotectants for increasing the viability of bacteria after freeze drying, improving the texture of the lyofilized cake for easy grinding and improving the long term stability of the freeze dried bacteria at different temperature conditions. The invention further relates to such freeze dried bacteria for use in food industry or in human or animal health applications. More in particular, the invention relates to the increased viability and long-term storage of recombinant bacteria capable of expressing heterologous proteins or peptides and administered to humans or animals for therapeutic or vaccination purposes.
Owner:INTREXON ACTOBIOTICS NV
Preservation of bioactive materials by freeze dried foam
ActiveUS7135180B2Improve permeabilityReduce residual moistureSsRNA viruses negative-senseOrganic active ingredientsPreservativeFreeze-drying
This invention provides methods and compositions to preserve bioactive materials in a dried foam matrix. Methods provide non-boiling foam generation and penetration of preservative agents at temperatures near the phase transition temperature of the membranes.
Owner:MEDIMMUNE LLC
Comparative phenotype analysis of two or more microorganisms using a plurality of substrates within a multiwell testing device
InactiveUS6046021ABioreactor/fermenter combinationsBiological substance pretreatmentsEscherichia coliPlant cell
The present invention relates to growing and testing microorganisms in a multitest format which utilizes a gel forming matrix for the rapid screening of clinical and environmental cultures. The present invention is suited for the characterization of commonly encountered microorganisms (e.g., E. coli, S. aureus, etc.), as well as commercially and industrially important organisms from various and diverse environments (e.g., the present invention is particularly suited for the growth and characterization of the actinomycetes and fungi). The present invention is also particularly suited for comparative analysis of phenotypic differences between cell types, including strains of microorganisms that have been designated as the same genus and species, as well as other cell types (e.g., mammalian, insect, and plant cells).
Owner:BIOLOG
Stabilized compositions comprising a probiotic
InactiveUS20050100559A1Improve long-term stabilityBiocideBacterial antigen ingredientsBacterial compositionMicrobiology
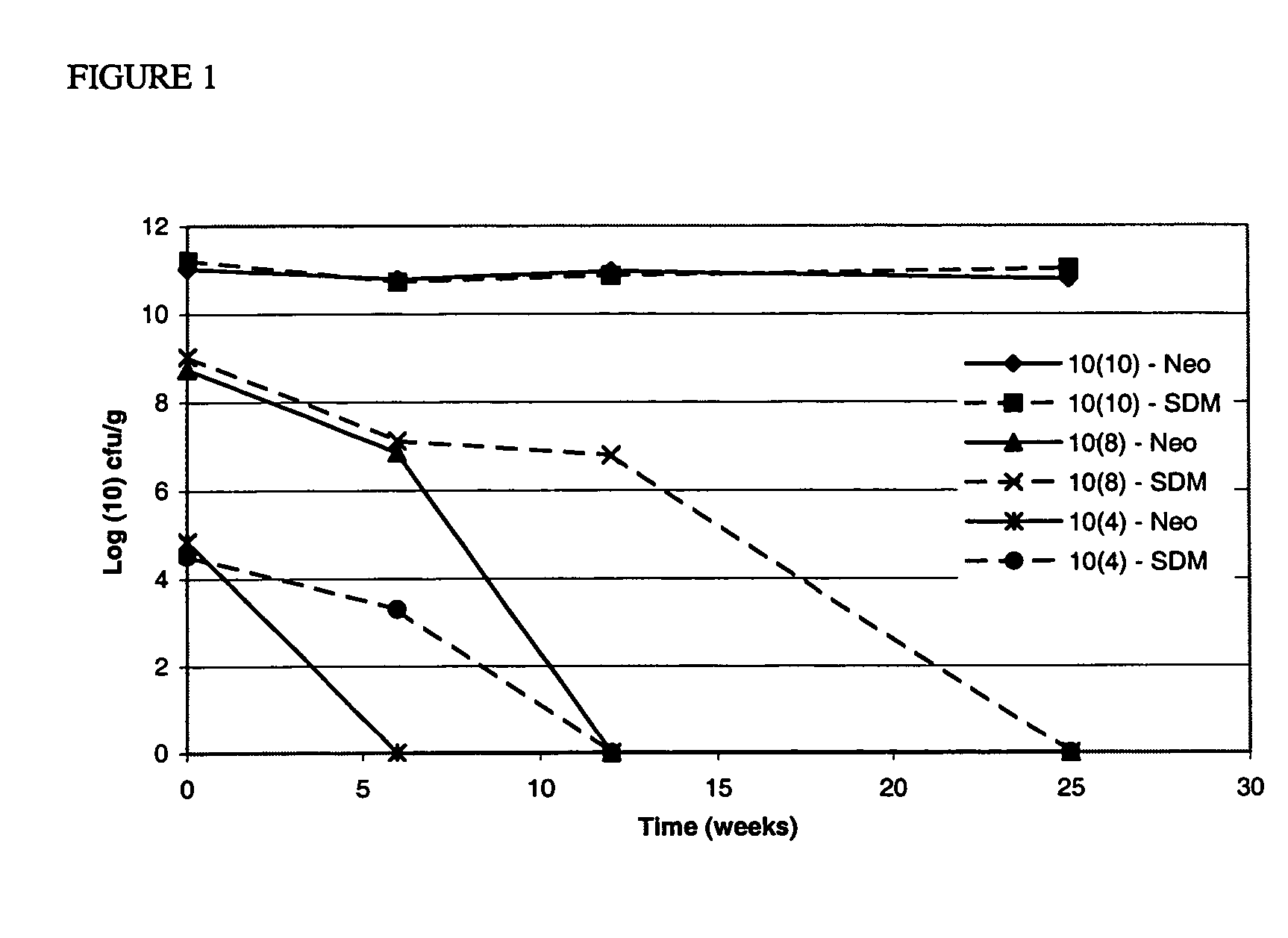
Stabilized dry bacterial compositions comprising greater than 10% dried bacterial concentrate having a concentration of bacteria of at least 1×108 cfu / g are provided. The compositions have improved stability. Also provided are packaged bacterial compositions, unit-dose compositions and methods of manufacturing the compositions provided herein.
Owner:THE PROCTER & GAMBLE COMPANY +1
Method of preparing biological materials and preparations produced using same
InactiveUS20050019417A1Simple methodDesired characteristicOrganic active ingredientsSenses disorderMicroparticleSolid particle
Methods for preparing products containing moisture-sensitive materials, including biological materials such as proteins, peptides or live cells, comprising at least the steps: (i) providing a coating liquid comprising at least one active, a sugar polymer and a water soluble / miscible solvent; (ii) providing a quantity of microparticles comprising at least water soluble gel forming solid particles; (iii) fluidizing said quantity of microparticles within a processing chamber of a of a suitable apparatus to form a fluidized bed of said microparticles; (iv) spraying said coating liquid onto said fluidized bed from beneath the fluidized bed to coat said microparticles therewith under saturated moisture conditions; and (vi) allowing coated microparticles to dry, are described. Also described are compositions and uses.
Owner:PFIZER INC
Bacterial leader sequences for increased expression
ActiveUS7618799B2Reduce environmental pollutionHigh expressionBacteriaAntibody mimetics/scaffoldsADAMTS ProteinsAmino acid
Compositions and methods for improving expression and / or secretion of protein or polypeptide of interest in a host cell are provided. Compositions comprising a coding sequence for a bacterial secretion signal peptide are provided. The coding sequences can be used in vector constructs or expression systems for transformation and expression of a protein or polypeptide of interest in a host cell. The compositions of the invention are useful for increasing accumulation of properly processed proteins in the periplasmic space of a host cell, or for increasing secretion of properly processed proteins from the host cell. In particular, isolated secretion signal peptide-encoding nucleic acid molecules are provided. Additionally, amino acid sequences corresponding to the nucleic acid molecules are encompassed. In particular, the present invention provides for isolated nucleic acid molecules comprising nucleotide sequences encoding the amino acid sequences shown in SEQ ID NO: 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24, and the nucleotide sequences set forth in SEQ ID NO: 1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 21, and 23, as well as variants and fragments thereof.
Owner:PELICAN TECH HLDG INC
Storage and delivery of micro-organisms
InactiveUS20030165472A1Improve abilitiesEasy to storeBiocideAnimal watering devicesBiotechnologyOrganism
We describe a method of delivering a micro-organism to an animal, the method comprising providing a formulation comprising a micro-organism suspended in or on a matrix; providing a feed stream for the animal; detaching micro-organisms from the matrix; and entraining detached micro-organisms into the feed stream. An apparatus for delivering a micro-organism to an animal is also described.
