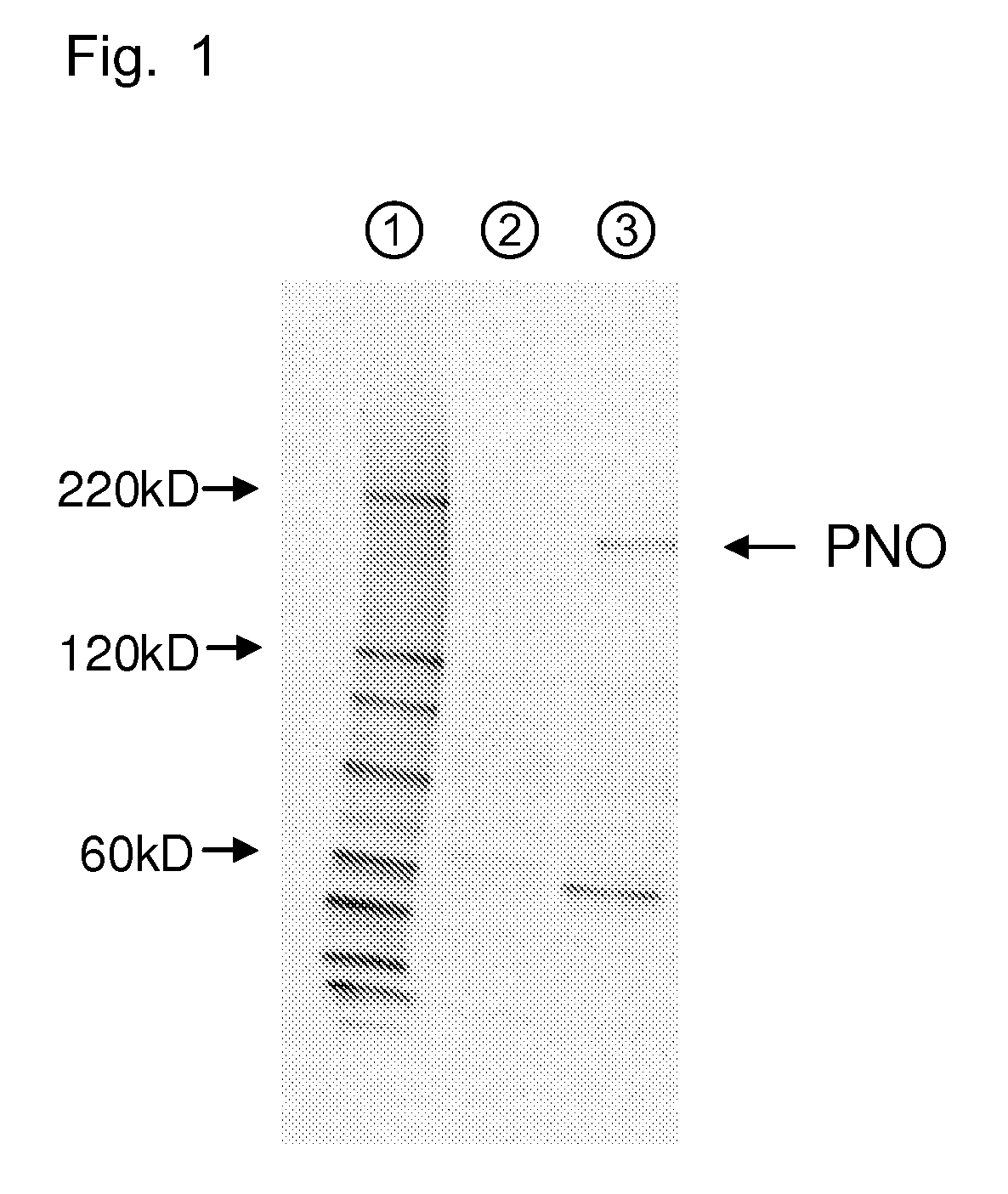
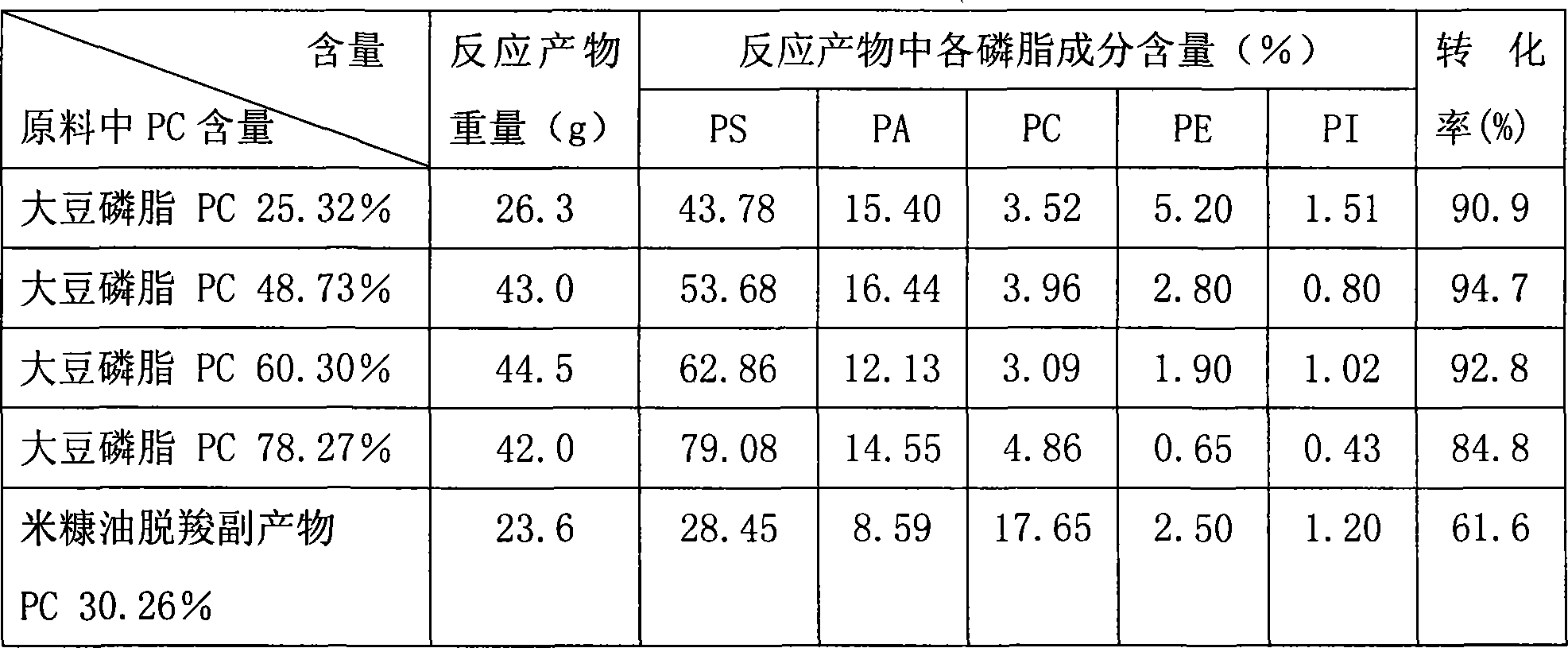
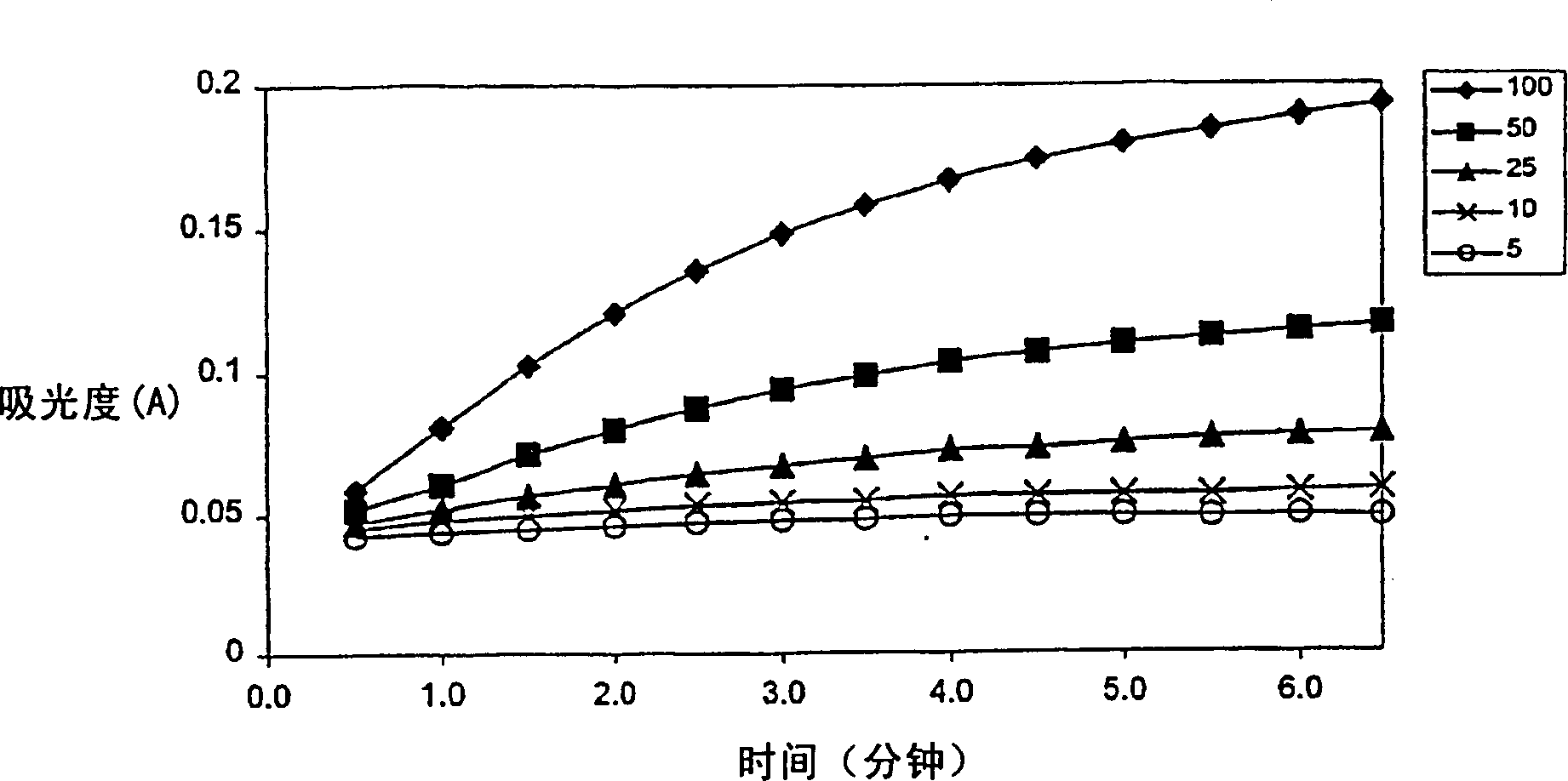
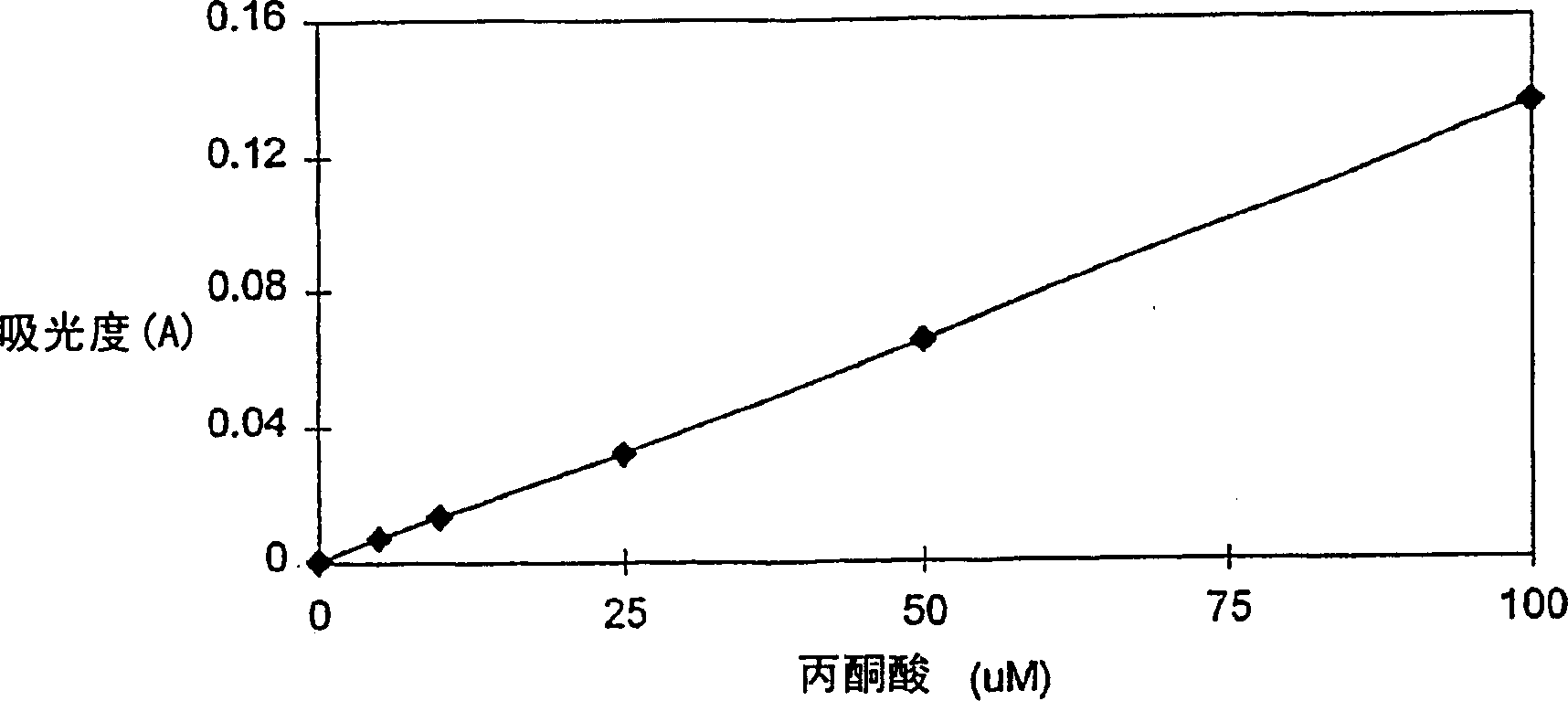
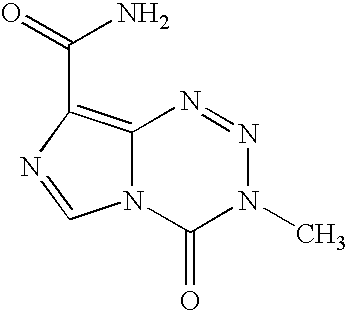
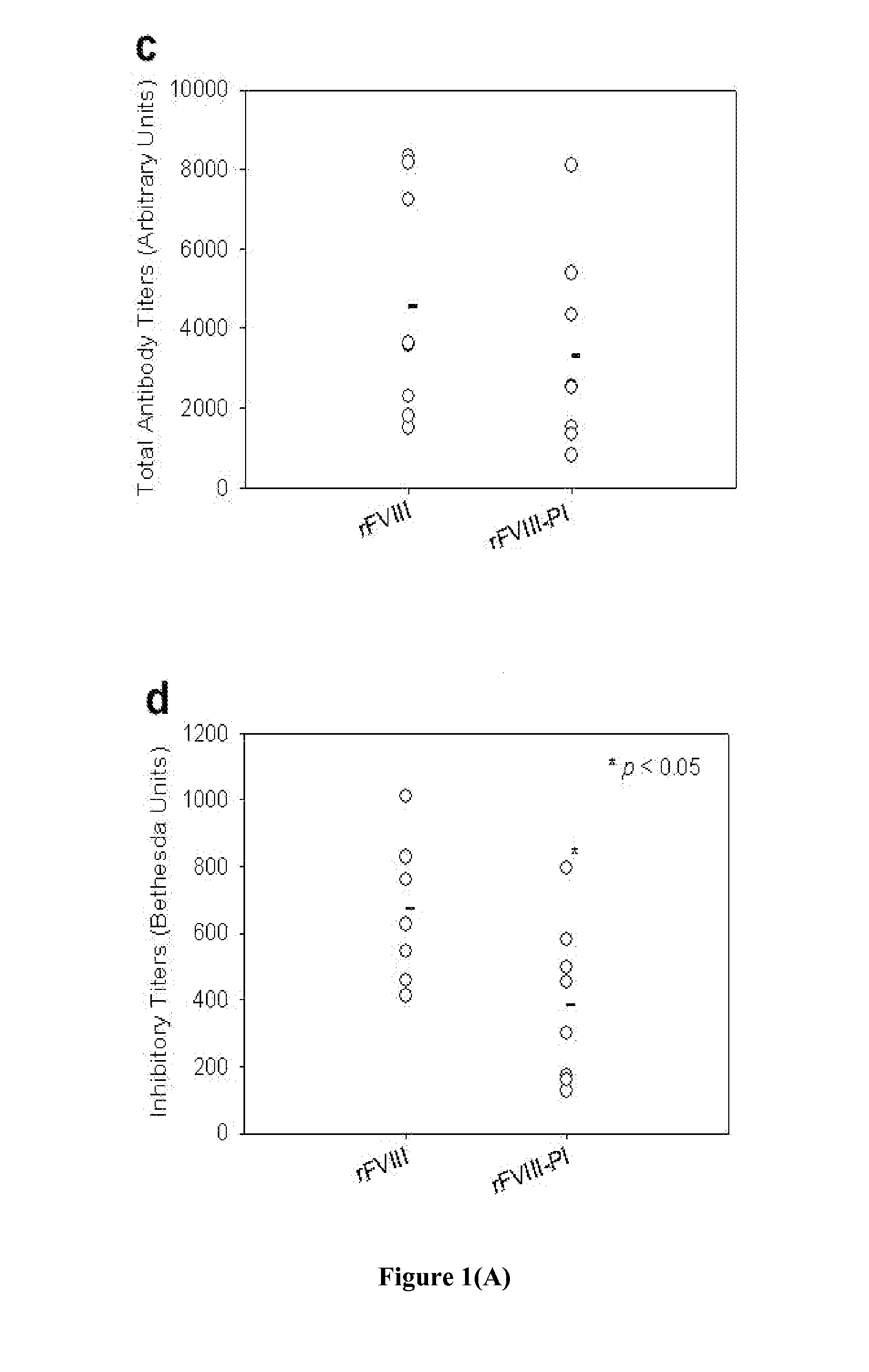
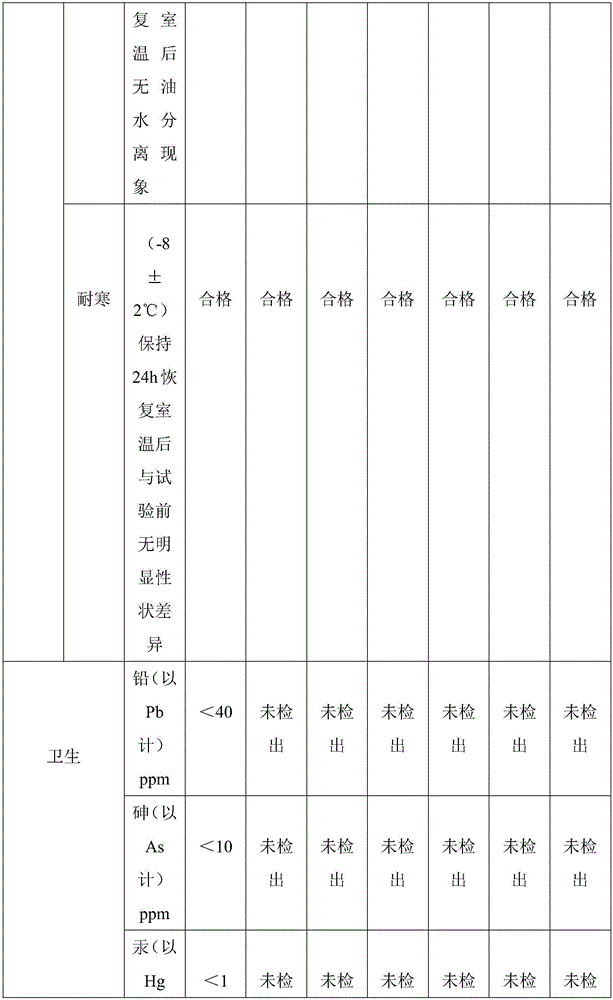
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
234 results about "L serine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Composition for an in vitro fertilization medium
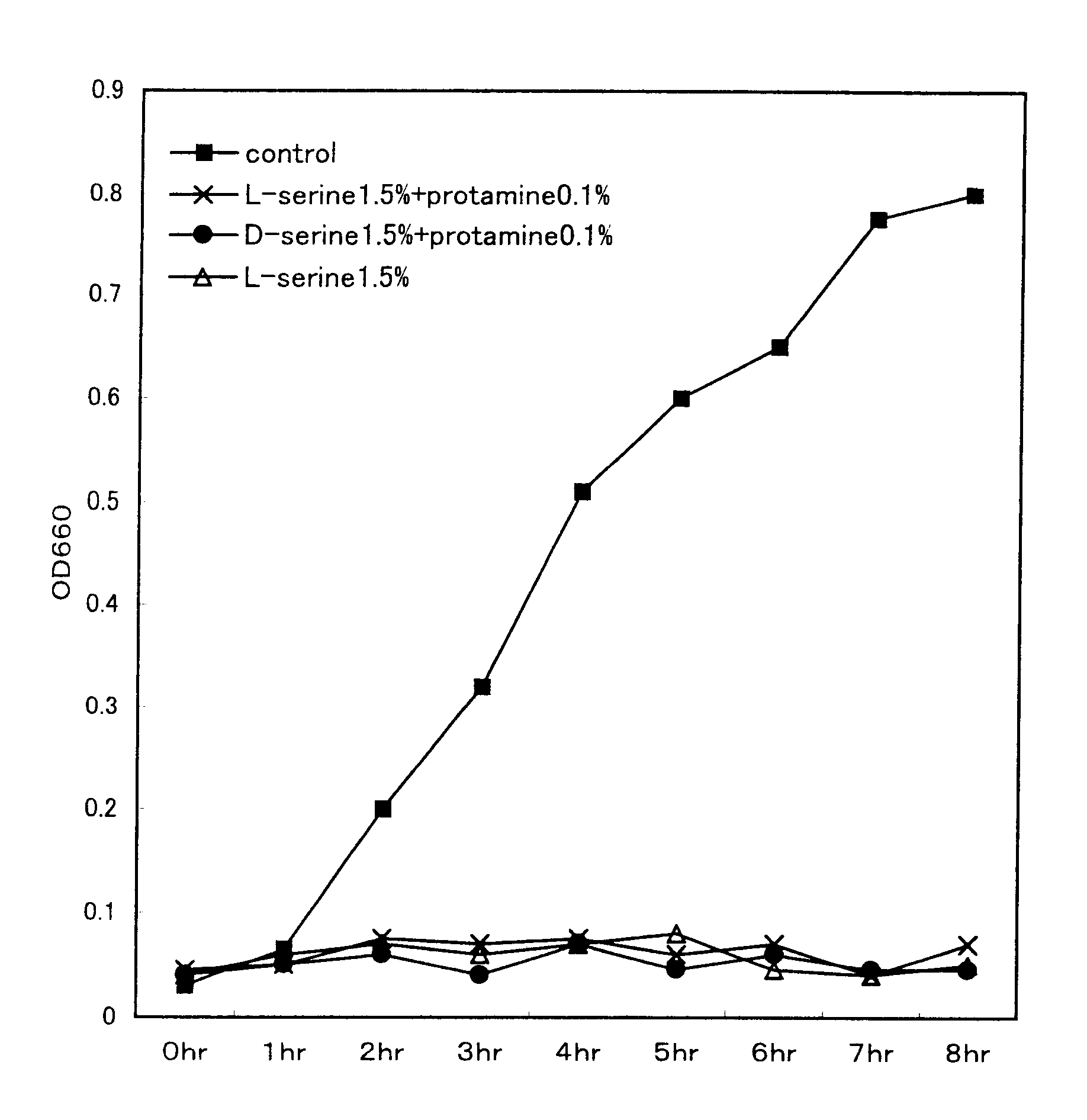
InactiveUS6130086AImprove stabilityIncrease stimulationCulture processMedical devicesArginineTryptophan
PCT No. PCT / JP96 / 02503 Sec. 371 Date Mar. 2, 1998 Sec. 102(e) Date Mar. 2, 1998 PCT Filed Sep. 4, 1996 PCT Pub. No. WO97 / 08946 PCT