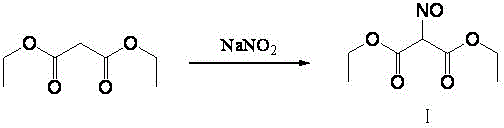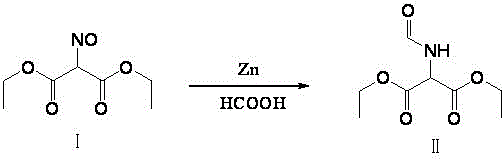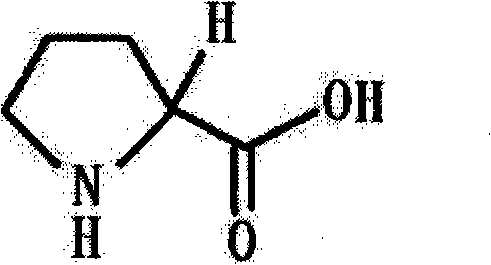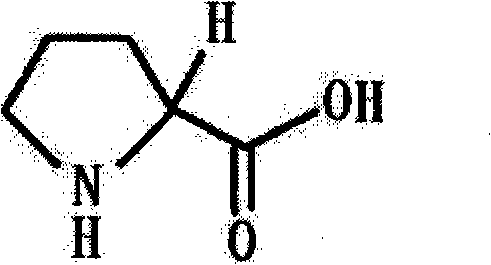Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
42 results about "Diethyl aminomalonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing 2-para octylphenyl ehtyl-2-amino propanediol
InactiveCN1528738AEasy to manufactureEfficient manufacturingOrganic compound preparationAmino-hyroxy compound preparationSodium iodidePropanediol
The invention provides a method to prepare a compound method, including the steps: ethylbenzene and capryl chloride make Friedel-Crafts reaction to generate p-capryl chloroethylbenzene; convert capryl chloroethylbenzene under the action of sodium iodide into p-capryl iodoethylbenzene; p-capryl iodoethylbenzene and acetylamino diethyl malonate condense under the action of alkali to generate 2-(p-capryl phenethyl)-2-acetylamino diethyl malonate; or p-capryl iodoethylbenzene makes elimination reaction under the action of alkali to generate p-capryl styrene, which together with acetylamino diethyl malonate condenses under the action of alkali into 2-(p-capryl phenethyl)-2- acetylamino diethyl malonate; a compound is reduced into 2-[4-(1-hydroxyoctyl) phenethyl-]2- acetylamino propylene alcohol; the other coumpound makes hydrogenolysis to obtain 2-(p-octyl phenethyl)-acetylamino propylene alcohol; make alkali hydrolyzation and then acidifies them into salt, so as to obtain it. It also provides a method to prepare intermediate in the above preparing course.
Owner:马启明
Method for preparing 2-P-octyl-phenenl-2-amino-propanediol hydrochloride
InactiveCN1814583AEasy to manufactureEfficient manufacturingOrganic compound preparationAmino-hyroxy compound preparationSilanesPropanediol