Owner:UUTECH
Encapsulation of bacteria and viruses in electrospun fibers
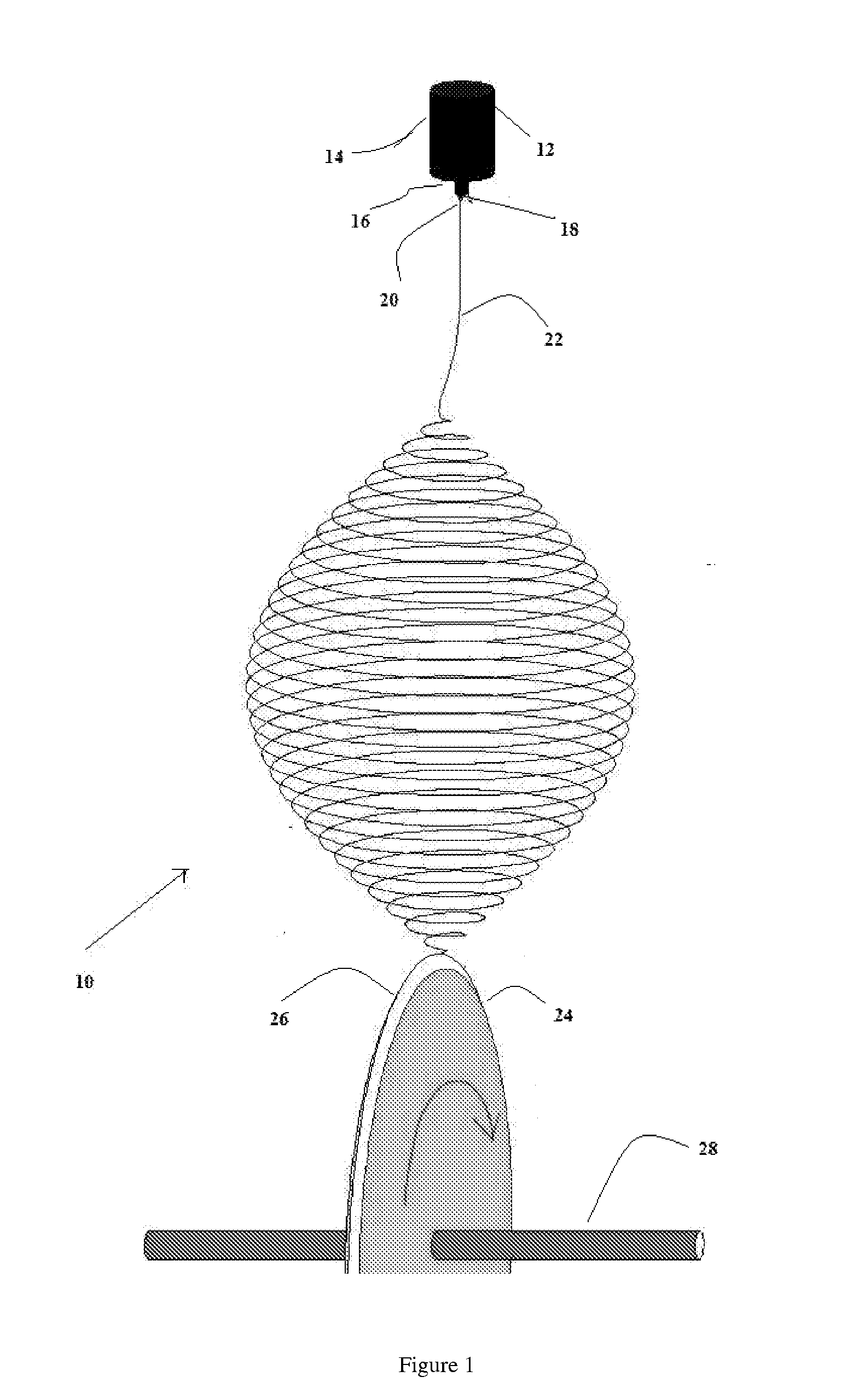
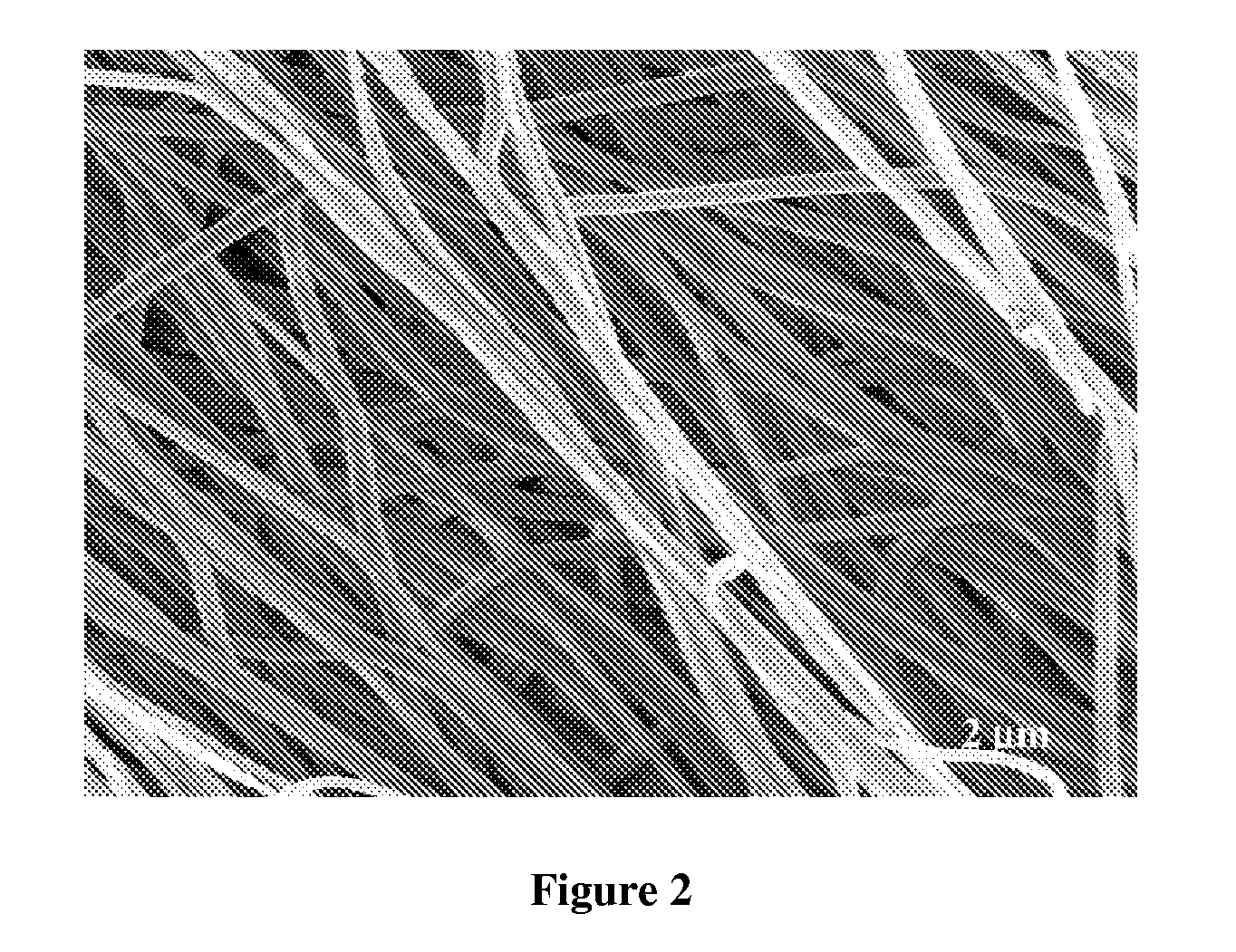
A method of preserving organisms in viable form, the method comprising: suspending organisms in a solution of electrospinnable polymer; drawing droplets of said solution through a spinneret; applying an electrostatic field to said droplets under electrospinning conditions; so as to form fibers having a diameter no greater than about 5 μm within which distinct organisms are encapsulated in viable form.
Owner:TECHNION RES & DEV FOUND LTD
Acellular dermal matrix and method of use thereof for grafting
InactiveUS8067149B2Reduce harmSsRNA viruses positive-senseViral antigen ingredientsMedicineAcellular Dermis
A method for processing and preserving an acellular collagen-based tissue matrix for transplantation is disclosed. The method includes the steps of processing biological tissues with a stabilizing solution to reduce procurement damage, treatment with a processing solution to remove cells, treatment with a cryoprotectant solution followed by freezing, drying, storage and rehydration under conditions that preclude functionally significant damage and reconstitution with viable cells.
Owner:LIFECELL
Preservation solution
InactiveUS6794124B2Drawback can be obviatedBioreactor/fermenter combinationsBiological substance pretreatmentsColloidBiology
An improved preservation solution is described, which is intended for the preservation of organs and tissues, or parts thereof, from humans and animals. The improved preservation solution contains calcium, at least one colloidosmotically active substance, and nitroglycerin. Also described is a method for preserving organs and tissues, or parts thereof, from humans and animals in the improved preservation solution.