Pub. Date Mar. 13, 1997The present invention aims to provide a medium composition for in vitro fertilization, in particular, a composition usable in the culture of ova or early embryos which are fertilized eggs, the preparation or culture of sperm, and the pre-treatment of ova or sperm. The composition comprises, as its essential components, L-phenylalanine, L-tryptophan, L-lysine, L-threonine, L-valine, L-methionine, L-isoleucine, L-leucine, L-proline, glycine, L-alanine, L-tyrosine, L-histidine, L-arginine, L-taurine, L-aspartic acid, L-serine, L-asparagine, L-glutamic acid, L-glutamine and L-cystine, provided that at least a part of the L-cystine may be replaced by L-cysteine.
Owner:FUSO PHARMA INDS
Pharmaceutical formulations of antineoplastic agents and processes of making and using the same
In its several embodiments, this invention discloses a pharmaceutical formulation comprising at least one antineoplastic agent or a pharmaceutically acceptable salt thereof, and at least one dissolution enhancing agent sufficient to substantially dissolve said at least one antineoplastic agent in at least one aqueous diluent, wherein said dissolution enhancing agent is urea, L-histidine, L-threonine, L-asparagine, L-serine, L-glutamine or mixtures thereof; a lyophilized powder comprising said pharmaceutical formulation, and articles of manufacture thereof.
Owner:MERCK SHARP & DOHME LLC
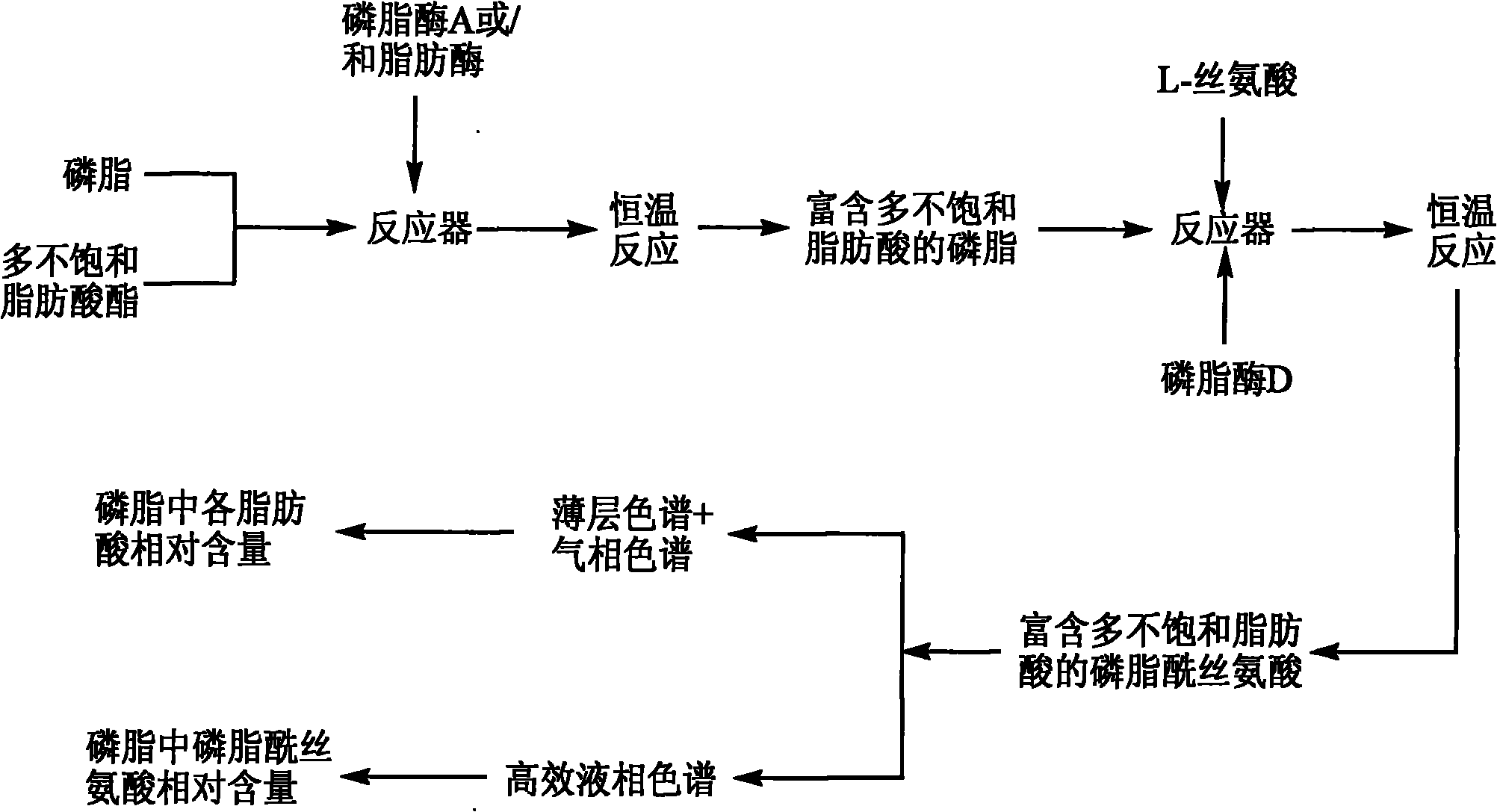
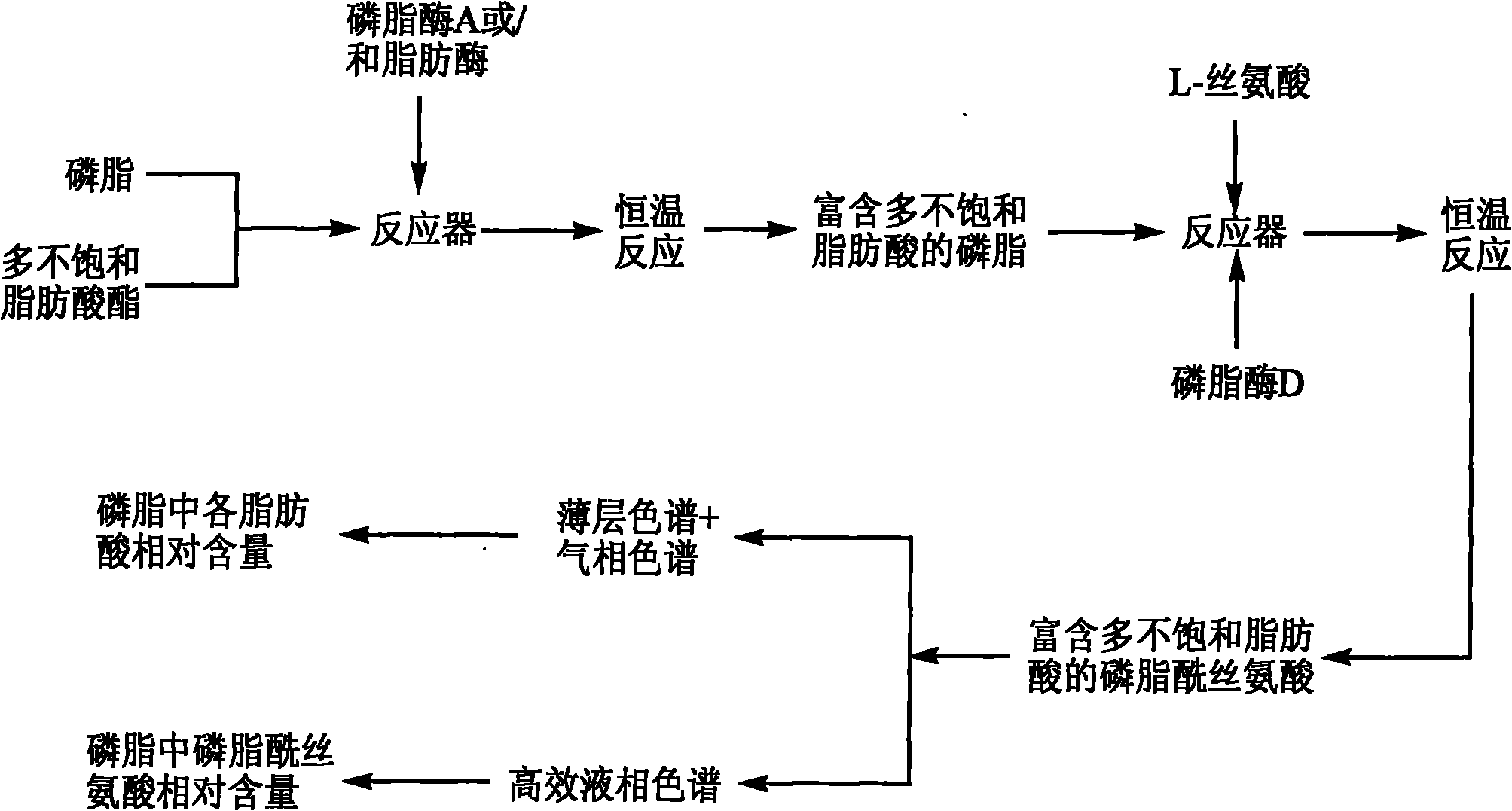