The invention offers 2-amphi capryl phenethyl-2-amino group propylene glycol hydrochloride preparation method. It includes the following steps: doing Friedel-Crafts reaction for styrene and capryl chloride to produce amphi capryl styrene; doing Michael addition reaction with kharophen diethyl malonate under alkali action to produce 2-amphi capryl phenethyl-2-kharophen diethyl malonate; reacting with triethyl silane to produce 2-amphi capryl phenethyl-2-kharophen diethyl malonate; reducing and hydrolyzing to produce 2-amphi capryl phenethyl-2-amino group propylene glycol; acidifying with hydrochloric acid to produce salt. It has the advantages of simple line, low cost, short period, little pollution, and high yield. It also offers the intermediate compound preparation method.
Owner:NANJING YOKO PHARMA GRP CO LTD
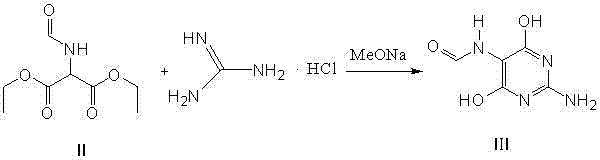
Method for preparing 2-amino-4,6-dichloro-5-formamido pyrimidine
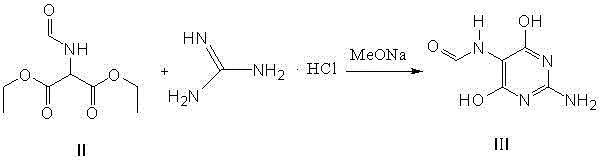
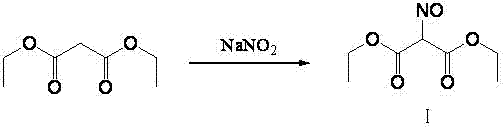
The invention relates to a method for preparing 2-amino-4,6-dichloro-5-formamido pyrimidine. The method comprises the following steps of: performing nitrosation on malonic acid diethyl ester and acetic acid serving as raw materials and sodium nitrite at first; then, performing reduction and formylation with formic acid in the presence of zinc powder to form formyl amino malonic acid diethyl ester; finally, performing condensation and cyclization with guanidine hydrochloride to produce 2-amino-4,6-dichloro-5-formamido pyrimidine; performing chlorination by using quaternary ammonium salt as a catalyst; fractionally performing hydrolysis under an alkali action to obtain a product. The method has easily-available raw materials, and is short in reaction time, simple in aftertreatment and high in hydrolysis selectivity; the cost is obviously reduced; the total yield is up to 74 percent and the purity of the product is up to 99.0 percent.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
Method for preparing 2-amino-2-[2-(4-alkyl phenyl) ethyl]-1,3-propanediol hydrochloride
ActiveCN102796022AOrganic compound preparationCarboxylic acid amides preparationAcetic anhydrideSilanes
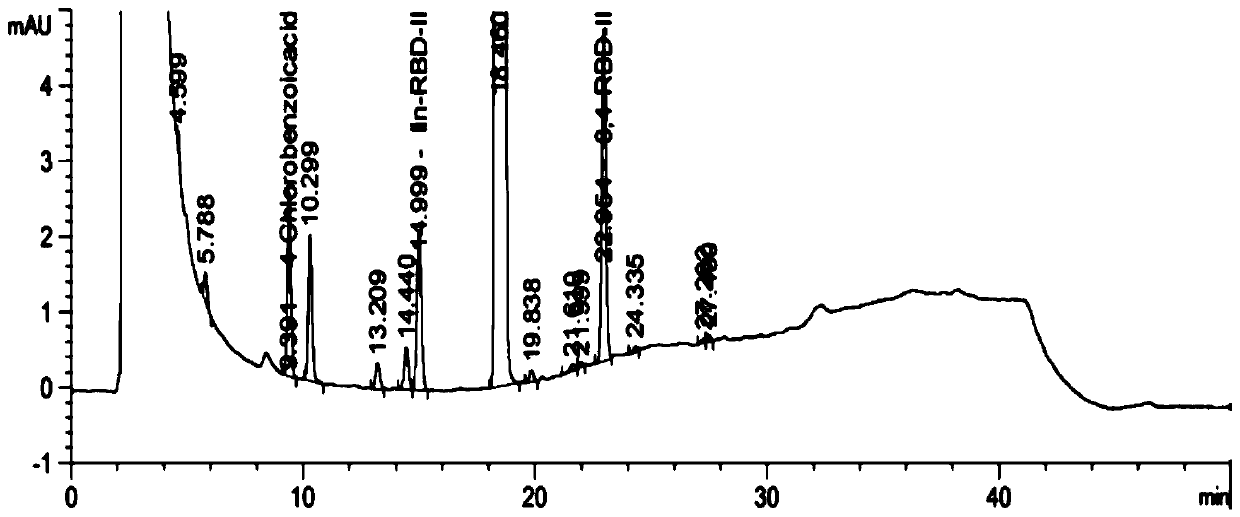
