A kind of sustained-release microsphere loaded with rifapentine and linezolid and its preparation method and application
A technology of linezolid and rifapentine, applied in the field of pharmaceutical preparations, can solve problems such as poor sustained release effect, poor patient compliance, and short maintenance time of effective drug concentration, and achieve strong antibacterial effect, good compliance, and adverse reactions little effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
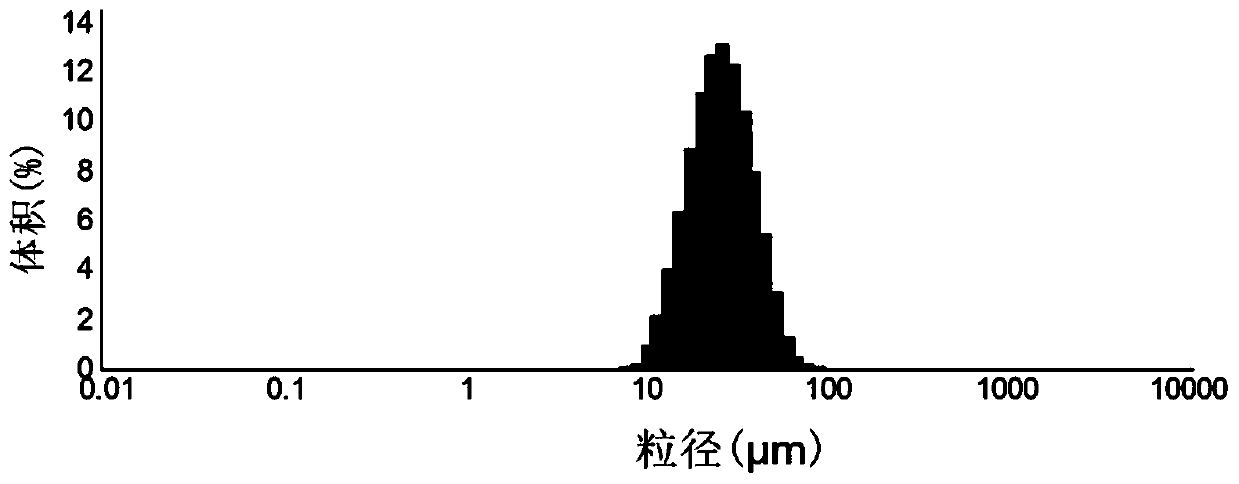
[0039] Add 600mg of polylactic acid-glycolic acid, 250mg of rifapentine and 250mg of linezolid into a volumetric flask filled with 10mL of dichloromethane, vortex for 2min to fully dissolve the medicine, and make a medicine solution. Add 2g of polyvinyl alcohol into a beaker containing 100mL of water and heat it to dissolve to prepare a 2% wt polyvinyl alcohol solution and place it in a constant temperature water bath at 30°C, and set the stirring speed of the rotor of the water bath to 800, 1000 , 1400 rpm. Then use a syringe with a specification of 2 mL to drop the medicinal solution into the polyvinyl alcohol aqueous solution drop by drop, and continue stirring for 3 hours to completely volatilize the dichloromethane in the polyvinyl alcohol aqueous solution. Use a disposable straw to suck off the foam and impurities on the surface of polyvinyl alcohol, centrifuge the remaining solution at a low speed of 1000 rpm for 5 minutes, collect the slow-release microspheres prepared...
Embodiment 2
[0048] 600mg polylactic acid-glycolic acid, (group 1: 250mg rifapentine and 250mg linezolid; group 2: 150mg rifapentine and 350mg linezolid; group 3: 200mg rifapentine and 300mg Linezolid) was added into a volumetric flask containing 10 mL of dichloromethane, and vortexed for 2 minutes to fully dissolve the drug to prepare a drug solution. Add 2g of polyvinyl alcohol into a beaker filled with 100mL of water and heat it to dissolve to prepare a 2%wt polyvinyl alcohol solution and place it in a constant temperature water bath at 30°C, and set the stirring speed of the rotors of the water bath to 1000 rpm / point. Then use a syringe with a specification of 2 mL to drop the medicinal solution into the polyvinyl alcohol aqueous solution drop by drop, and continue stirring for 3 hours to completely volatilize the dichloromethane in the polyvinyl alcohol aqueous solution. Use a disposable straw to suck off the foam and impurities on the surface of polyvinyl alcohol, centrifuge the re...