Owner:XENODEVICE +1
Process and composition for the manufacture of a microbial-based product
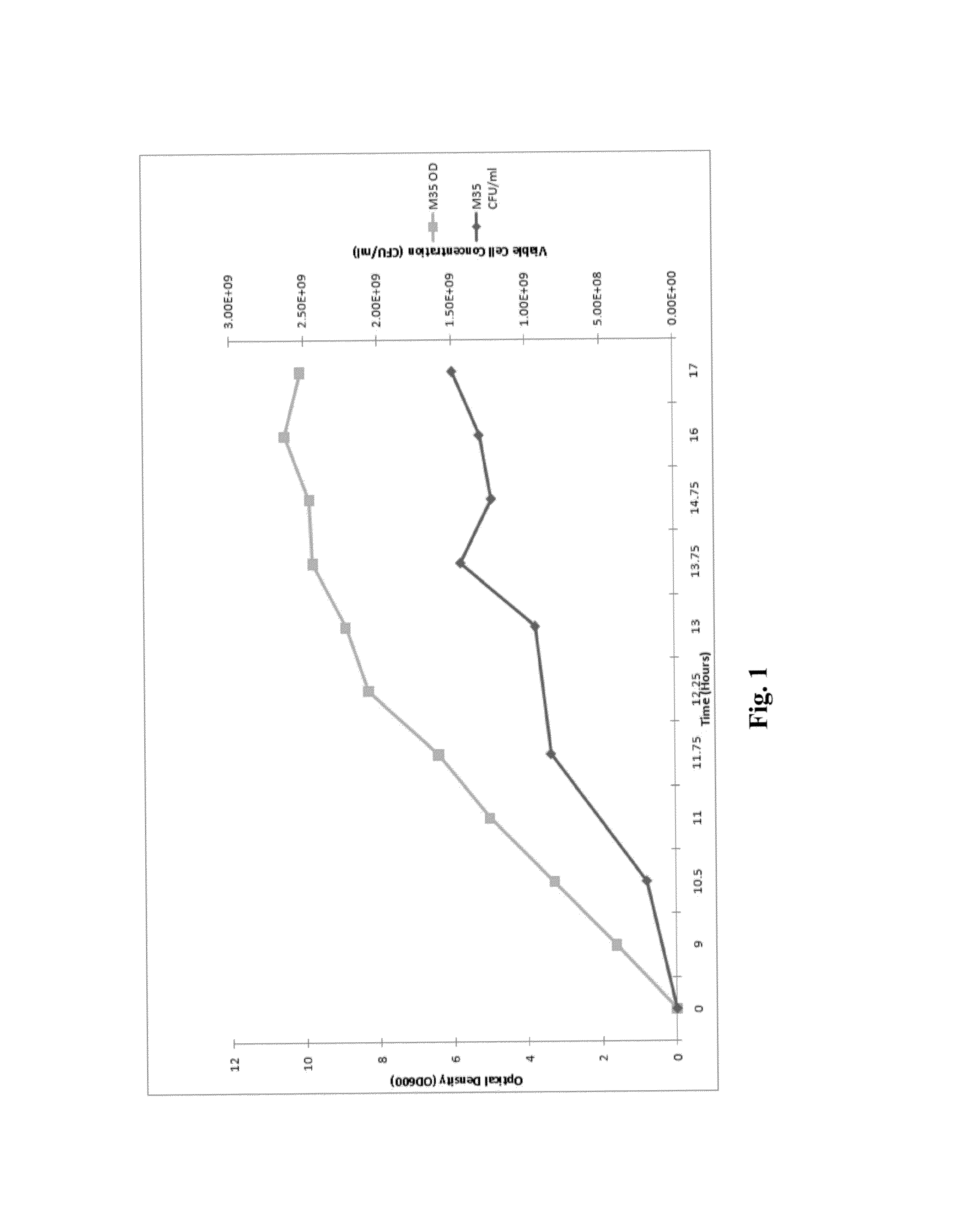
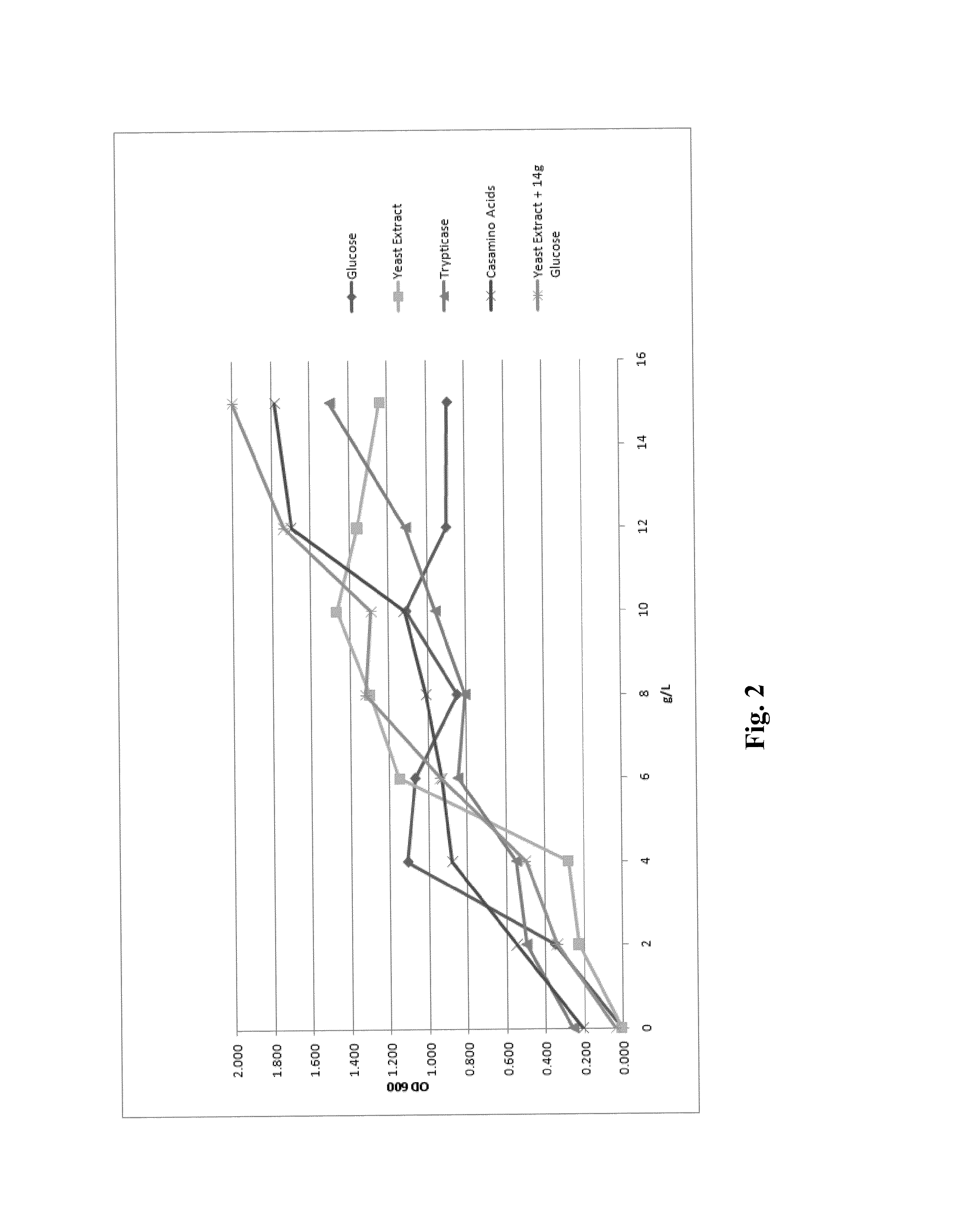
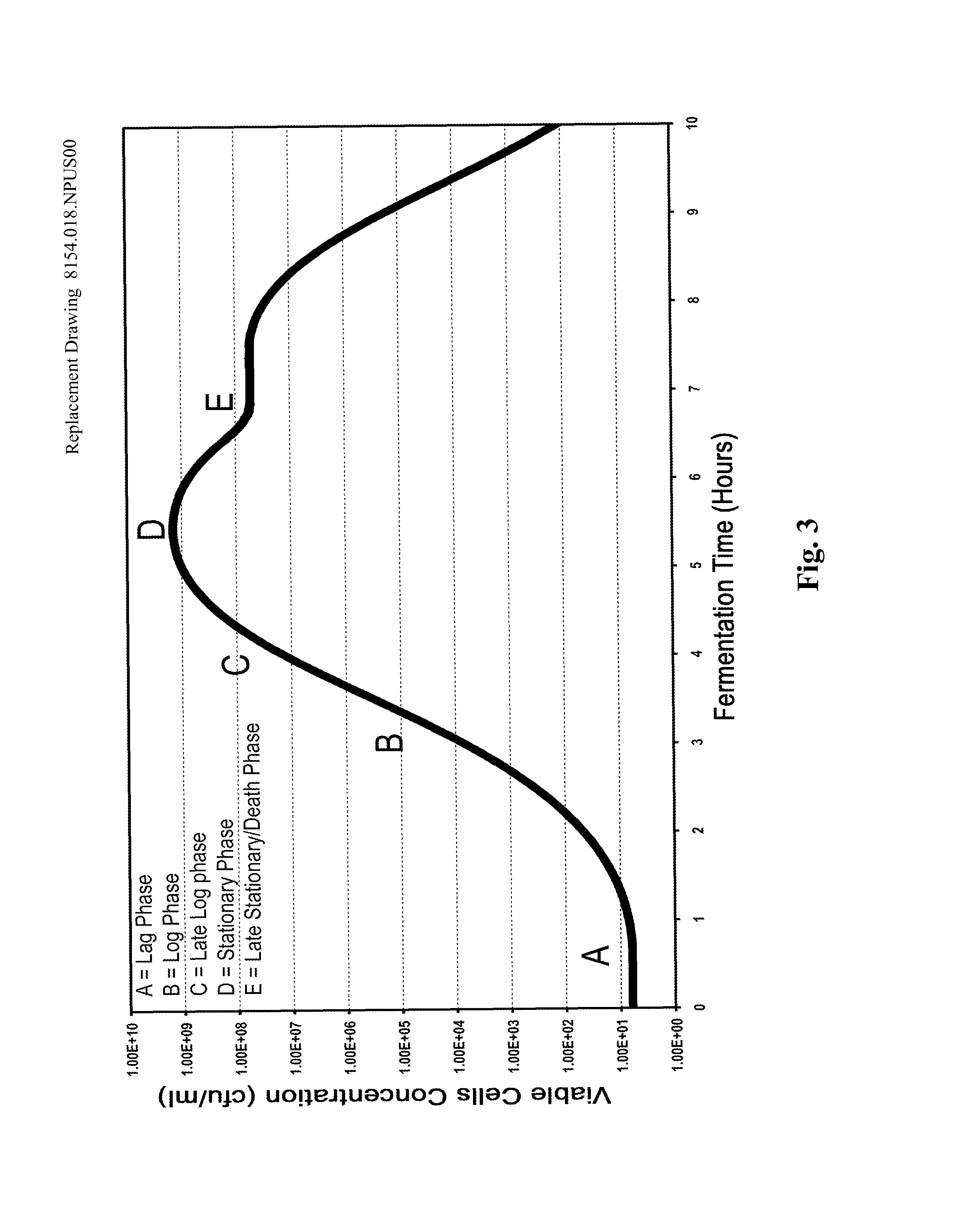
Processes to produce microorganisms that can be incorporated into a microbial-based product that results in high viable cell yields and shelf-stable products are disclosed. These microbial-based products are useful for inhibiting pathogenic growth and as a food additive. A preferred microorganism is the lactic acid producing bacteria, Lactobacillus amylovorus M35. In one embodiment, the process comprises inoculating a lactobacillus fermentation medium with M35 cells, harvesting the M35 cells at mid to late log phase, concentrating the M35 cells, and preserving the M35 cells at a concentration of at least 5×109 cfu / ml.