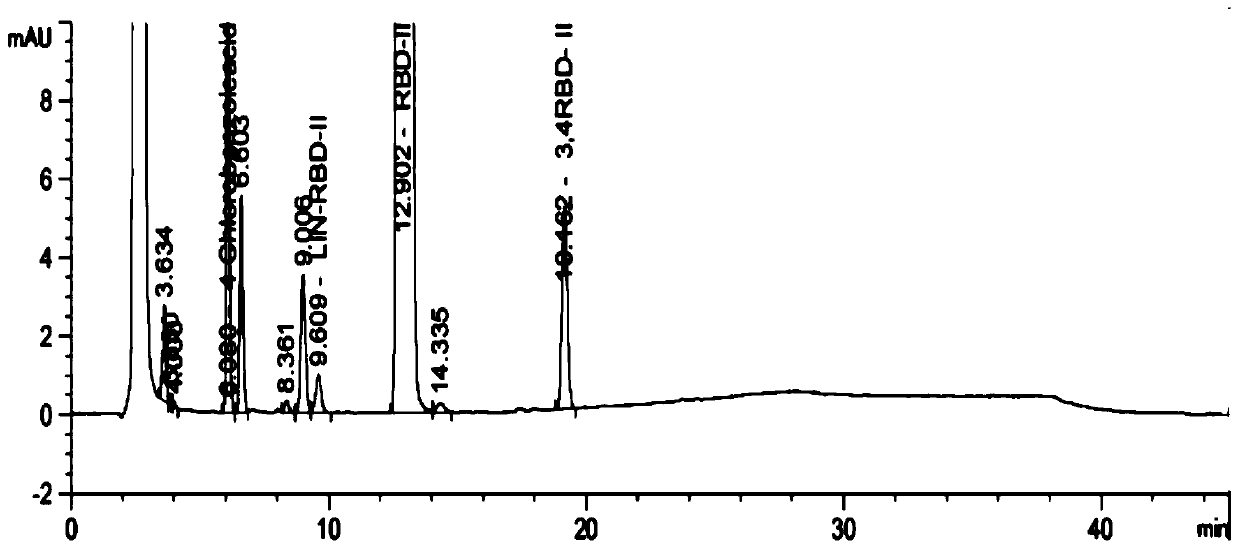
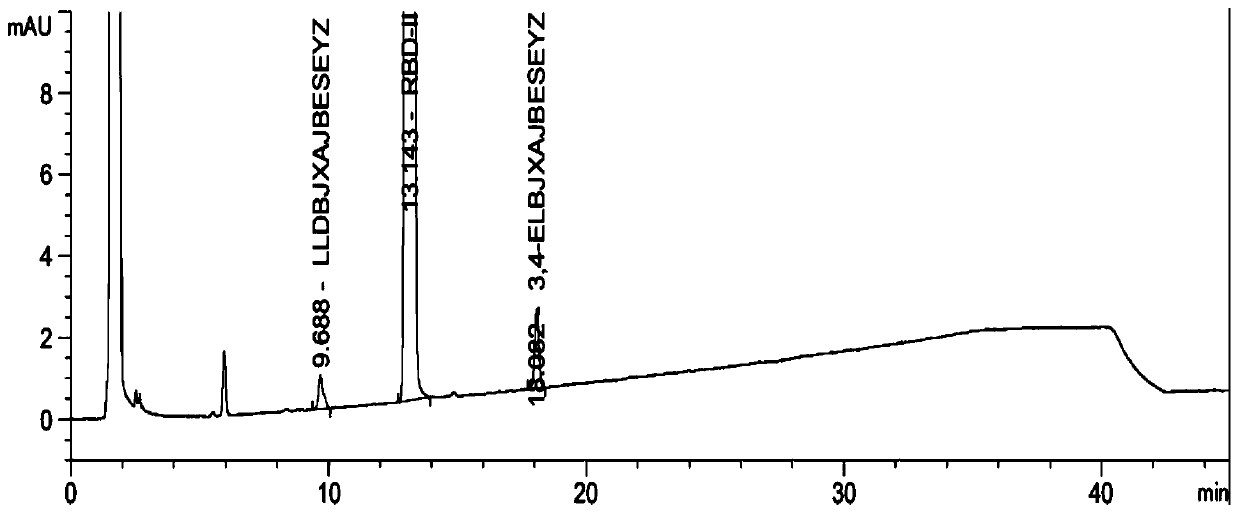
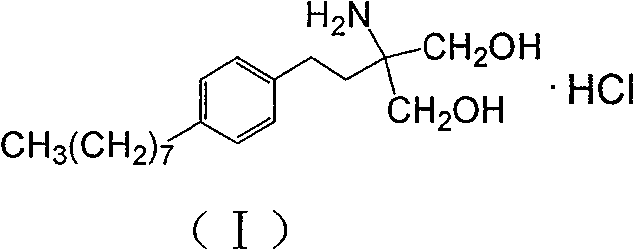
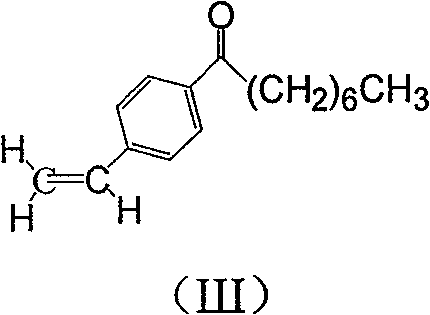
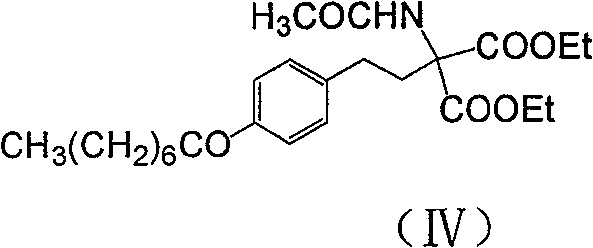
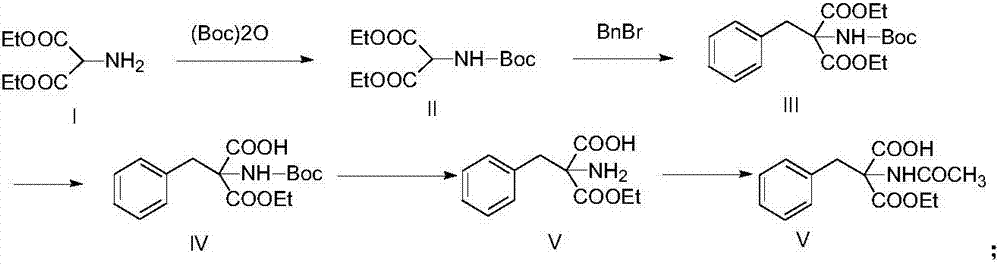
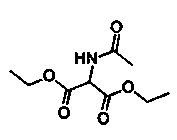
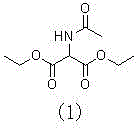
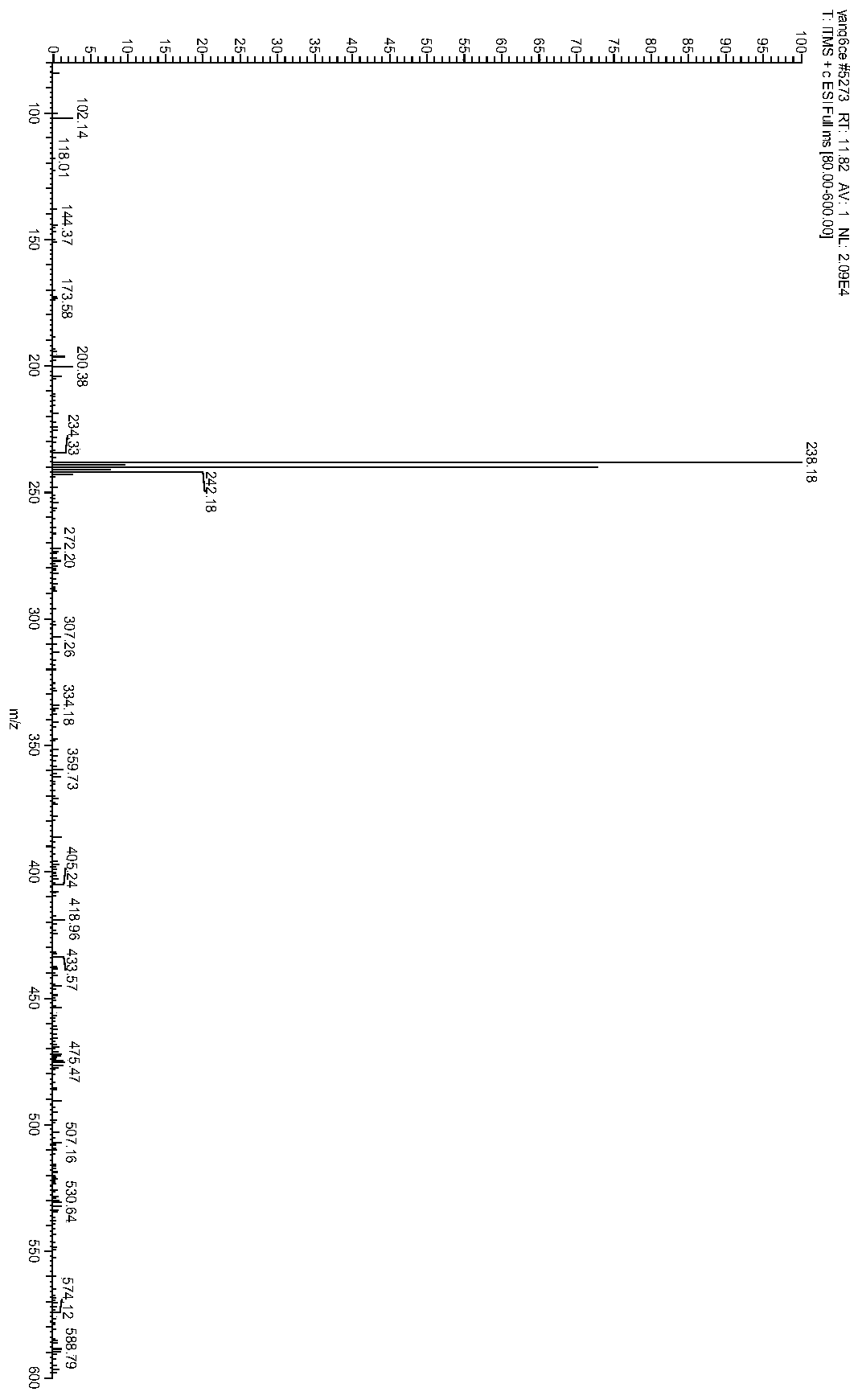
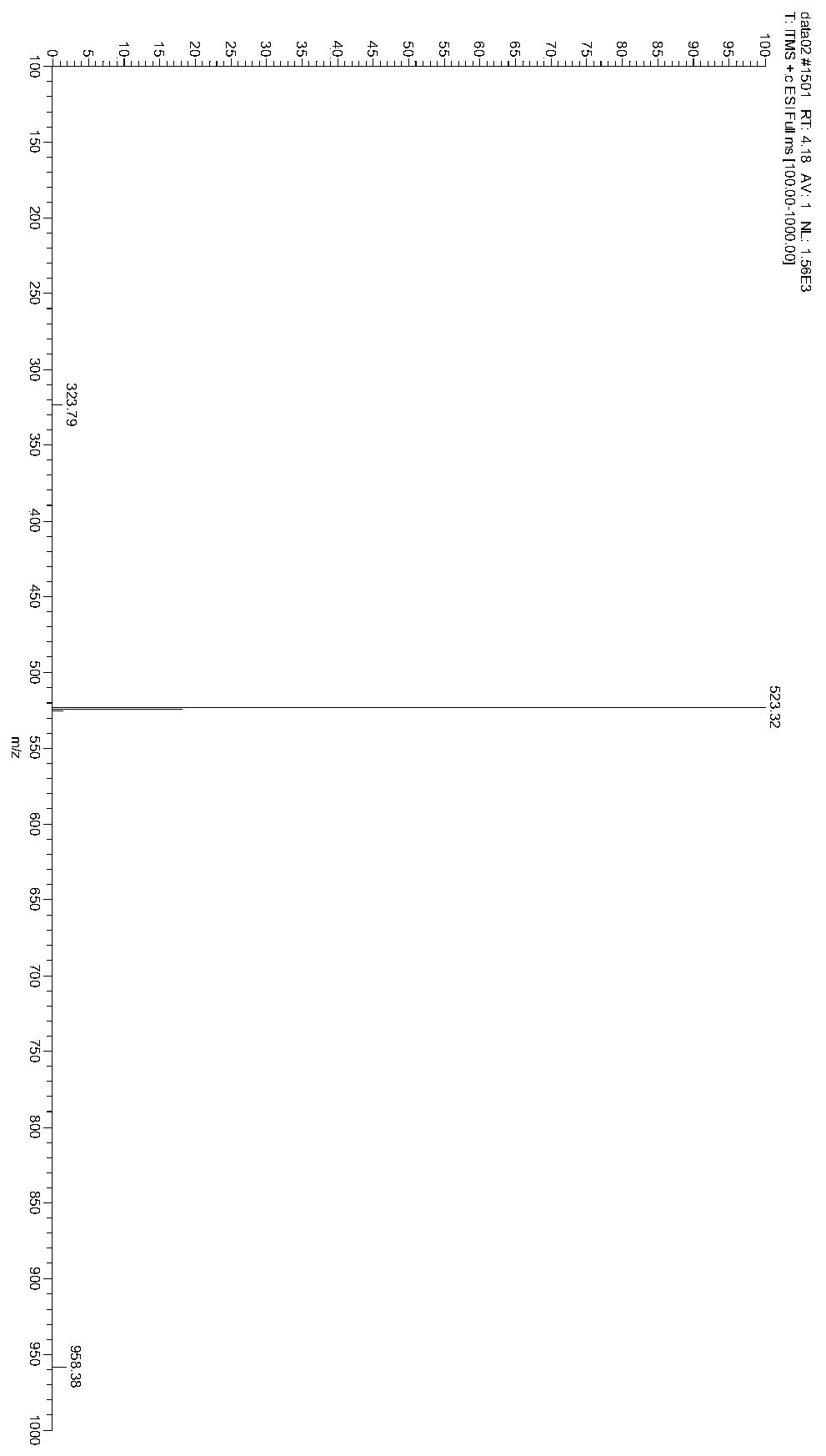
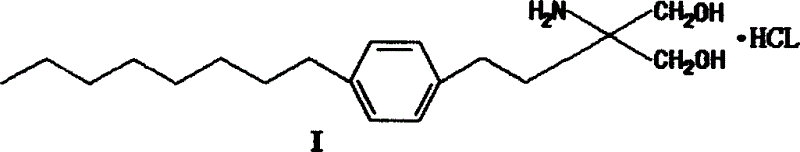
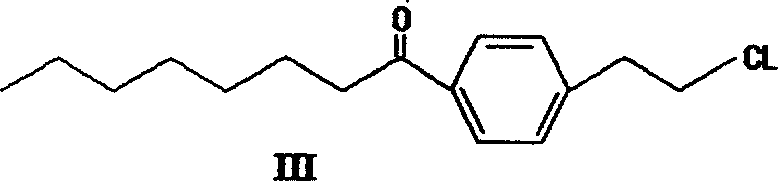
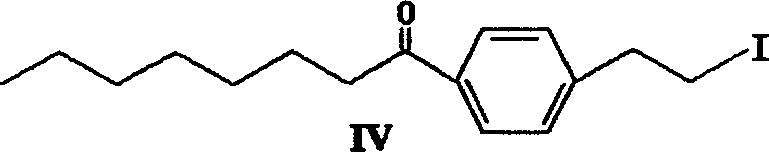
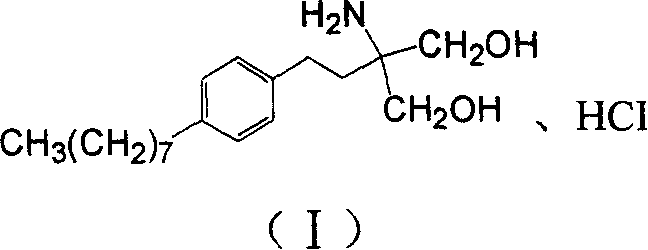
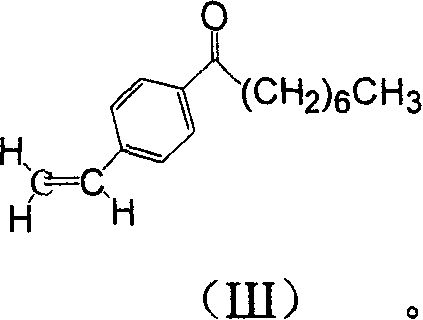
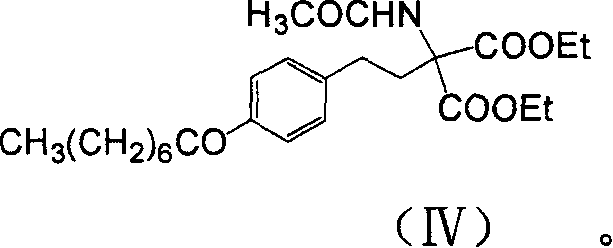
The invention discloses a method for preparing 2-amino-2-[2-(4-alkyl phenyl) ethyl]-1,3-propanediol hydrochloride. The method comprises the following steps of: firstly, enabling a compound shown as a formula (II) and diethyl acetamidomalonate to be subjected to a condensation reaction under the action of a catalyst to obtain a compound shown as a formula (III); enabling the compound shown as the formula (III) as well as triethyl silane and titanium tetrachloride to be subjected to a reaction to prepare a compound (IV); then, continuously enabling the compound (IV) as well as lithium aluminum hydride and acetic anhydride to be subjected to a reaction to prepare a compound (V); and finally, enabling the compound (V) as well as lithium hydroxide and concentrated hydrochloric acid to be subjected to a reaction to obtain a compound shown as the formula (I). A midbody (III) prepared by using the method provided by the invention has enough purity and high yield up to over 95%, and purification through column chromatography is not needed in the reaction process, so that the next reaction can be directly carried out. The final target compound is synthesized by using easily purchased raw materials in a short way, and the method is short in production period, high in yield, mild in reaction condition, simple and feasible and suitable for industrial production.
