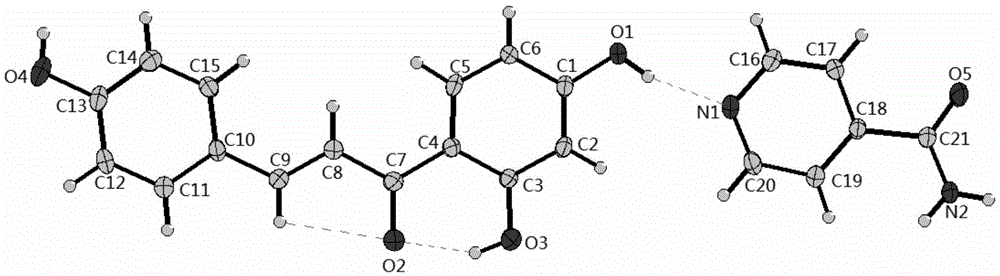
Isoliquiritigenin pyrazinamide eutectic crystal and preparation method thereof
A technology of isoliquiritigenin and isonicotinamide is applied in directions such as organic chemistry, can solve the problems of limited application, low solubility of isoliquiritigenin, unsatisfactory oral intake and the like, and achieves the effects of improving solubility, low cost and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Precisely weighed 128.17 mg of isoliquiritigenin and 61.06 mg of isonicotinamide were placed in a mortar, added 75 μl of methanol, and ground for 20 minutes to obtain isoliquiritigenin-isonicotinamide co-crystal.
Embodiment 2
[0031] Precisely weighed 135.49 mg of isoliquiritigenin and 64.56 mg of isonicotinamide were placed in a mortar, added 90 μl of ethanol, and ground for 40 minutes to obtain isoliquiritigenin-isonicotinamide co-crystal.
Embodiment 3
[0033] Precisely weigh 89.79 mg of isoliquiritigenin and 42.76 mg of isonicotinamide and place them in a mortar, add 60 μl of methanol, and grind for 35 minutes to obtain isoliquiritigenin-isonicotinamide co-crystal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com