Owner:MICROBIOS
Tubular electro-acoustic aggregation device
InactiveUS20140017758A1Efficient HarvestingEfficiently dewateredBioreactor/fermenter combinationsBiological substance pretreatmentsAcoustic energyElectric field
Described herein are systems, methods, and apparatuses for aggregating microorganism in an aqueous suspension. In particular, are systems, methods, and apparatuses that apply an electrical field and / or acoustic energy to an aqueous suspension comprising microorganisms as the aqueous suspension follows a flow path to cause aggregation of the microorganisms. The electrical field may be continuous or pulsed. In some embodiments, the flow path for the aqueous suspension may vary.
Owner:HELIAE DEVMENT
Methods for lyophilizing competent cells
This invention relates to a method for producing cells which are competent for transformation and which may be stably stored for extended periods of time at various temperatures. The method involves growing cells in a growth conducive medium, rendering said cells competent, and lyophilizing said competent cells. The invention further relates to competent cells produced by such a method, to methods of transforming said cells with a DNA molecule, and to a method of producing a desired protein or polypeptide from said transformed cells.
Owner:LIFE TECH CORP
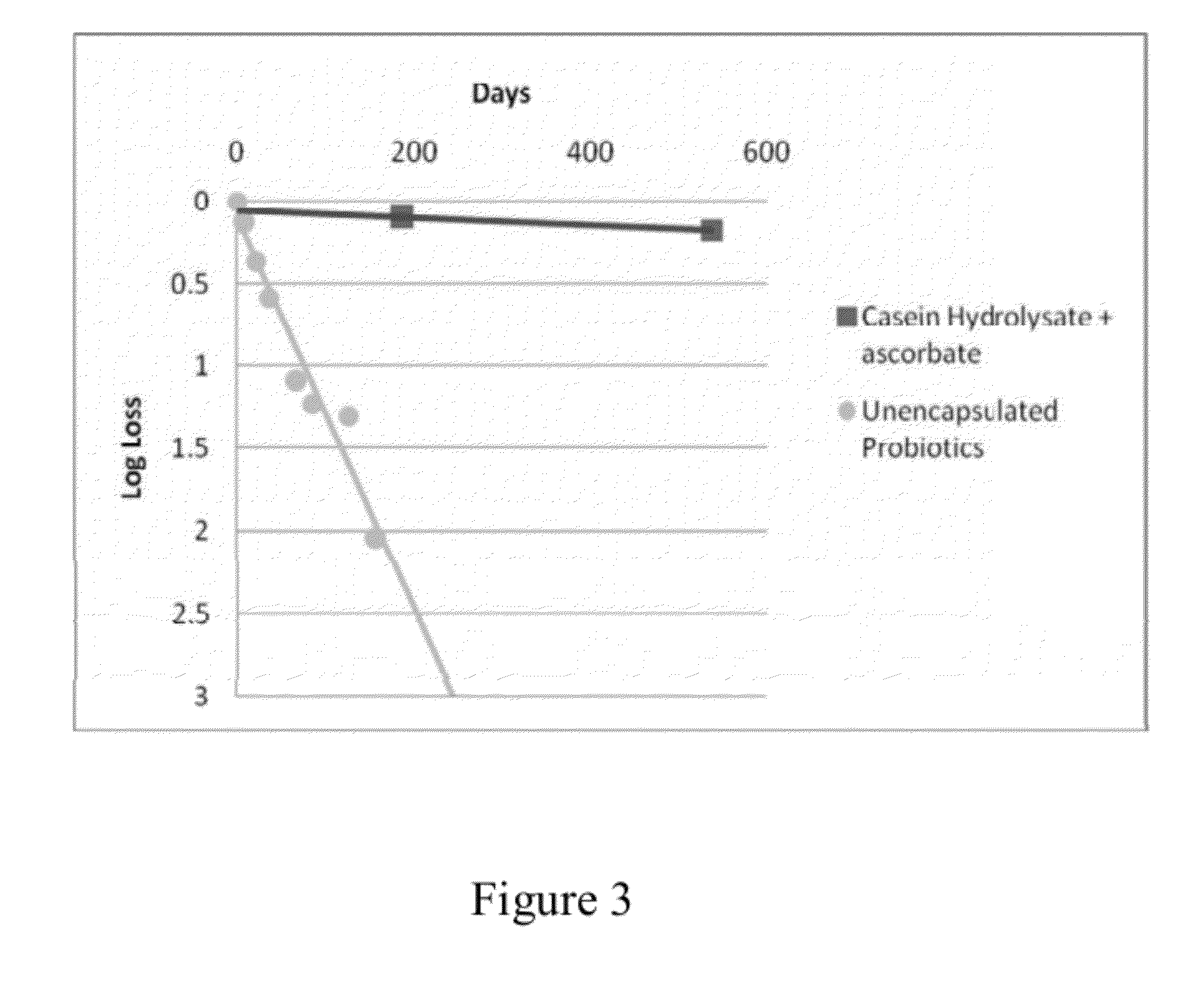
Encapsulation system for protection of probiotics during processing
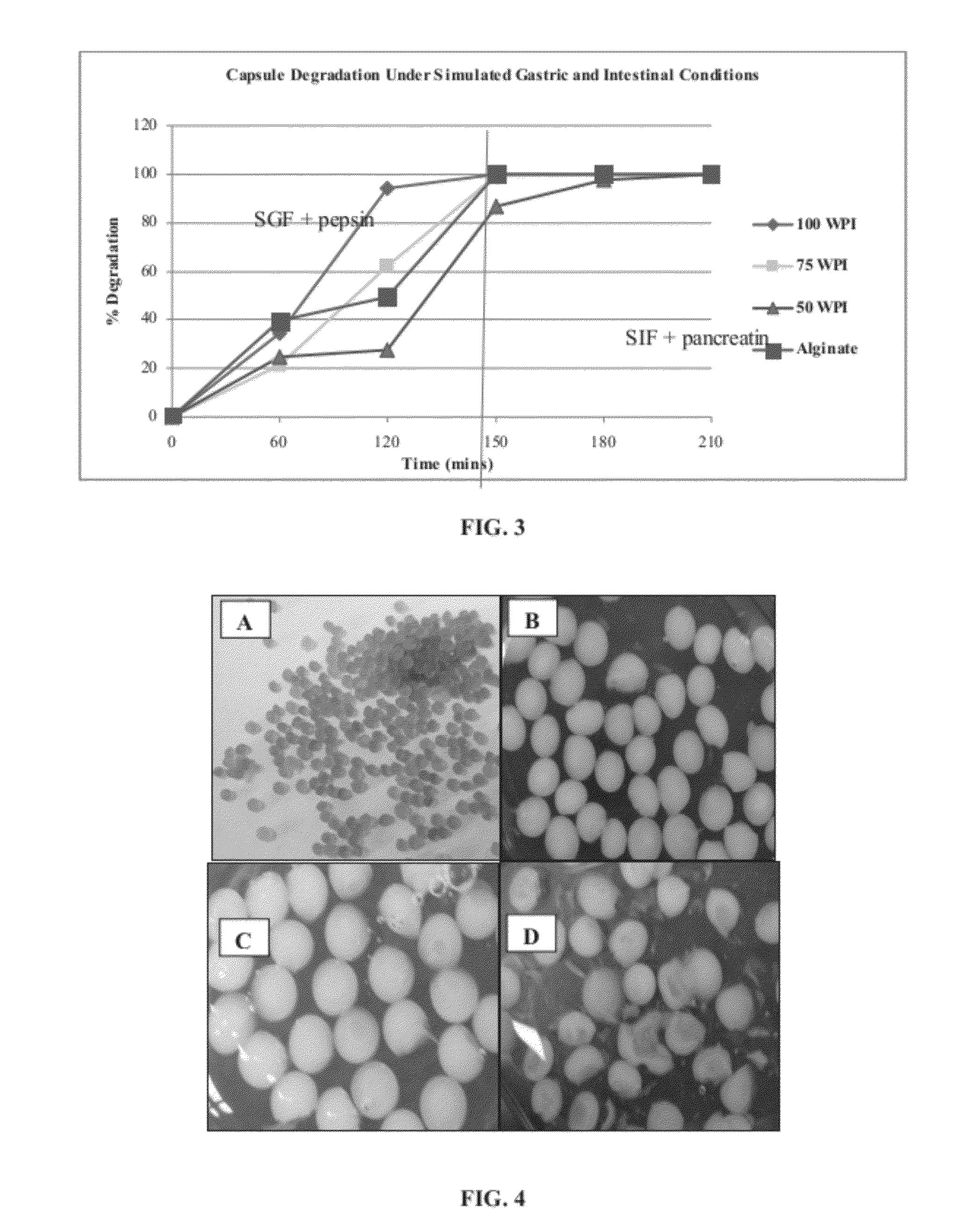
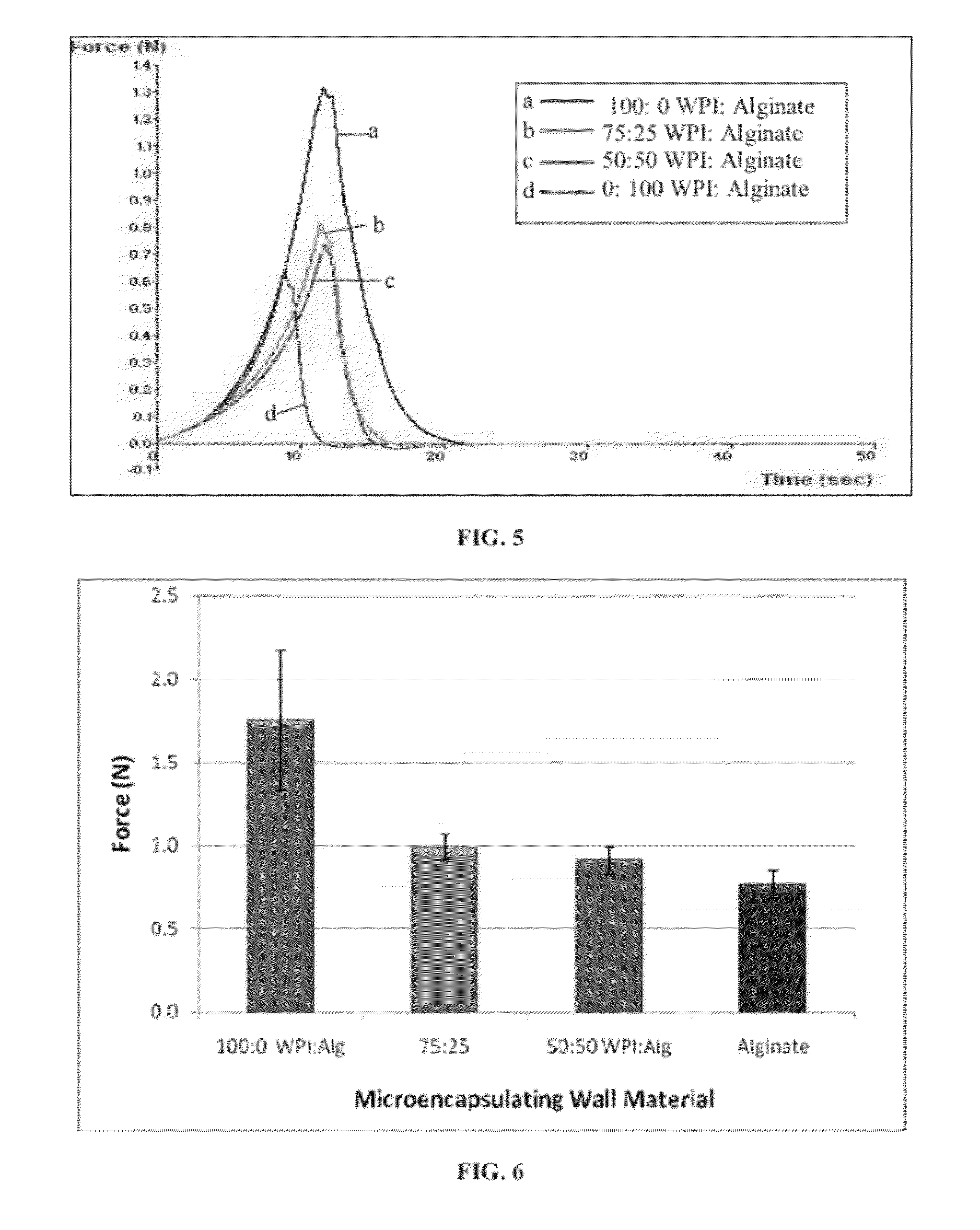