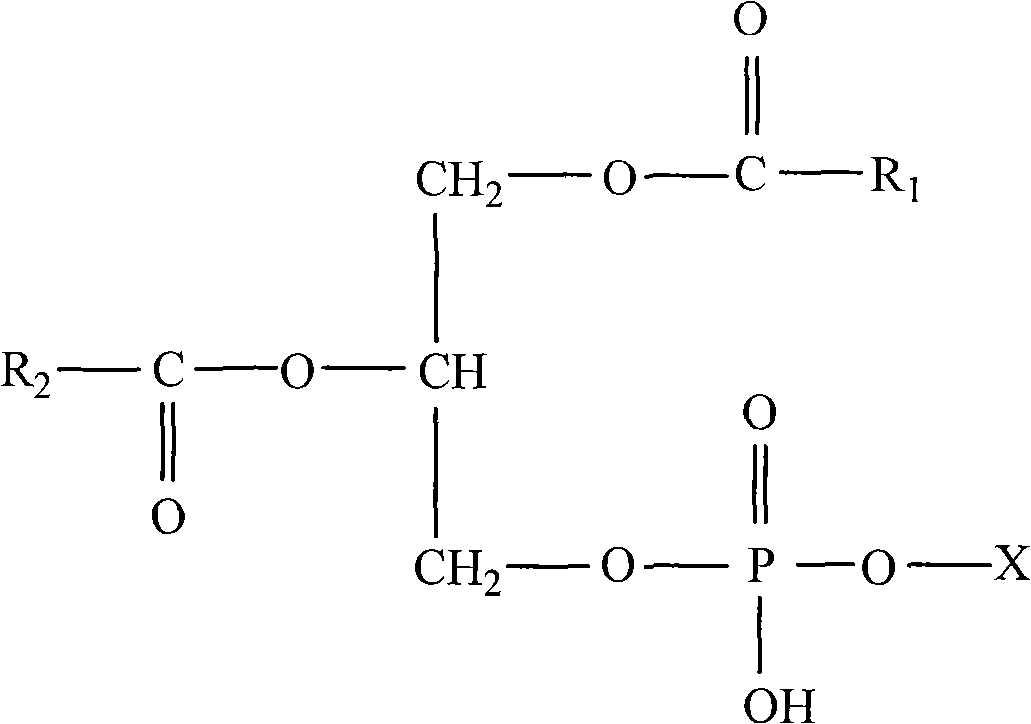
Method for preparing phosphatidylserine abundant in polyunsaturated fatty acid
InactiveCN101818179ANo emissionsImprove product qualityMicroorganism based processesFermentationUnsaturated fatty acid esterPhospholipid
The invention relates to a method for preparing phosphatidylserine abundant in polyunsaturated fatty acid and belongs to the technical field of bioengineering. The method is characterized by comprising the following steps of: firstly, catalyzing ester exchange reaction between phosphatide and polyunsaturated fatty acid ester by utilizing one or a mixture of phosphatidase A and lipase to generate phosphatide abundant in polyunsaturated fatty acid; and then catalyzing phosphor-transfer esterification reaction between the phosphatide abundant in the polyunsaturated fatty acid and L-serine by utilizing phosphatidase D to generate the phosphatidylserine abundant in the polyunsaturated fatty acid. The method has the advantages of no discharge of waste water, good product quality, no solvent residue, safe process operation, few reaction byproducts, no waste generation, cost reduction, simple production process and easy realization of scale production because of utilizing two enzymes to perform sub-step catalysis and perform reaction in the same reactor, and completing the reaction process in a non-solvent system. Therefore, the invention provides a good and feasible method for preparing the phosphatidylserine abundant in the polyunsaturated fatty acid.