Owner:NANJING HUAWE MEDICINE TECH DEV +1
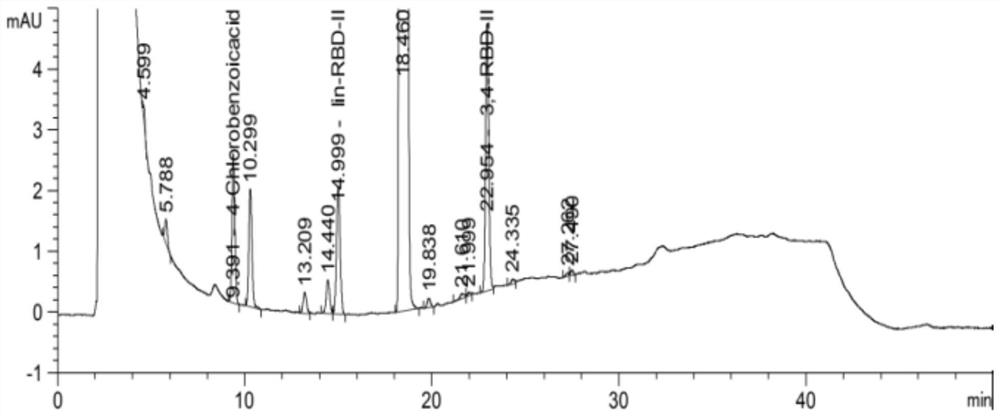
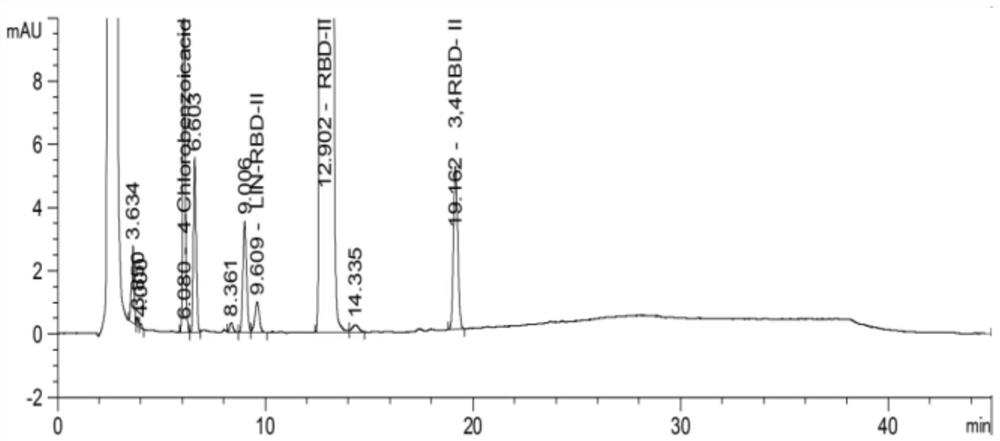
Method for detecting related substance in diethyl 2-(4-chlorobenzamido)malonate sample
ActiveCN110426463ASuitable for controlSuitable for researchComponent separationPropanoic acidMalonate
The invention relates to the technical field of pharmaceutical analysis, and specifically relates to a method for detecting a related substance in a diethyl 2-(4-chlorobenzamido) malonate sample. Themethod includes the following steps: dissolving the diethyl 2-(4-chlorobenzamido) malonate sample with an organic solvent to obtain a sample solution; and using reversed phase high performance liquidchromatography to detect the sample solution, where chromatographic conditions are as follows: a C18 chromatographic column is applied; a mobile phase A is 0.005-0.015M of a monopotassium phosphate aqueous solution having a pH value of 2-3; a mobile phase B is acetonitrile; and gradient elution is carried out. The method performs analysis with a reversed phase column using octadecylsilane chemically bonded silica as a filler, uses a specific saline solution and an organic solvent as mobile phases, and performs gradient elution for a sample solution of a 2-(4-chlorobenzoylamino)-3-[2(1H)-quinolinone-4-yl]propionic acid raw material. The method is rapid, simple, accurate, repeatable, and suitable for the control of many related substances and the study of impurities.
Owner:苏州正济药业有限公司
Synthesis method of ticagrelor
The invention discloses a synthesis method of ticagrelor. The method comprises the steps of 1, adding thiourea and alkali to a solution where bi-aminomalonic acid diethyl ester is dissolved, wherein the mole ratio of bi-aminomalonic acid diethyl ester, thiourea and alkali is 1:(1.0-1.5):(2-2.3), and performing a reaction under the protection of nitrogen at 25-100 DEG C for 5-72 h to obtain a compound 2; 2, adding bromopropane to a solution where the compound 2 is dissolved at -2 DEG C-2 DEG C, and conducting stirring at 25-50 DEG C for 2-72 h to obtain a compound 3; 3, adding organic alkali and a chloride agent to the compound 3, raising the temperature to 20-75 DEG C, and performing a reaction for 3-8 h to obtain a compound 4; 4, synthesizing ticagrelor, wherein the compound ticagrelor is obtained by conducting substitution, loop closing, substitution and a hydrolysis reaction on the compound 4 (4,6-dichloro-2-propylthiopyrimidine-5-amine), the operation steps are greatly simplified, and the yield is drastically increased. The synthesis method of ticagrelor is simple in operation and high in reaction yield.
Owner:JINGCHU UNIV OF TECH +1
Novel process for preparing acetamino diethyl malonate
InactiveCN1876621AReduce dosageReduce generationOrganic compound preparationCarboxylic acid amides preparationAcetic acidSolvent
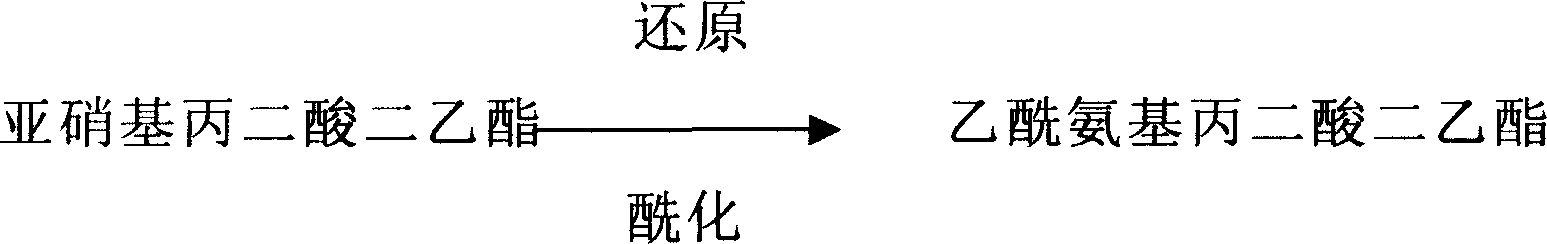
The invention relates the new technology of preparing acylated amino-compound, especially preparing acetamino-diethyl malonate with diethyl malonate. The method comprises the following steps: using diethyl malonate and sodium nitrite as raw material to prepare nitroso- diethyl malonate, mixing nitroso- diethyl malonate, acylating agent and solvent at the ratio of 1:1-2:1-2, adding reducer, stirring, after acylating, filtering to remove fouled catalyst, filter liquor carrying out vacuum distillation to recover acetic acid, when a great amount of crystals separate out, cooling, filtering, drying and getting product. The productivity of acetamino-diethyl malonate is above 95%. The technology is fit for mass production.
Owner:安徽省恒锐新技术开发有限责任公司
Polybasic nitrogen heterocyclic non-natural chiral amino acid and synthesis method thereof
ActiveCN108752253ASimple processSave raw materialsOrganic chemistry methodsBulk chemical productionAlkaneSynthesis methods
The invention relates to a polybasic nitrogen heterocyclic non-natural chiral amino acid and a synthesis method thereof. The amino acid can be applied to molecule building for antibiotic synthesis. According to the synthesis method, 2-aminodiethyl malonate and halogenated alkanes carry out substitution reactions, cyclization reactions, and decarboxylation reactions, and the reaction products are split to obtain the polybasic nitrogen heterocyclic non-natural chiral amino acid. The provided novel synthesis method has the advantages of simple synthesis route, low cost, convenient operation, andeasiness for commercial production, the chiral purity of obtained products is high, and the application prospect is good.