Comestible products, for example beverage products, are disclosed containing encapsulated probiotic bacteria having resistance to subjection to at least thermal and acidic conditions. Beverage products include at least one aqueous liquid and capsules comprising a gelled mixture of alginate and denatured protein, and probiotic bacteria entrapped within the gelled mixture. The average particle size of the capsules is optionally less than 1000 microns (μm) in diameter, such as less than 500 μm in diameter. Methods are provided for making such encapsulated probiotics by providing a mixture comprising sodium alginate, denatured protein and active probiotic cells, and combining the mixture with a divalent cation to initiate cold gelation of the sodium alginate and denatured protein to form a second mixture. The second mixture is passed through an opening having a diameter of less than 1000 μm to form capsules. The weight ratio of protein to alginate is from 1:1 to 9:1.
Owner:PEPSI COLA TECHNICAL OPERATIONS INC +1
Methods of isolation, expansion and differentiation of fetal stem cells from chorionic villus, amniotic fluid, and placenta and therapeutic uses thereof
InactiveUS20070116682A1Sufficient supplyRaise the potentialBiocideNervous disorderAmniotic fluidBiology
Owner:CHILDRENS MEDICAL CENT CORP
Methods of isolation, expansion and differentiation of fetal stem cells from chorionic villus, amniotic fluid, and placenta and therapeutic uses thereof
InactiveUS20070116684A1Sufficient supplyRaise the potentialBiocideNervous disorderAmniotic fluidBiology
The present invention is directed to pluripotent fetal stem cells derived from chorionic villus, amniotic fluid, and placenta and the methods for isolating, expanding and differentiating these cells, and their therapeutic uses such as manipulating the fetal stem cells by gene transfection and other means for therapeutic applications.
