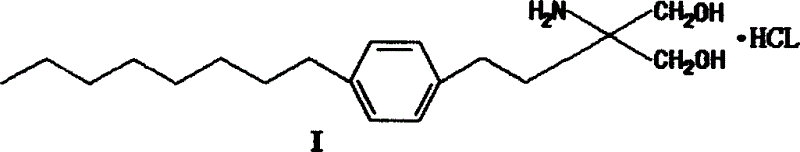
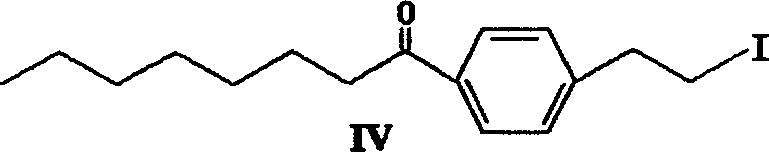
Method for preparing 2-para octylphenyl ehtyl-2-amino propanediol
A technology of aminopropanediol and octylphenethyl, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxy compounds, etc., can solve the problems of labor protection, high price, low yield, etc., and achieve low price. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
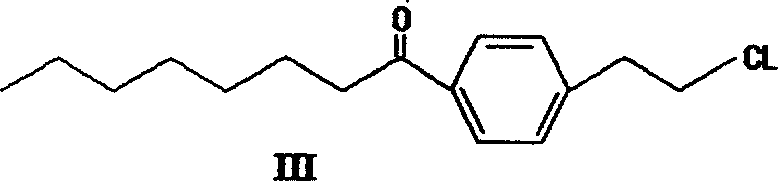
[0046] One. The preparation of compound p-octanoyl chloride ethylbenzene (III):
[0047] Under the cooling of cooling water, after putting 365.6g (2.25mol) of octanoyl chloride into the reactor, control the inner temperature not to exceed 50°C and put in 118g of anhydrous aluminum trichloride in batches, stir for 30 minutes after adding, and cool the temperature with ice-salt water. At -2°C, 140.5 g (1 mol) of chloroethylbenzene (II) was slowly added dropwise. After the addition, keep warm for 4 hours, pour into hydrochloric acid for hydrolysis, extract twice with chloroform, and combine the chloroform layers. The organic layer was washed twice with 10% hydrochloric acid, the chloroform layer was separated, washed with saturated brine until neutral, dried with anhydrous sodium sulfate, and concentrated to obtain about 252 g of p-octanoyl chloride ethylbenzene (III) as a red oil. The rate is 94%.
[0048] 1 H NMR (CDCl 3 )δ: 7.89(d, J=9.6Hz, 2H), 7.26(d, J=9.6Hz, 2H), 4.15~...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com