Owner:广西茵诺圣药业有限公司
Synthesis method of double different protected amino acids
InactiveCN109824547AImprove general performanceLow priceCarbamic acid derivatives preparationOrganic compound preparationSynthesis methodsMethanesulfonyl chloride
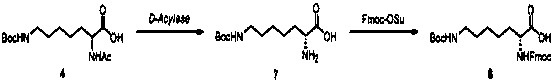
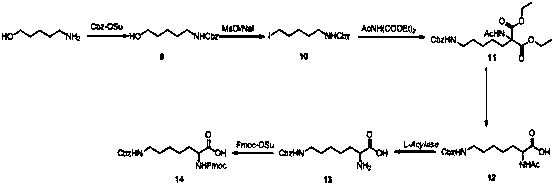
The invention relates to a synthesis method of double different protected amino acids.The technical problems of harsh reaction conditions, inapplicability of production enlarging and the like in an existing synthesis method are mainly solved. According to the technical scheme, the synthesis method of double different protected amino acids comprises the following steps: one of Boc20, Alloc-Cl or Cbz-Osuis added to amino alcohol under the action of an alkaline reagent to obtain a compound 1; the compound 1 reacts with methanesulfonyl chloride or paratoluensulfonyl chloride to obtain an intermediate, then a halide is added into acetone, heating and refluxing are executed to obtain a compound 2; the compound 2 is condensed with diethyl acetamidomalonate under the action of an alkaline agent togenerate a compound 3; the compound 3 is dissolved in alcohol and water, an inorganic base is added, heating, hydrolyzing and decarboxylating are executed to obtain a compound 4; acetylase is added into deionized water to obtain a compound 5 through enzymolysis; amino acid protection is executed, wherein one of Fmoc-Osu, Cbz-OSu, Alloc-Cl or Boc20 is added into thecompound 5 under the action of an alkaline agent to generatea target compound A.
Owner:KANGHUA SHANGHAI DRUG RES DEV CO LTD
Process for synthesizing rebamipide
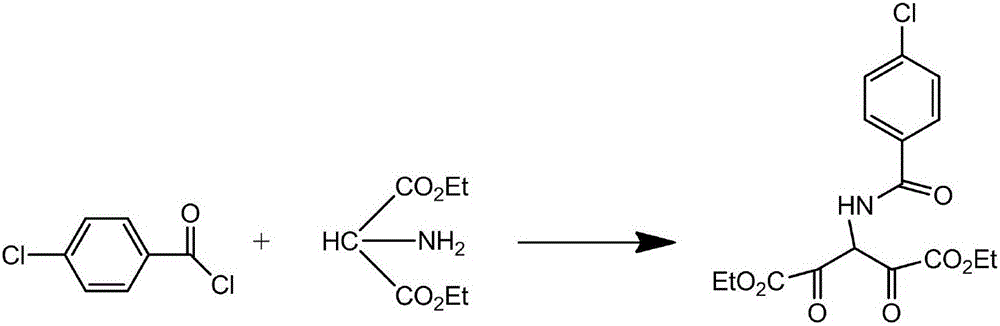
The invention provides a process for synthesizing rebamipide. The process comprises the following steps: taking aminomalonic acid diethyl ester and 4-chlorobenzoyl chloride as raw material substrates so as to prepare an intermediate 4-chlorobenzoyl diethyl aminomalonate; reacting with 4-bromomethylcarbostyril so as to obtain 2-(4-chlorobenzhylamino)-2-ethoxycarbonyl-3-[2(1H)-quinolinone-4-yl] ethyl propionate; and finally, heating and refluxing in a sodium alcoholate solution, concentrating, and re-crystallizing, thereby obtaining the final product. The rebamipide prepared by the process disclosed by the method is high in yield, and the purity can reach 99.2%.
Owner:大桐制药(中国)有限责任公司
Preparation method of 2-amino-4,6-dichloro-5-carboxamidopyrimidine
The invention relates to a method for preparing 2-amino-4,6-dichloro-5-formamido pyrimidine. The method comprises the following steps of: performing nitrosation on malonic acid diethyl ester and acetic acid serving as raw materials and sodium nitrite at first; then, performing reduction and formylation with formic acid in the presence of zinc powder to form formyl amino malonic acid diethyl ester; finally, performing condensation and cyclization with guanidine hydrochloride to produce 2-amino-4,6-dichloro-5-formamido pyrimidine; performing chlorination by using quaternary ammonium salt as a catalyst; fractionally performing hydrolysis under an alkali action to obtain a product. The method has easily-available raw materials, and is short in reaction time, simple in aftertreatment and high in hydrolysis selectivity; the cost is obviously reduced; the total yield is up to 74 percent and the purity of the product is up to 99.0 percent.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
Method for preparing L-serine-**N
InactiveCN101130503AIncrease profitOrganic compound preparationIsotope introduction to organic compoundsL serineDiethyl aminomalonate
The invention discloses a making method of L-serine-15N, which comprises the following steps: adopting Na15NO2 and diethyl malonate as raw material to synthesize acetamino-diethyl malonate-15N; using the obtained acetamino-diethyl malonate-15N to synthesize N-acetyl-DL-serine-15N; detaching through enzyme method to obtain the product with optical purity over 98. 5% and chemical purity over 98%. The invention improves the utility of raw material of 15N, which makes the abundance of 15N in the product over 98%.
Owner:SHANGHAI RES INST OF CHEM IND
Method for preparing 2-P-octyl-phenenl-2-amino-propanediol hydrochloride
InactiveCN100548968CEasy to manufactureEfficient manufacturingOrganic compound preparationAmino-hyroxy compound preparationMalonateHydrolysis
The invention provides a method for preparing 2-p-octylphenethyl-2-aminopropanediol hydrochloride: the method comprises the following steps: styrene (II) and octanoyl chloride carry out Friedel-Crafts reaction to generate p-octanoyl Styrene (III); (III) carries out Michael addition reaction with acetamidodiethyl malonate under alkali action, generates 2-to-octanoylphenethyl-2-acetamidodiethyl malonate (IV ); (IV) reacts with triethylsilane to generate 2-to-octylphenethyl-2-acetamidodiethyl malonate (V); (V) generates 2-to-octylphenethyl through reduction and hydrolysis Base-2-aminopropanediol (VI), then acidified with hydrochloric acid to form a salt to obtain the product of the present invention. The invention has the advantages of short route, low cost, short cycle, less pollution and high yield. At the same time, the preparation method of the intermediate in the above preparation process is also provided.
Owner:NANJING YOKO PHARMA GRP CO LTD
Synthetic method of monoamino inhibitor intermediate monoethyl 2-acetylamino-2-benzylmalonate
ActiveCN106946724ARaw materials are easy to getLow costCarbamic acid derivatives preparationOrganic compound preparationBenzoyl bromideTert-Butyloxycarbonyl protecting group
The invention discloses a synthetic method of a monoamino inhibitor intermediate monoethyl 2-acetylamino-2-benzylmalonate. The method comprises the following steps: 1, carrying out amino protection on a compound I diethyl aminomalonate and di-tert-butyl dicarbonate to obtain a compound II diethyl-2-Boc-aminomalonic acid; 2, reacting the compound II with benzyl bromide to obtain a compound III diethyl 2-(N-(tertbutyloxycarbonyl)amino)-2-benzylmalonate; 3, removing monoester from the compound III to obtain a compound IV monoethyl 2-(N-(tertbutyloxycarbonylamino)-2-benzyl-malonate; 4, carrying out amino deprotection on the compound IV to obtain a compound V monoethyl 2-amino-2-benzyl-malonate; and 5, carrying out an acetylation reaction on the compound V to prepare a compound VI monoethyl 2-(N-acetylamino)-2-benzyl-malonate. The method has the advantages of simple reactions, easily available raw materials, simple post-treatment, high yield, low cost, high operationality, and suitableness for industrial production.
Owner:苏州汉德创宏生化科技有限公司
Method for preparing intermediate diethyl acetamidomalonate through organic synthesis
InactiveCN103922959AReduce pollutionHigh purityOrganic compound preparationCarboxylic acid amides preparationAcetic anhydrideOrganic synthesis
The invention discloses a method for preparing an intermediate diethyl acetamidomalonate through organic synthesis. The preparation method is characterized by comprising the following steps of preparing oximinodiethyl malonate by using water as a solvent, using diethyl malonate, sodium nitrite and acetic acid as raw materials and adding a phase transfer catalyst; extracting oximinodiethyl malonate by using an organic solvent, and then distilling to remove the organic solvent; adding acetic acid into the system obtained in the third step, and adding zinc powder at 40-50 DEG C to perform reduction and acetic anhydride acylation; then removing the acetic acid solvent under reduced pressure, and crystallizing with water to obtain the diethyl acetamidomalonate product, wherein the reaction temperature of the reduction and acetic anhydride acylation is 40-50 DEG C, and the reaction time is 1-6.5 hours. The yield of diethyl acetamidomalonate obtained through the preparation method reaches over 80%, the purity of the product is high, the energy consumption is low, the environmental pollution is low, and the cost is low.