Owner:DALIAN UNIV OF TECH
Amino acid producing microorganism and a method for producing an amino acid
ActiveUS20090068712A1Efficient productionImprove productivityBacteriaOxidoreductasesTryptophanTert-leucine
A microorganism is provided which has an ability to produce an L-amino acid such as L-lysine, L-tryptophan, L-phenylalanine, L-valine, L-leucine, L-isoleucine and L-serine, and has been modified to increase the activity of pyruvate synthase or pyruvate:NADP+ oxidoreductase. This microorganism is cultured in a medium containing ethanol or an aliphatic acid as the carbon source to produce and accumulate the L-amino acid in the medium or cells, and the L-amino acid is collected from the medium or the cells.
Owner:AJINOMOTO CO INC
Method for preparing powdered phosphatidyl serine
The invention relates to a method of preparing compounds, in particular to a method of preparing powder phosphatidylserine. The invention has to overcome the problems that the prior art inputs a lot of equipment and costs a lot, and the transformation ratio of the reaction is hard to be guaranteed. The invention has the technical proposal that a method of preparing powder phosphatidylserine takes natural lecithin as the reaction substrate; and after being separated by water, the reaction substrate can be added with an inorganic system prepared by L-serine, calcium salt and buffer solution to produce rough product under the effect of phospholipase D. Concentrate can be obtained by conducting the solvent extraction to the rough product, and then can be purified by solvent to obtain phosphatidylserine. The invention is characterized in that to separate the reaction substrate needs water three to ten times (w / v) of reaction substrate; and the separating