Embodiment 3
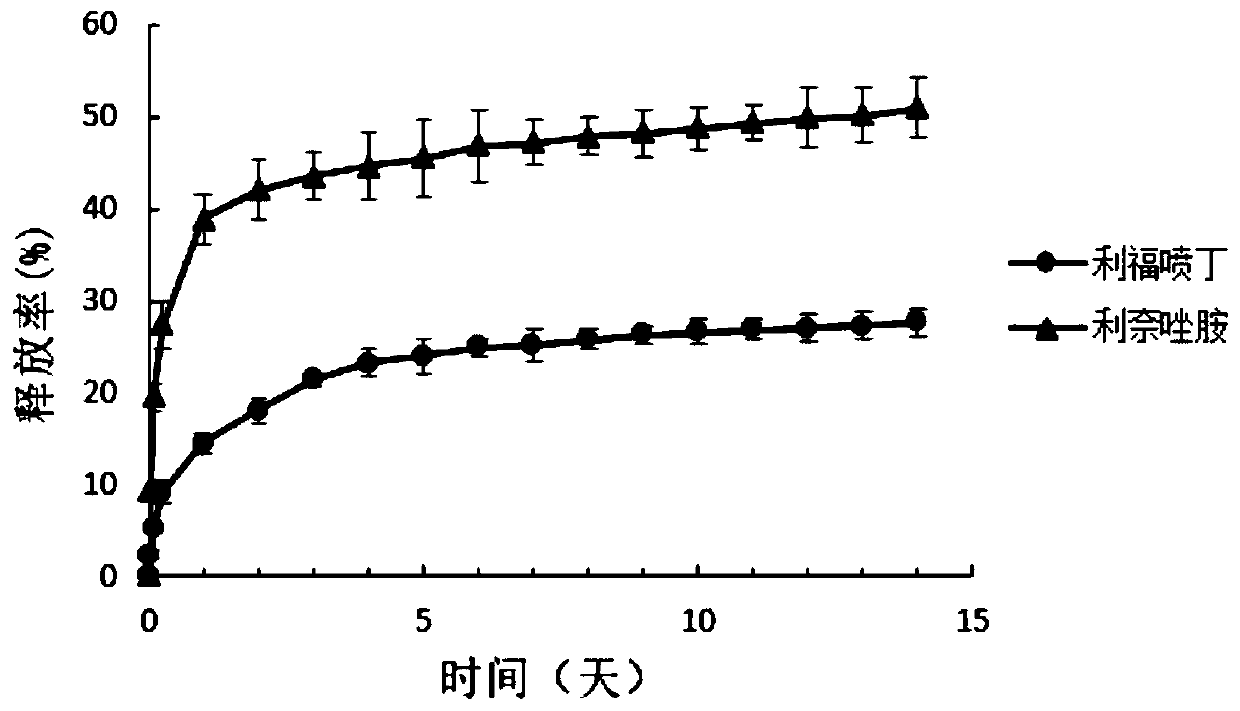
[0059] Embodiment 3 draws release curve in vitro
[0060] Put 10 mg of microspheres prepared under optimal conditions into a sealed dialysis bag (molecular weight cut-off 8000 or more) and place the dialysis bag in 50 mL full of release medium-phosphate buffer (PBS, pH=7.4, 0.1 mg / mL vitamin C) in a test tube with a screw cap. Put the test tube into a dissolution tester that shakes back and forth at a frequency of 100 rpm, and maintain the water temperature at 37±0.5°C. At 30min, 2h, 6h, 1d, 2mL samples were taken at 1d intervals, and the same volume of fresh release medium was added at the same time. The release medium was filtered with a microporous membrane and the drug content was measured by HPLC. The experiment was repeated three times. Finally, the in vitro cumulative release rate and release rate curves of rifapentine and linezolid were drawn respectively. The vitamin C in it can prevent rifapentine from being oxidized and decomposed and maintain the stability of rif...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com