Owner:SUZHOU TIANMA SPECIALTY CHEM
Pyrrolecarboxylic acid derivative synthesis method
InactiveCN110357804ARaw materials are easy to getMild reaction conditionsOrganic chemistrySynthesis methodsCarboxylic acid
The invention discloses a pyrrolecarboxylic acid derivative synthesis method, which comprises: obtaining a group substituted formylacetone derivative by using a group substituted acetyl derivative andethyl acetate as raw materials; carrying out cyclization with diethyl aminomalonate hydrochloride to generate a pyrrolecarboxylic acid derivative ethyl ester; and carrying out a hydrolysis reaction to obtain the target product pyrrolecarboxylic acid derivative. According to the present invention, the method has characteristics of mild reaction conditions, simple purification, high yield and highproduct purity, and is suitable for process amplification and mass production.
Owner:上海毕得医药科技股份有限公司
Synthetic method for diethyl acetamidomalonate
ActiveCN107602408AImprove conversion rateReduce manufacturing costOrganic compound preparationCarboxylic acid amides preparationAcetic acidSolvent
The invention discloses a synthetic method for diethyl acetamidomalonate. The method comprises the following steps: firstly, performing a reaction in the presence of a metal salt and a ligand to prepare a mixed liquid containing the diethyl acetamidomalonate by taking diethyl malonate and acetamide as raw materials, air as an oxidant, and acetic acid as a solvent, performing distilling, and performing repeated extraction and recrystallization to obtain the diethyl acetamidomalonate in a white crystalline powder state. The method has the advantages that the metal salt and the ligand are cheap and easy to obtain, after the reaction is finished, the metal salt and the ligand can be further recycled through treatment, the air is taken as the oxidant, and therefore the production costs are greatly reduced; the yield of the product is more than 90%, the purity of the product is more than 99%, the conversion rates of the raw materials are high, and the reaction is complete; and the by-productis only water, no ''three waste'' (waste gas, waste water and solid waste) pollution problems exist, and the method meets environmental protection requirements.
Owner:NANTONG NABAIYUAN CHEM
Method for preparing beta-N-methylamino-L-alanine
ActiveCN109369442ALow costEasy to operateCarbamic acid derivatives preparationOrganic compound preparationMannich reactionAlkaline hydrolysis
The invention relates to a method for preparing beta-N-methylamino-L-alanine. The technical problems that the conventional preparation method is low in yield, long in route and difficult in chiral control and the like are mainly solved. According to the technical scheme of the invention, the method for preparing beta-N-methylamino-L-alanine comprises the following steps: carrying out a Mannich reaction on diethyl acetamidomalonate, N-methylbenzylamine and an aqueous solution of formaldehyde to obtain an intermediate I; performing alkaline hydrolysis on the intermediate I, and performing decarboxylation to obtain an intermediate 2; performing palladium carbon catalytic debenzylation on the intermediate 2 in the presence of Boc anhydride, and performing Boc protection to obtain an intermediate 3; reacting the intermediate 3 in the presence of L-acetylase so as to obtain an L-isomer, and performing Boc protection to obtain an intermediate 4; and performing Boc- deprotection on the intermediate 4 in the presence of hydrogen chloride, and forming hydrochloride, thereby obtaining the chemically pure and optically pure final product beta-N-methylamino-L-alanine.
Owner:KANGHUA SHANGHAI DRUG RES DEV CO LTD
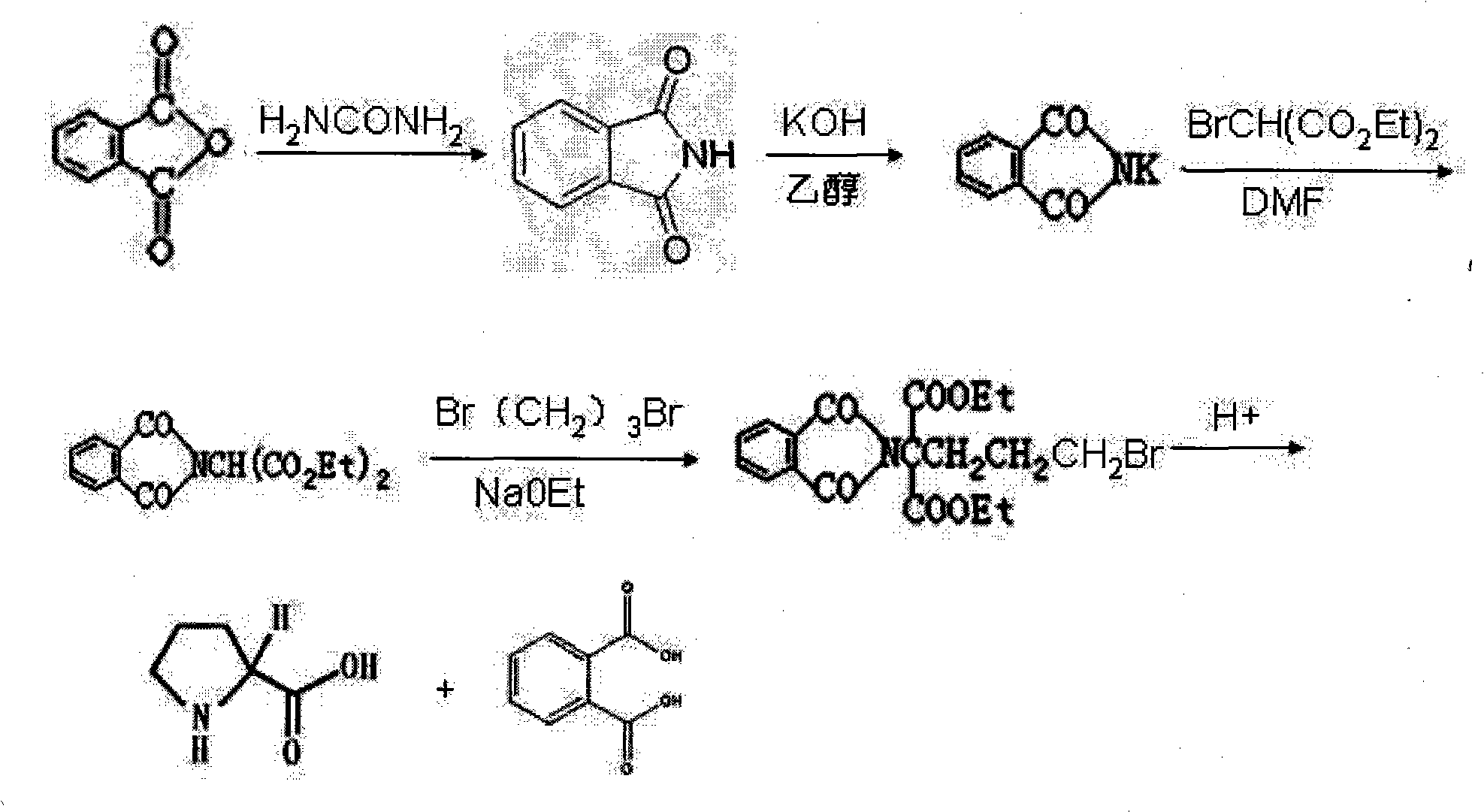
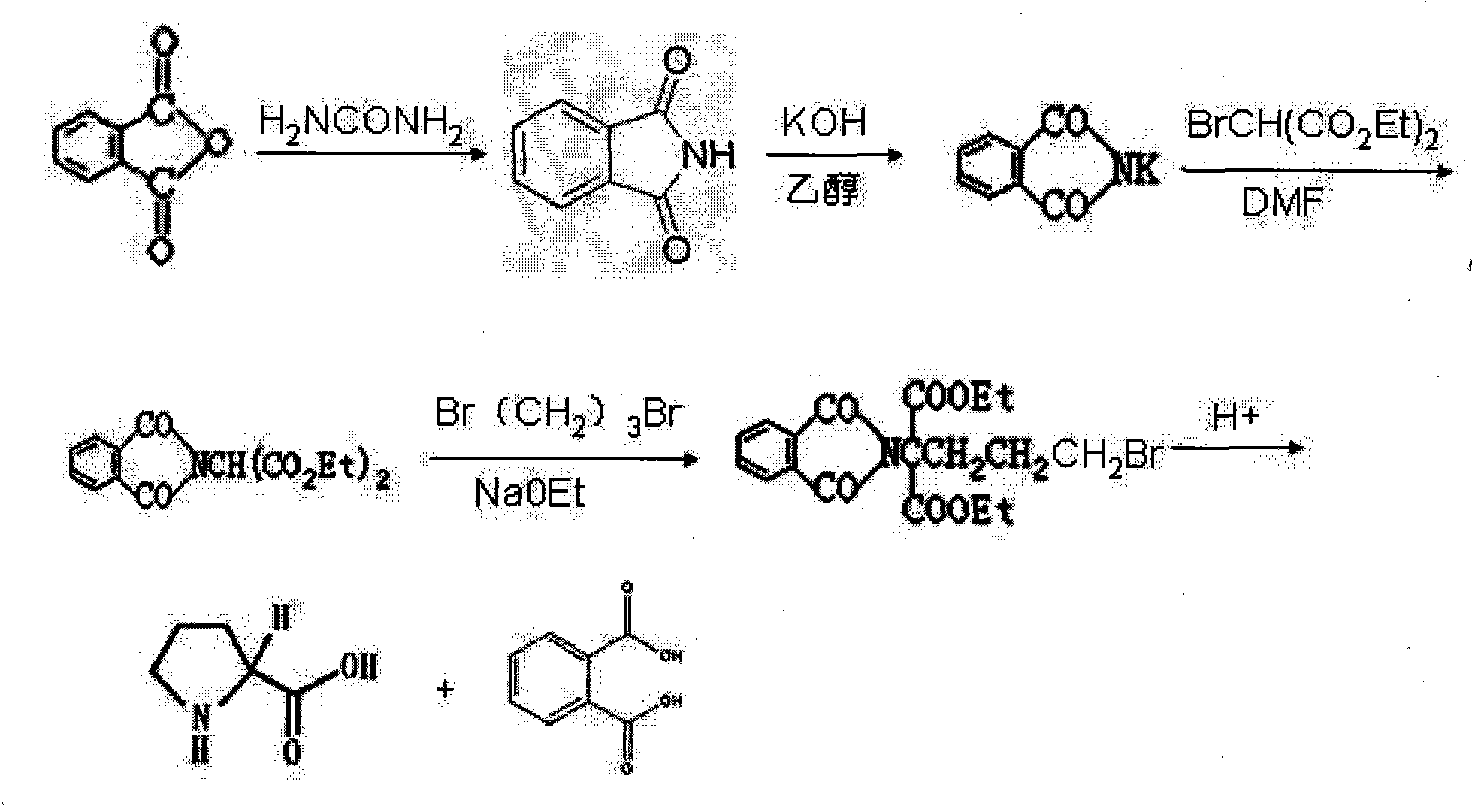
Preparation method of functional amino proline
ActiveCN101830842ALow costRaw materials are easy to getOrganic chemistryPotassiumPotassium hydroxide
The invention discloses a preparation method of functional amino proline. The invention adopts the technical scheme that phthalic anhydride and urea are reacted to obtain phthalimide, the obtained phthalimide is dissolved into ethanol, the mixture is regulated to alkalinity by potassium hydroxide to generate phthalimide potassium, the phthalimide potassium is reacted with bromodiethyl malonate to obtain phthalyl diethyl malonate, the phthalyl diethyl malonate is reacted with 1,3-dibromopropane to generate phthalyl-2-bromopropyl diethyl malonate, the phthalyl-2-bromopropyl diethyl malonate is dissolved into the ethanol to be regulated to the alkalinity, and then the mixture is reacted with acid to generate a product of proline. Compared with the traditional preparation method of the proline, the invention can be repeatedly utilized by adopting phthalic anhydride as raw materials, lower the cost and also save expensive sodium borohydride reducing agent needed by the traditional prepared proline, therefore, the invention has the advantages of low cost, simple process, high yield and high purity and is more beneficial to mass production.