Owner:CHILDRENS MEDICAL CENT CORP
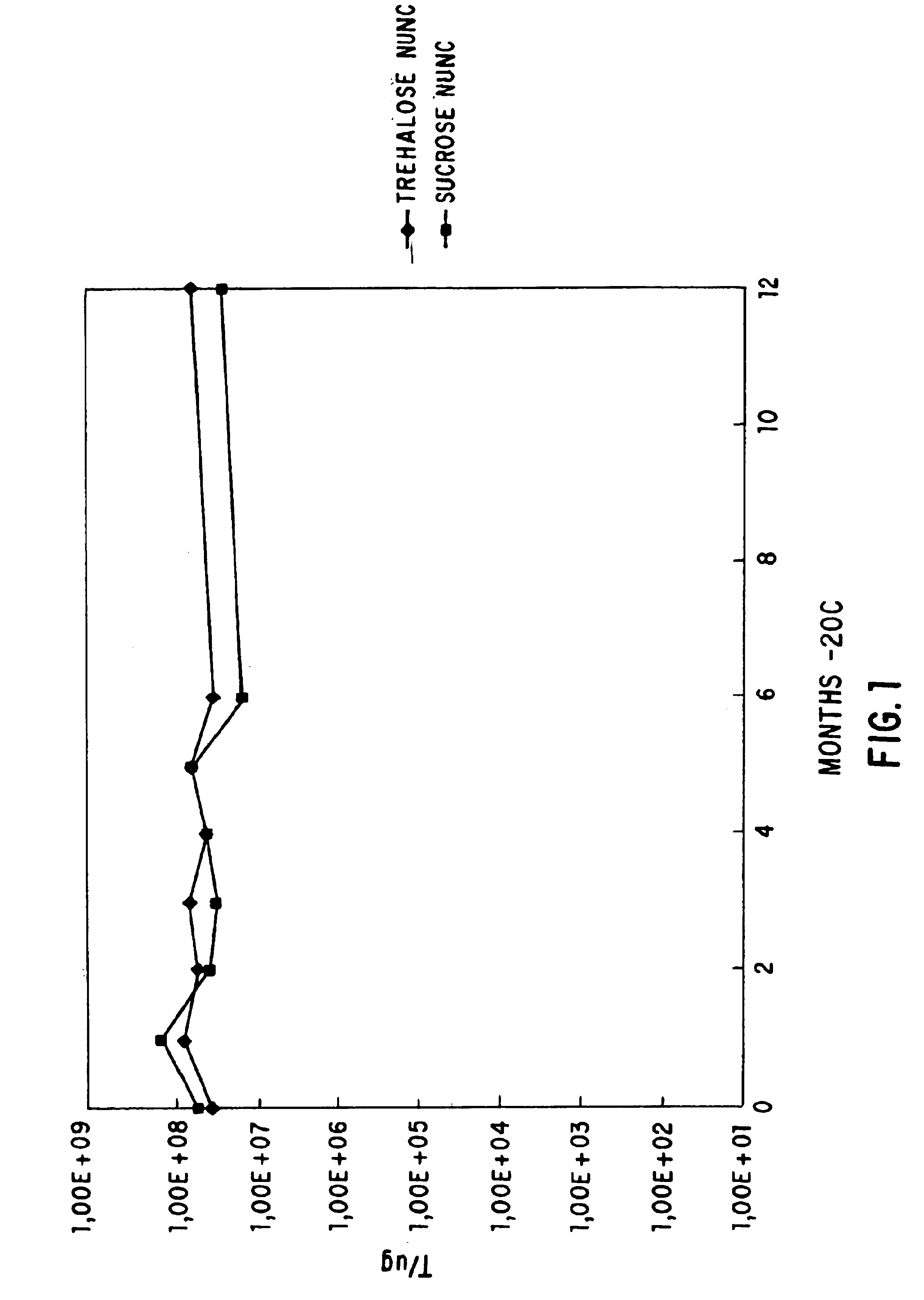
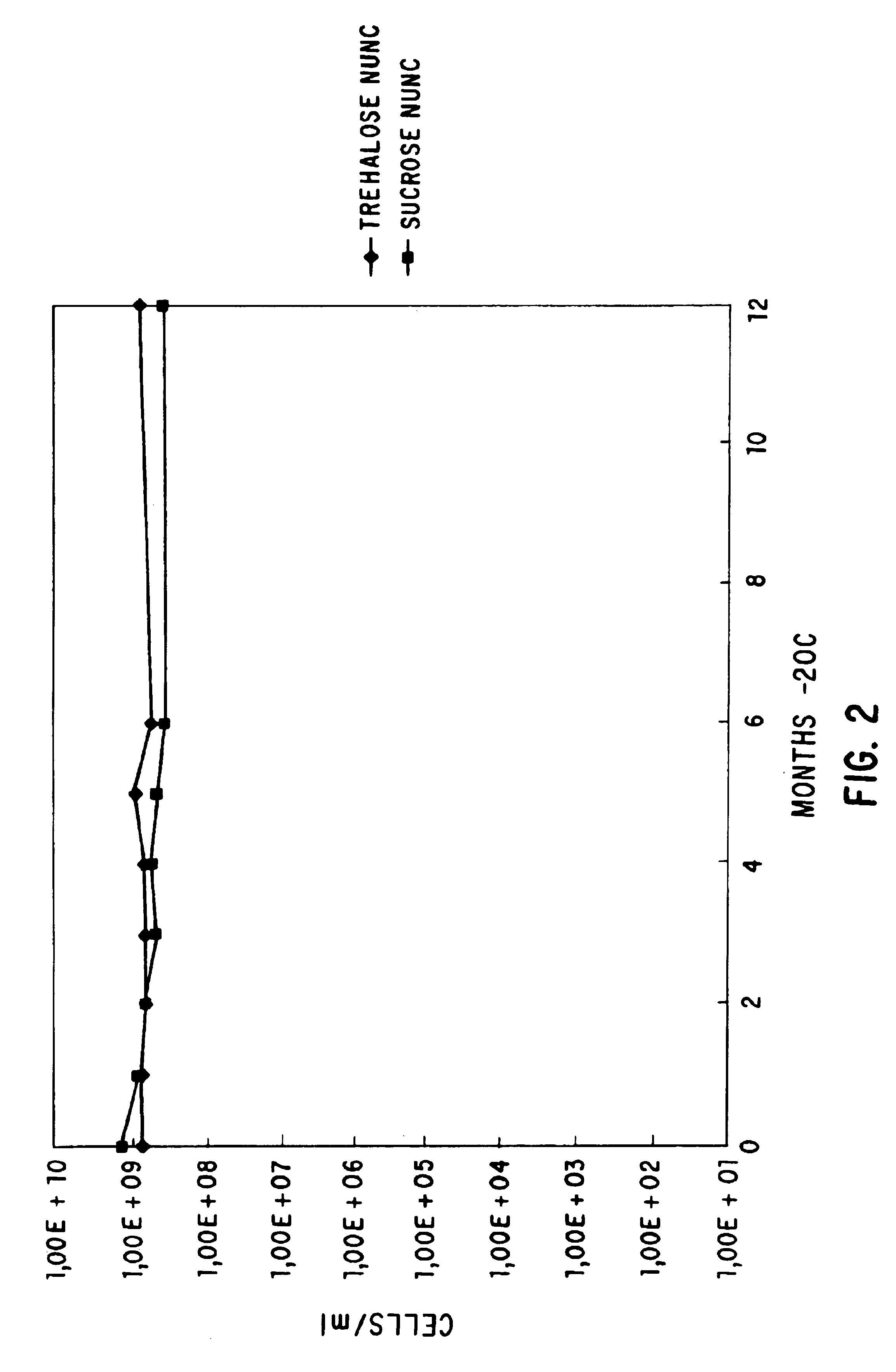
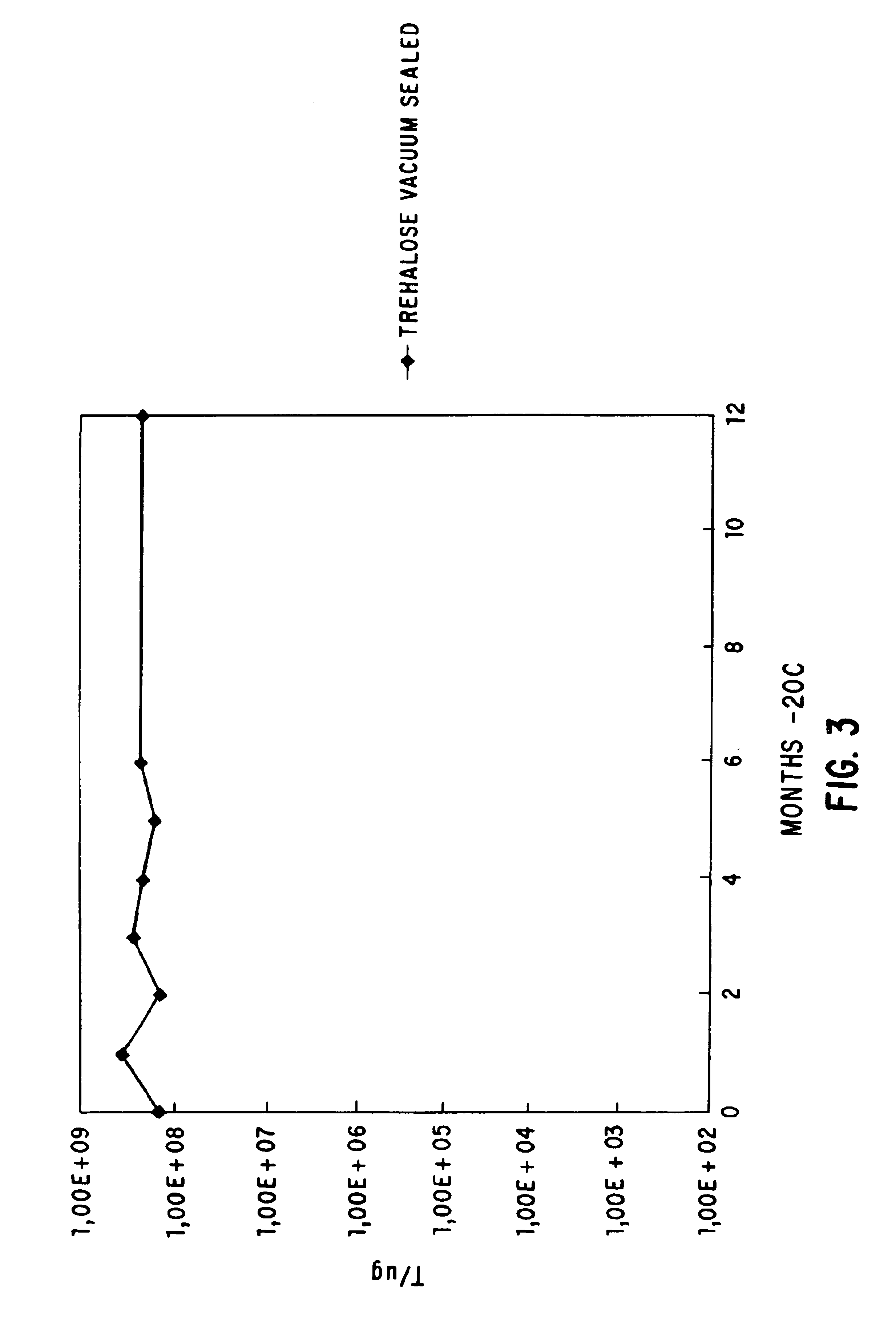
Viable dried bacteria produced by drying in the presence of trehalose and divalent cation
A method is provided for preserving live bacteria by subjecting an aqueous system containing the growing bacteria to drying without special equipment, in the presence of trehalose with or without the addition of divalent cations as stabilizing agents. Further, a dried composition for preservation of aerobic bacteria in a viable state is provided. The dried composition consists essentially of dried viable aerobic bacteria and an appropriate growth medium. The bacteria and growth medium are initially placed in an aqueous solution of 10 mM to 200 mM trehalose and a divalent cation, and dried at room temperature.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC OF THE NAVY NAVAL RES LAB WASHINGTON
Method for stably incorporating substances within dry, foamed glass matrices
Owner:QUADRANT DRUG DELIVERY
Porous freeze-dried hydrocolloid beads containing viable microorganisms for biological control
The present invention provides cellular solid carriers comprising viable microorganisms capable of controlling plant pathogens. The cellular solid carriers are formed from water-soluble hydrocolloid beads dried under conditions which preserve their porosity, thereby allowing efficient release of microorganisms or diffusion of products derived from the microorganisms from the beads to the surrounding environment.