temperature is ranged from 20 DEG C to 60 DEG C. In the system, the weight of the L-serine is two to ten times of the reaction substrate, and the weight of water used in the inorganic system is three to ten times(w / v) of reaction substrate. Ph value of the inorganic system can be adjusted between 4.5 and 6.0 by the buffer solution.
Owner:杨凌萃健生物工程技术有限公司 +1
Enzymatic cycling assays for homocysteine and cystathionine
InactiveCN1612937APrecise and consistent resultsAccurate multi-point calibration curveMicrobiological testing/measurementEnzymesIsolation proceduresChemistry
The present invention provides an enzymatic cycling assay for assessing the amount of homocysteine and / or cystathionine in a solution such as blood, blood derivatives, or urine. The assay comprises the steps of contacting the solution containing homocysteine and / or cystathionine to form a reaction mixture, with CBS, or a derivative thereof, L-serine, and CBL, or a derivative thereof, for a time period sufficient to catalyze the cyclical conversion of homocysteine form to cystathionine and the reconversion of cystathionine to homocysteine with the production of pyruvate and ammonia; determining the amount of homocysteine and / or ammonia present in the reaction mixture; and determining the amount of homocysteine and / or cystathionine present in the solution based on the amount of pyruvate and / or ammonia formed. Expression vectors and isolation procedures for CBS, or derivatives thereof, and CBL, or derivatives thereof, are also provided as well as test kits for carrying out the assay. In preferred embodiments, the assays can be conducted in 15 minutes or less, with a minimum of enzyme usage.
Owner:AXIS SHIELD DIAGNOSTICS