Owner:卫仕宠物营养研究院(芜湖)有限公司
Preparation method of diethyl acetamidomalonate
InactiveCN104610082AReduce pollutionHigh product contentOrganic compound preparationCarboxylic acid amides preparationSodium acetateNitroso
The invention discloses a preparation method of diethyl acetamidomalonate. The preparation method comprises the following steps: adding diethyl malonate and sodium nitrite to organic solvent, dropwise adding acetic acid at the temperature of 0-5 DEG C, carrying out heat preservation on a reaction system for 10-15 h at the temperature of 35-45 DEG C after adding, filtering solid after the reaction, recycling the organic solvent after filter liquor is concentrated to obtain nitroso-diethyl malonate, adding the nitroso-diethyl malonate to acetic acid and acetic anhydride, slowly adding zinc powder to carry out reductive acylation reaction, carrying out heat preservation on feed liquid for 2 h at the temperature of 50-60 DEG C after the zinc powder is added, filtering zinc acetate generated by the reaction after the reductive acylation reaction, recycling the acetic acid after the filter liquor is concentrated, carrying out recrystallization on concentrate by using purified water, and drying to obtain the diethyl acetamidomalonate. The preparation method is low in production cost, sodium nitrite can react completely, and the sodium nitrite is not contained in byproduct sodium acetate generated by nitrosation reaction, so that subsequent treatment is facilitated.
Owner:NANTONG NABAIYUAN CHEM
Method of producing DL-tyrosine -**N
InactiveCN101153013AIncrease profitSimple methodOrganic chemistryRadioactive preparation carriersNitrogenIsotope
The present invention relates to a preparation method of DL-tyrosine-15 N; the Na15NO2 is used as nitrogen isotope to synthesize DL-tyrosine-15N. The method comprises: (1) the synthesis of) oximide malonic ester diethyl oximinomalonate-15N; (2) the synthesis of acetylamino malonic ester -15N; (3) the synthesis of 2-acetyl-2-(p-methoxybenzyl) malonic ester (p-p) Diethyl - 15; (4) the synthesis of DL-tyrosine- SUP>15N; and (5) the purification of DL-tyrosine- SUP>15N. compared with the prior art, the present invention is simple in method, simple in device, high in utilization rate of raw materials, high in collection rate of product D-tyrosine-15 N, high in chemical purity and of no decrease in abundance.
Owner:SHANGHAI RES INST OF CHEM IND
Preparation of medical intermediate AMD by electro-reduction
InactiveCN101100759BReduce dosageReduce generationElectrolysis componentsElectrolytic organic productionSodium acetateAcetic acid
Owner:安徽省恒锐新技术开发有限责任公司
A kind of synthetic method of ticagrelor
The invention discloses a synthesis method of ticagrelor. The method comprises the steps of 1, adding thiourea and alkali to a solution where bi-aminomalonic acid diethyl ester is dissolved, wherein the mole ratio of bi-aminomalonic acid diethyl ester, thiourea and alkali is 1:(1.0-1.5):(2-2.3), and performing a reaction under the protection of nitrogen at 25-100 DEG C for 5-72 h to obtain a compound 2; 2, adding bromopropane to a solution where the compound 2 is dissolved at -2 DEG C-2 DEG C, and conducting stirring at 25-50 DEG C for 2-72 h to obtain a compound 3; 3, adding organic alkali and a chloride agent to the compound 3, raising the temperature to 20-75 DEG C, and performing a reaction for 3-8 h to obtain a compound 4; 4, synthesizing ticagrelor, wherein the compound ticagrelor is obtained by conducting substitution, loop closing, substitution and a hydrolysis reaction on the compound 4 (4,6-dichloro-2-propylthiopyrimidine-5-amine), the operation steps are greatly simplified, and the yield is drastically increased. The synthesis method of ticagrelor is simple in operation and high in reaction yield.
Owner:JINGCHU UNIV OF TECH +1
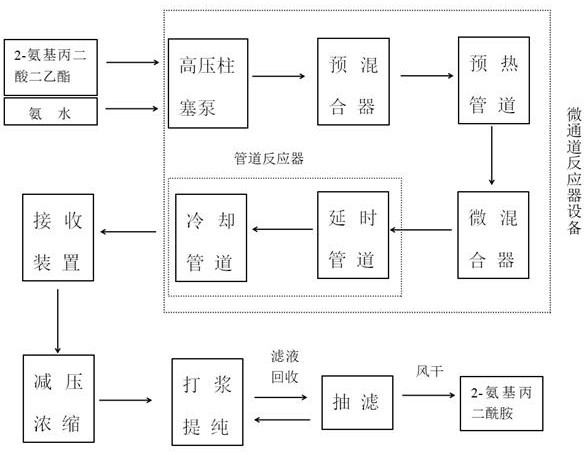
Continuous preparation method of 2-aminomalonamide
PendingCN113429310AShort reaction timeImprove reaction efficiencyOrganic compound preparationCarboxylic acid amides preparationMalonic acidOrganic synthesis
The invention discloses a continuous preparation method of 2-aminomalonamide, and belongs to the field of organic synthesis. The method comprises the steps of by taking diethyl 2-aminomalonate as a raw material and ammonia water as an ammonolysis reagent, reacting through a micro-channel reactor at a certain temperature and pressure, and carrying out vacuum concentration to obtain 2-aminomalonamide. The invention provides a continuous preparation process method of 2-aminomalonamide. The method is simple, continuous production can be realized, the reaction time is short, the product yield reaches 80-90%, and the productivity is improved.