Owner:YISSUM RES DEV CO OF THE HEBREWUNIVERSITY OF JERUSALEM LTD
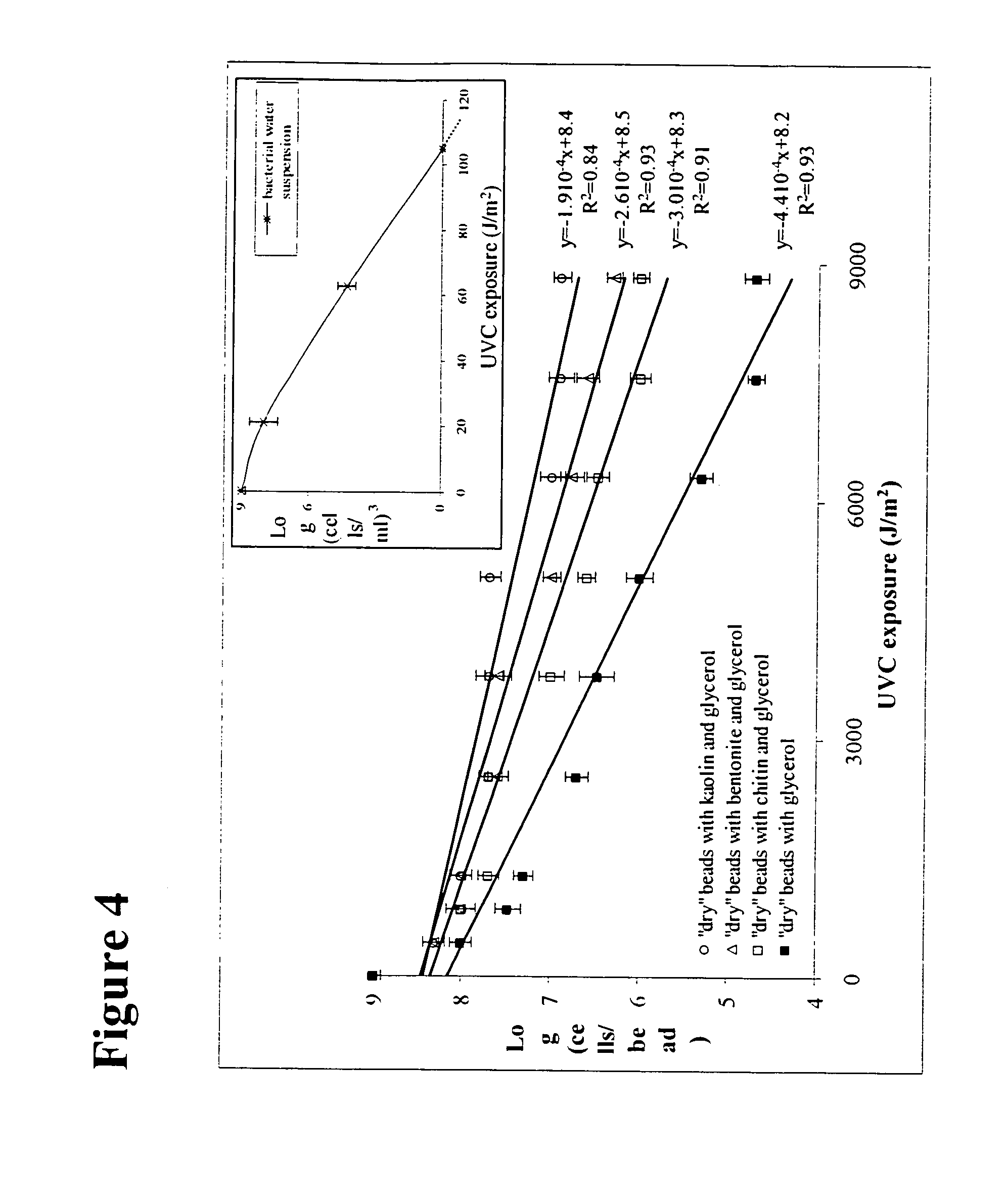
Dry storage stabilizing composition for biological materials
ActiveUS20120039956A1Improve biostabilityBenefit of stabilizingPowder deliveryVirusesBiotechnologyBiological materials
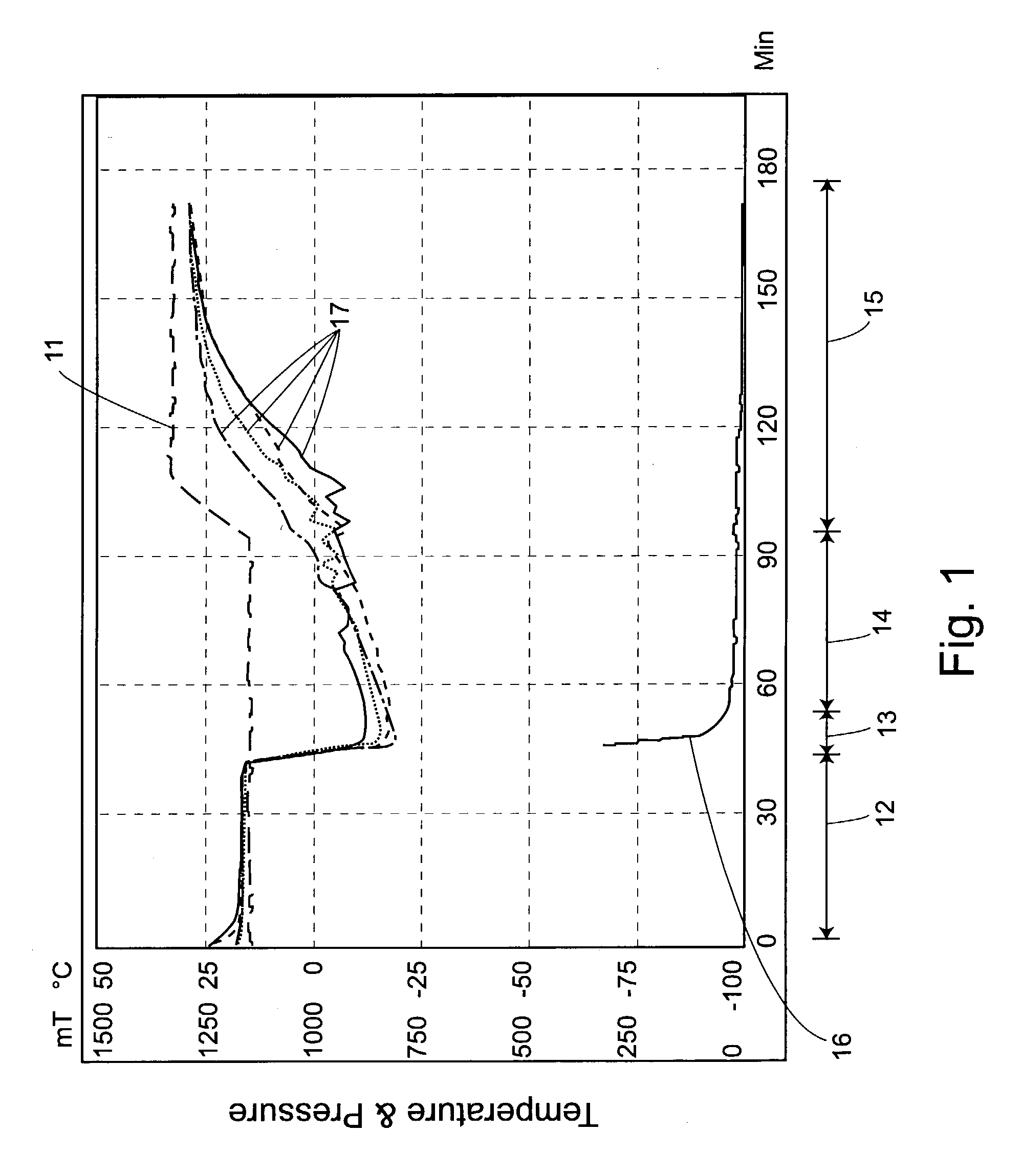
The present invention includes compositions and drying methods for preserving sensitive bioactive materials, such as peptides, proteins, hormones, nucleic acids, antibodies, drugs vaccines, yeast, bacteria (probiotic or otherwise), viruses and / or cell suspensions, in storage. The compositions include a carbohydrates component and a glass enhancer component, wherein the carbohydrate component includes a mixture of di-, oligo- and polysaccharides and the glass enhancer includes ions of organic acid and protein hydrolysates. The composition is prepared by dispersing all the solid components in a solution and then snap-frozen to form small beads, strings or droplets. The preferred drying method of the frozen beads, strings or droplets is initiated by a short purging and structure stabilizing step of the frozen particles under a vacuum pressure of less than <2000 mTORR followed by a primary drying step under vacuum pressure of more than >2000 mTORR and at a desired temperature. During the secondary and final drying step of the material a full vacuum pressure and elevated temperature are applied, to achieve a final desirable water activity of the dry material.
