Pyrazinamide derivatives as well as preparation method and uses thereof
A technology of bispyrazinamide and pyrazinecarboxylic acid, which is applied in the fields of organic compound synthesis and nano-device regeneration manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
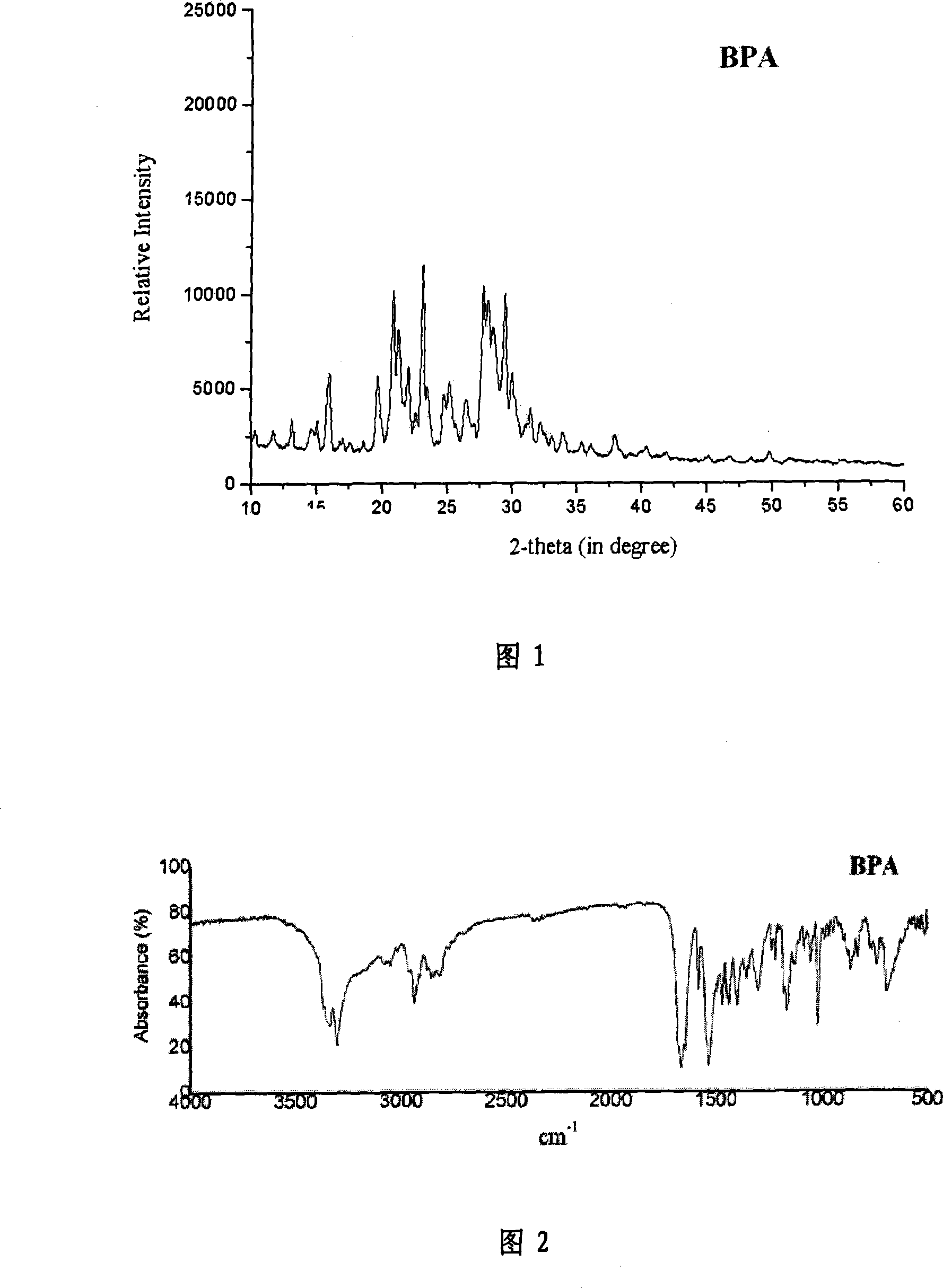
[0059] (BPA) Synthesis of N,N'-(4-aza-1,7-heptyl)bispyrazinamide
[0060] Add 3.0 g (21.7 mmol) of methyl pyrazinecarboxylate into dry 20 mL of methanol under nitrogen protection, then add 10.0 mmol of dipropylenetriamine dropwise under nitrogen protection and vigorous stirring, and the reaction solution is heated to reflux under stirring for 10 h, spin Dry solvent, add methanol until the solute dissolves, add a small amount of activated carbon to decolorize, heat filter, pour into 20 times the amount of ice water, precipitate a white solid, vacuum filter under reduced pressure, wash the filter cake with a small amount of water, and use 100ml methanol: water to 10 :1 solution was recrystallized, allowed to stand at room temperature, crystals were precipitated naturally, filtered, and dried to obtain colorless crystals with a yield of 45%, melting point: 78.0-80.0°C.
Embodiment 2
[0062] The (BPA) synthesis of compound N, N'-(4-aza-1,7-heptyl)bispyrazinamide added 4.8g (35mmol) methyl pyrazinecarboxylate into dry 70mL methanol under nitrogen protection, Then under nitrogen protection and vigorous stirring, 17.0mmol dipropylenetriamine was added dropwise, the reaction solution was heated to reflux for 10h under stirring, the solvent was spin-dried, methanol solution was added until the solute was dissolved, a small amount of activated carbon was added for decolorization, hot filtration, poured into 20 times the amount A white solid was precipitated in ice water, filtered under reduced pressure, and the filter cake was washed with a small amount of water, recrystallized with a solution of 100ml methanol:water 10:1, left to stand at room temperature, and crystals were naturally precipitated, filtered, and dried to obtain Colorless crystals, yield 57%, melting point: 79.5-80.0°C.
[0063] Infrared analysis results:
[0064] The main infrared absorption pea...
Embodiment 3
[0071] Preparation of Hydrous Organic Compound Crystals
[0072] Dissolve 0.01mmol of N,N'-(4-aza-1,7-heptyl)bispyrazinamide in 10mL of methanol, add 0.01-1.0mL of water, magnetically stir to dissolve, mix well, and place it. Two weeks later, a colorless bulk crystal suitable for X-ray single crystal structure analysis was obtained (see accompanying drawing 4). Yield: 46%, melting point: 60-61°C.
[0073] Infrared analysis results:
[0074] The main infrared absorption peak is: IR(KBr, cm -1 ): 3255(m), 2868(m), 2740(m), 1649(s), 1515(s), 1477(m), 1424(m), 1317(m), 1161(s), 1058(s ), 865(m), 704(m), 657(s).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com