Owner:DALIAN UNIV OF TECH
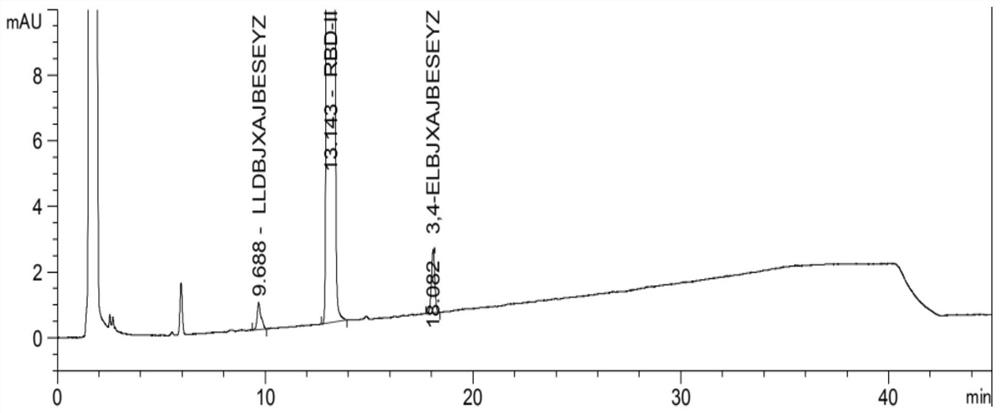
A kind of detection method of related substances in diethyl p-chlorobenzamidomalonate sample
ActiveCN110426463BSuitable for controlSuitable for researchComponent separationChlorobenzenePhosphate
The invention relates to the technical field of drug analysis, in particular to a method for detecting related substances in a sample of diethyl p-chlorobenzamidomalonate. The method comprises: dissolving a sample of diethyl p-chlorobenzamidomalonate in an organic solvent to obtain a sample solution; using reverse-phase high-performance liquid chromatography to detect the sample solution, and the chromatographic conditions are as follows: C18 chromatographic column; Phase A is 0.005-0.015 M potassium dihydrogen phosphate aqueous solution, pH value is 2-3; mobile phase B is acetonitrile; gradient elution. The present invention uses octadecylsilane-bonded silica gel as filler for reverse-phase chromatographic column analysis, and uses specific salt solution and organic solvent as mobile phase to carry out gradient elution to the sample solution of rebamipide raw material. The method is fast, simple, accurate and reproducible, and is suitable for the control of various related substances and the research of impurities.
Owner:苏州正济药业有限公司
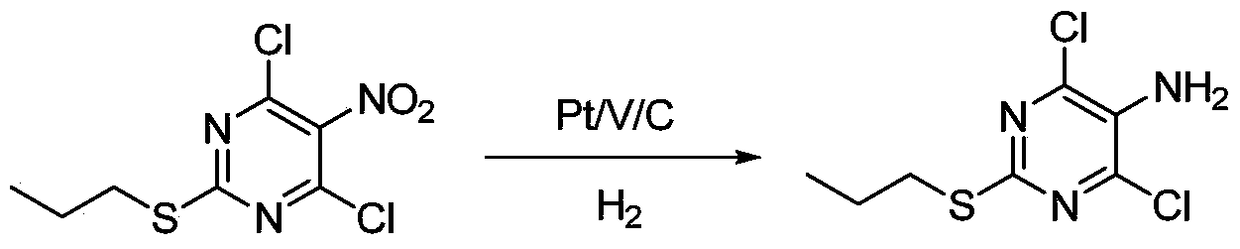
A method for synthesizing 4,6-dichloro-2-(propylthio)-5-aminopyrimidine
Owner:XIAMEN MEDICAL COLLEGE
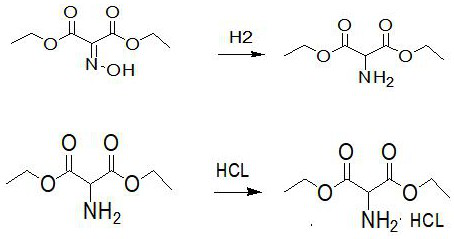
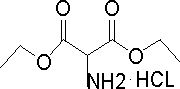
Preparation method of diethyl aminomalonate hydrochloride
PendingCN113735728AAvoid the disadvantages of expensive, easy poisoning and inactivationThe reaction conditions are mild and safeOrganic compound preparationAmino-carboxyl compound preparationPtru catalystNitration
The invention discloses a preparation method of aminomalonic acid diethyl ester hydrochloride, which comprises the following steps: 1, carrying out nitrosation on malonic acid diethyl ester in acetic acid by using a nitrous acid aqueous solution to obtain oximido malonic acid diethyl ester; 2, carrying out catalytic hydrogenation reaction on the oximido diethyl malonate in an alcohol solvent by using a nickel-containing three-way catalyst to obtain aminodiethyl malonate; 3, filtering out the catalyst from the hydrogenated liquid, salifying the compound by using hydrogen chloride ethanol under cooling, desolventizing the solution, and crystallizing the liquid by using acetone to obtain the diethyl aminomalonate hydrochloride. The method has the characteristics of milder and safer reaction conditions, simplicity and convenience in operation, high yield, low cost, good quality and the like, and has a wide application prospect. In addition, according to the hydrogenation technology used in the method, waste residues, waste acid and the like generated by reduction of zinc powder are avoided, and the defects that a palladium-carbon catalyst is high in price and prone to poisoning and inactivation are overcome.
Owner:SUZHOU JINGYE MEDICINE & CHEM
Method for preparing L-serine-15N
InactiveCN101130503BIncrease profitOrganic compound preparationAmino-carboxyl compound preparationL serineDiethyl aminomalonate
Owner:SHANGHAI RES INST OF CHEM IND
Synthesis method of diethyl acetamidomalonate
ActiveCN113121378AHigh yieldOrganic compound preparationCarboxylic acid amides preparationPotassium borohydrideAcetic anhydride
The invention discloses a synthesis method of diethyl acetamidomalonate, which comprises the steps of sequentially adding glacial acetic acid and a sodium nitrite solution into diethyl malonate, carrying out heat preservation reaction, standing for layering, and taking an oil layer solution; and sequentially adding glacial acetic acid, potassium borohydride, methanol and acetic anhydride into the oil layer solution, stirring to react, washing, and distilling to obtain the diethyl acetamidomalonate. The potassium borohydride reducing agent is introduced, so that the yield of the diethyl acetamidomalonate obtained by the reaction is obviously improved.
Owner:湖北宇阳药业有限公司
Preparation method of functional amino proline
ActiveCN101830842BLow costRaw materials are easy to getOrganic chemistryPotassiumPotassium hydroxide
Owner:卫仕宠物营养研究院(芜湖)有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
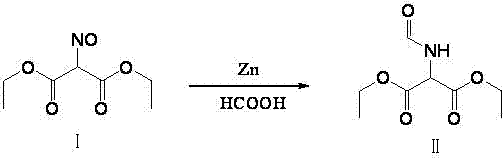
![Method for preparing 2-amino-2-[2-(4-alkyl phenyl) ethyl]-1,3-propanediol hydrochloride Method for preparing 2-amino-2-[2-(4-alkyl phenyl) ethyl]-1,3-propanediol hydrochloride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ac7b744b-5fc2-45f8-af7d-47b3b0805817/BDA00002058578400011.PNG)
![Method for preparing 2-amino-2-[2-(4-alkyl phenyl) ethyl]-1,3-propanediol hydrochloride Method for preparing 2-amino-2-[2-(4-alkyl phenyl) ethyl]-1,3-propanediol hydrochloride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ac7b744b-5fc2-45f8-af7d-47b3b0805817/BDA00002058578400021.PNG)
![Method for preparing 2-amino-2-[2-(4-alkyl phenyl) ethyl]-1,3-propanediol hydrochloride Method for preparing 2-amino-2-[2-(4-alkyl phenyl) ethyl]-1,3-propanediol hydrochloride](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ac7b744b-5fc2-45f8-af7d-47b3b0805817/BDA00002058578400031.PNG)