Owner:ADVANCED BIONUTRITION CORP
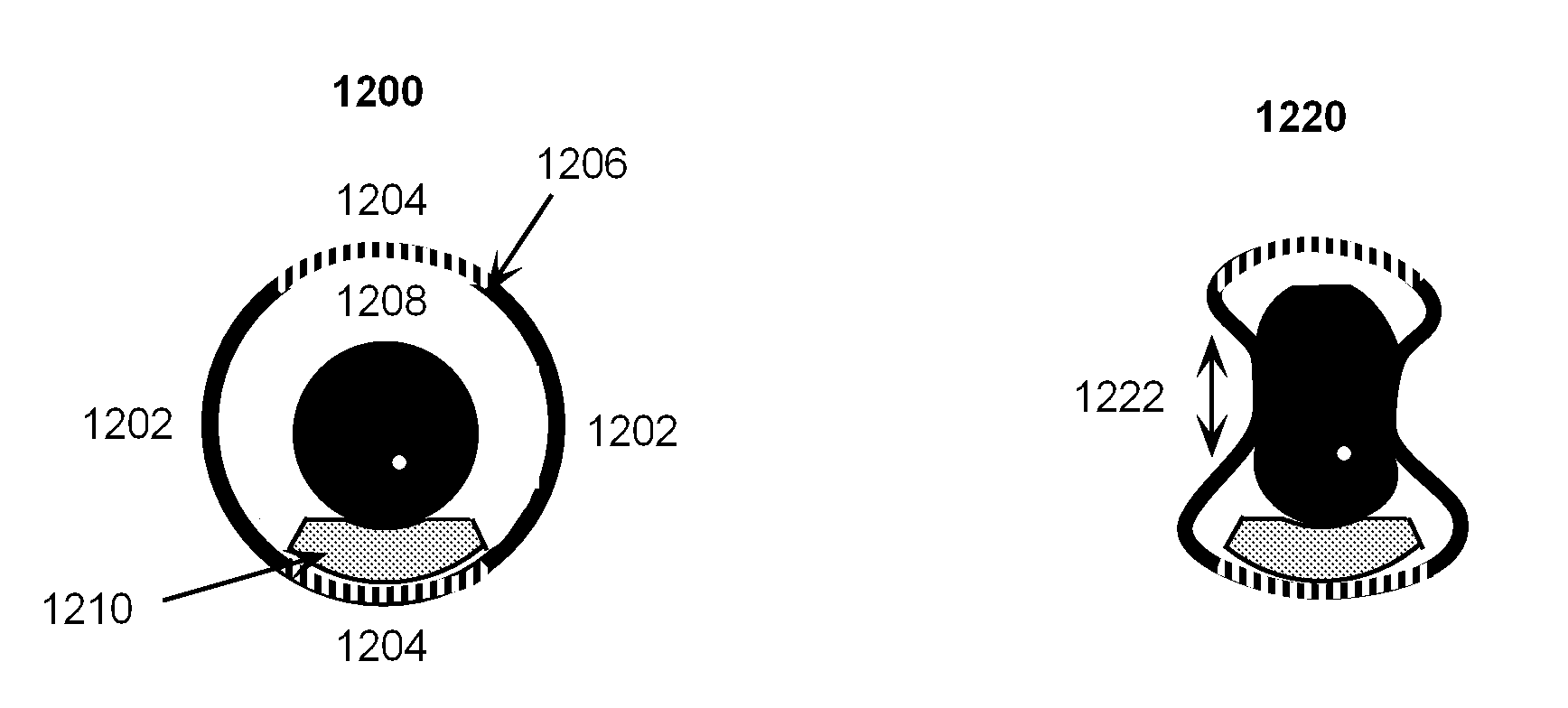
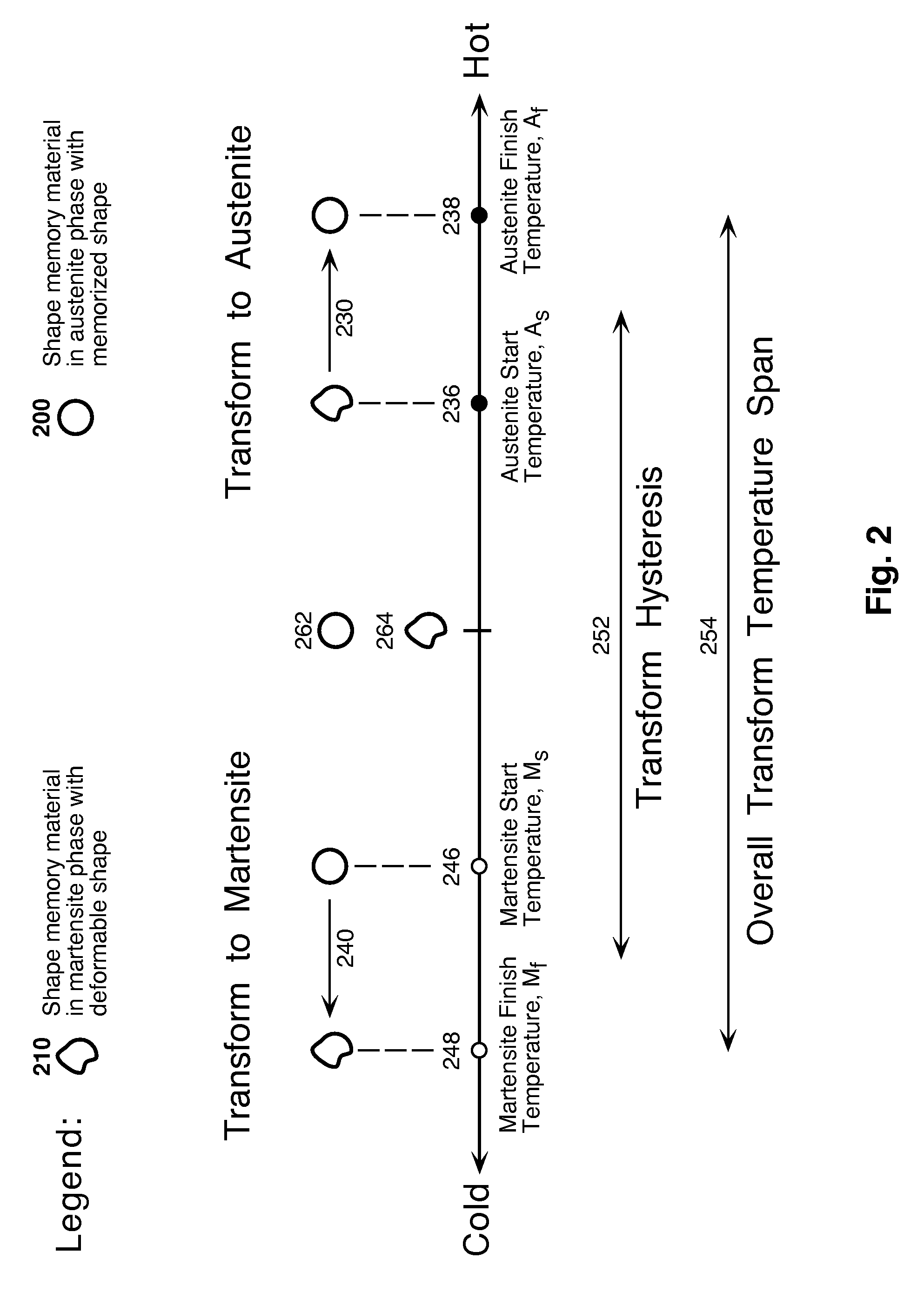
Shape-Shifting Vitrification Device
InactiveUS20090123992A1Ease of unloadingRapid coolingBioreactor/fermenter combinationsBiological substance pretreatmentsVitrificationCryopreservation
This invention is a storage device (cryocontainer) for the vitrification method of cryopreservation that uses shape memory materials to create a novel shape-shifting feature in which the relevant heat transfer zone of the cryocontainer can be thermally morphed between a shape conducive to biological specimen handling and to a shape conducive to rapid heat transfer. This feature utilizes the temperature induced phase transformation of shape memory materials. The temperature inducement occurs naturally within the normal temperature changes that occur during vitrification.
Owner:COOK MEDICAL TECH LLC
Dehydrated polysaccharide gel containing microorganisms, a sugar and a polyol for producing fermented drinks
Improved fermentation activity of microorganisms in a polysaccharide gel such as an alginate gel is obtained after dehydration, staorage and rehydration by soaking the gel containing the microorganisms prior to dehydration in a sugar solution to provide in the gel an amount of sugar of at least 100 g / kg and less than 500 g / kg of gel, preferably less than 300 g / k of gel. The dehydration may be carried out in a fluidized bed or by lyophilization. The gel may be in the form of beads or fibers having a double layer structure formed by an internal layer or core of gel containing the microorganisms and an external lay er or envelope of gel essentially devoid of the microoraganisms. The sugar is preferably xylose, glucose, fructose, lactose or sucrose, and the sugar solution may contain a polyol such as sorbitol, inositol or glycerol to provide in the gel an amount of polyol of at least 30 g / kg of gel. The sugar solution may also contain a non-ionic surfactant such as sorbitan monostearate as a protecting substance to fur ther improve fermentation activity. The microorganisms in the gel are preferably yeast, and after rehydration the yeast containing gel is used in producing a fermented drink such as in secondary fermentaion of wine to produce sparkling wine or champagne.
Owner:MOET & CHANDON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com