Medicine composition containing 15 kinds of amino acid and preparation method thereof
ActiveCN101357118ASolve the problem of trace oxygenSolve the problem of oxygen incorporationOrganic active ingredientsPharmaceutical delivery mechanismArginineTryptophan
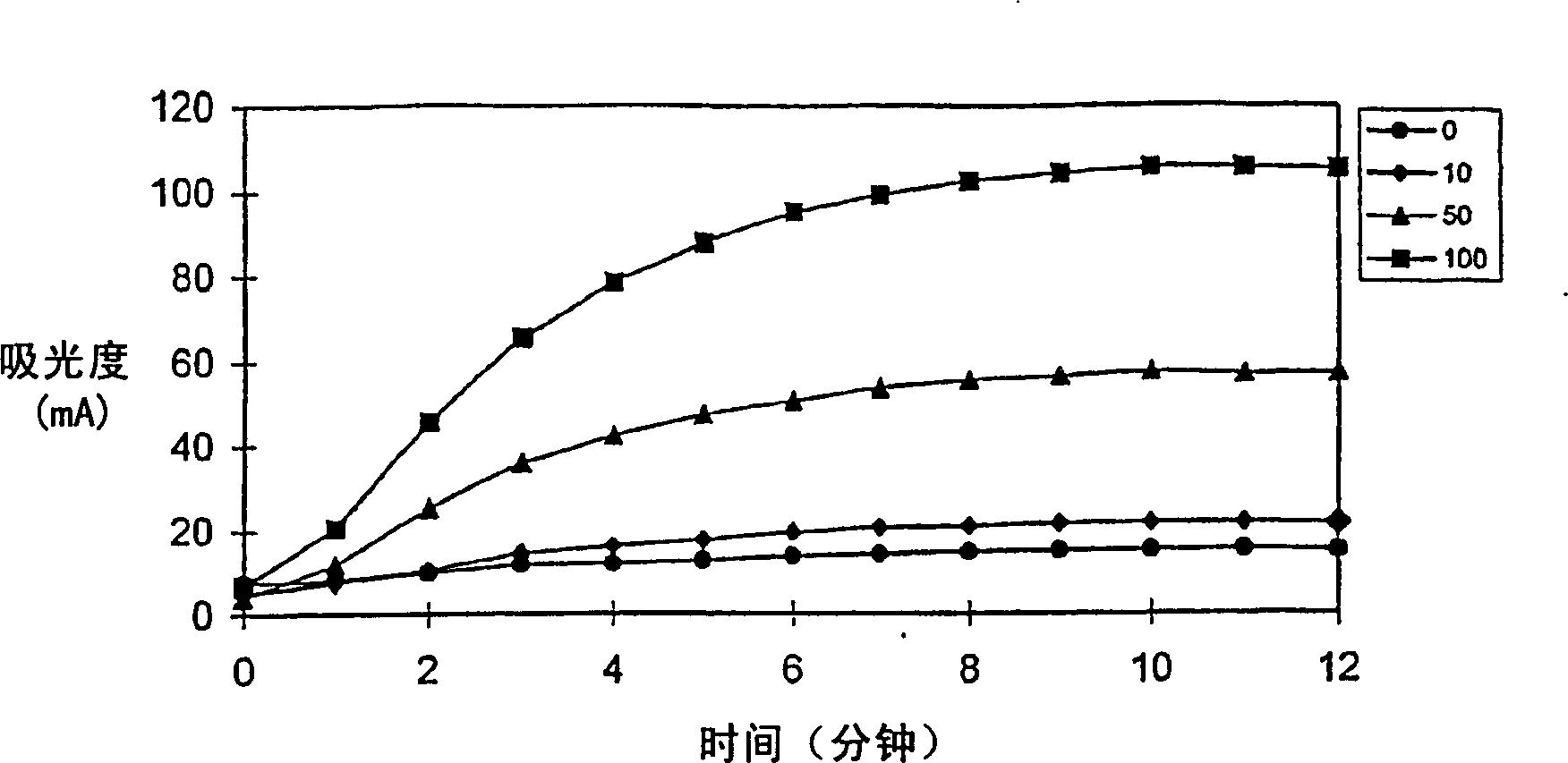
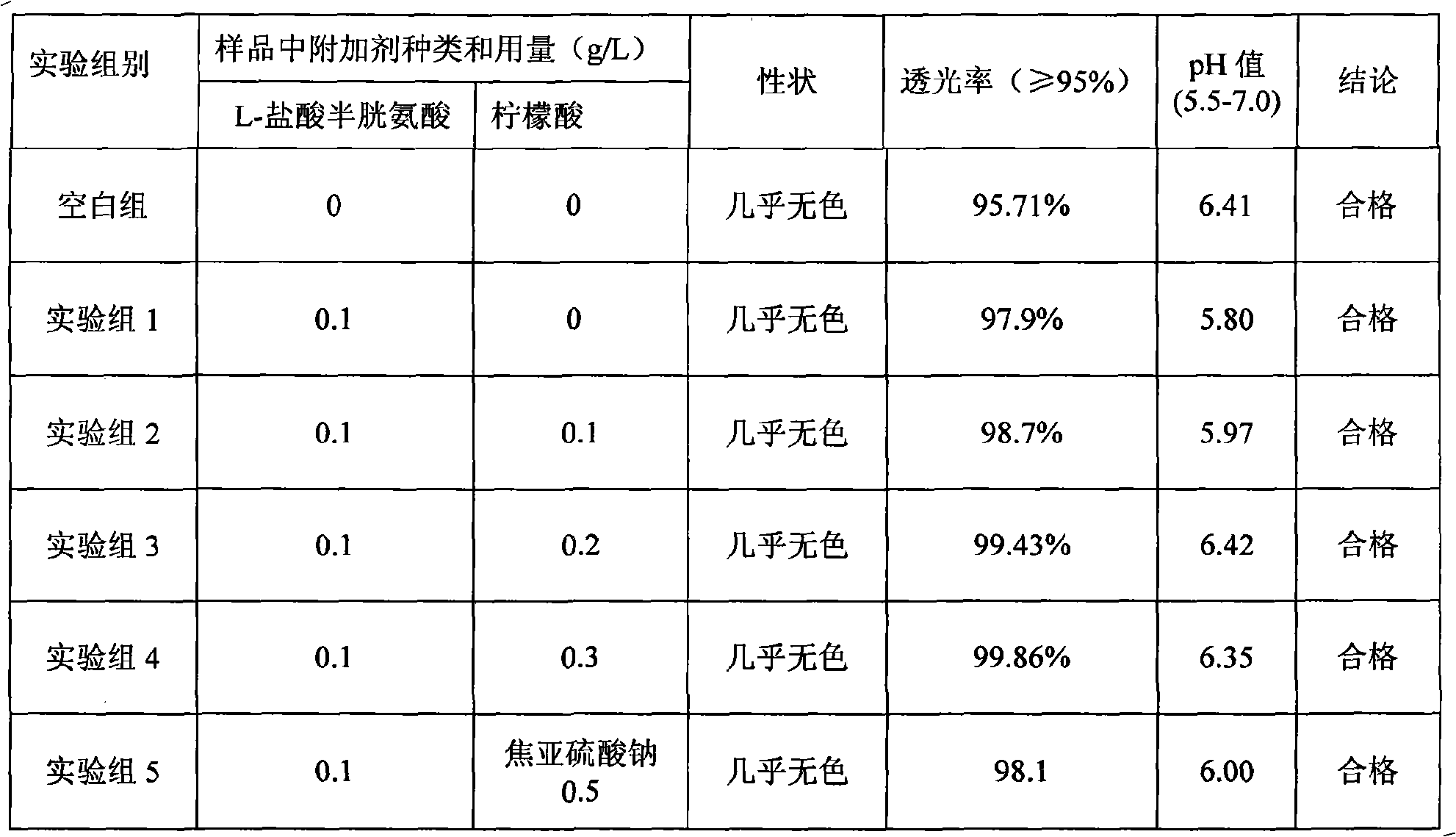
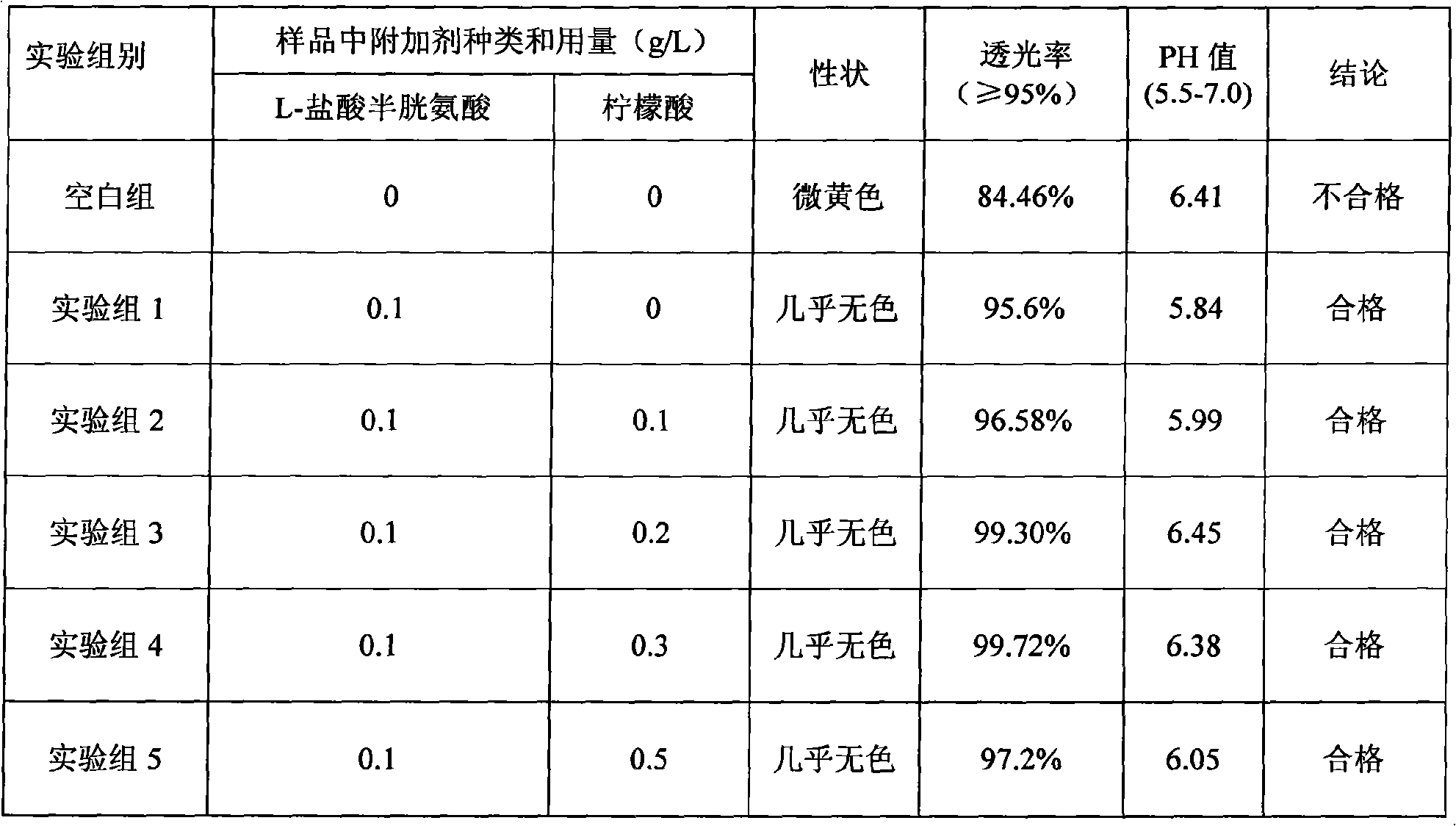
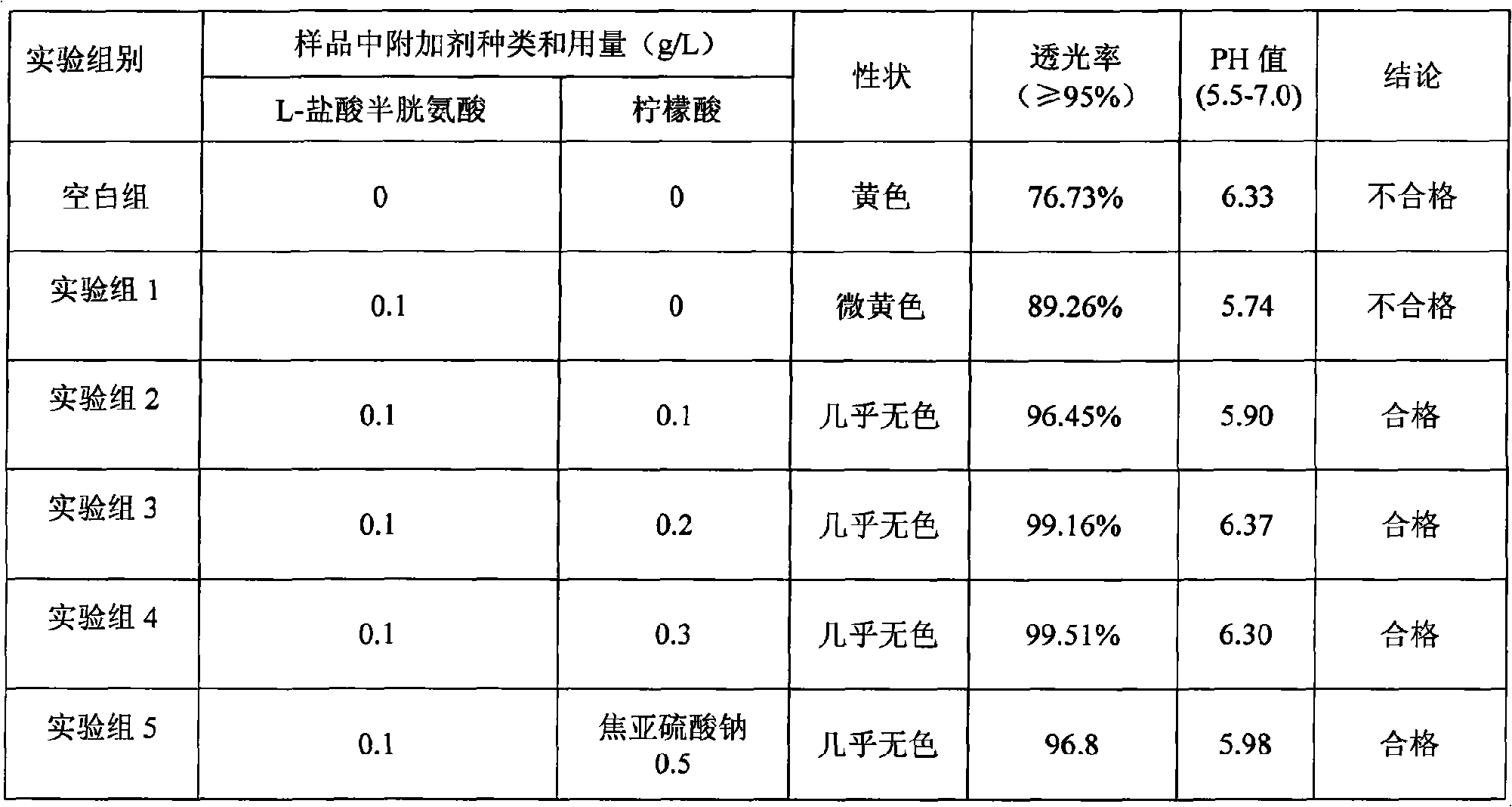
The invention discloses a medicine combination which contains 15 amino acids and the preparation method thereof; the medicine combination is characterized in that a compound amino acid injecta with different concentrations are prepared by the 15 amino acids which serve as raw materials and admixture according to the following parts by weight: 6.1-10.8 parts of L-Isoleucine, 8.8-16.6 parts of L-Leucine, 4.6-10.4 parts of L-Lysine Acetate, 0.8-3.0 parts of L-Methionine, 0.8-3.9 parts of L-Phenylalanine, 1.6-5.4 parts of L-threonine, 0.5-1.1 parts of L-Tryptophan, 6.7-10.7 parts of L-Valine, 3.2-9.3 parts of L-alanine, 4.6-7.2 parts of L-arginine, 1.2-2.9 parts of L-Histidine, 5.0-9.6 parts of L-proline, 2.6-6.0 parts of L-serine, 2.6-10.8 parts of glycin, 0.1-1.0 parts of L-Cysteine hydrochloride, 0.1-0.5 parts of citric acid and moderate water for injection. The injecta does not contain sulphite type chemical inhibitor, thoroughly solves the harm of sulphite type on human body, and ensures that the obtained products are safer. The PH value of the injecta is 5.5-7.0. By the accelerated test and quality test, the results show that the stability of the medicine combination which contains 15 amino acids is the same as or better than like products which contains sulphite type sold on market.
Owner:郑飞雄
Pharmaceutical formulations of antineoplastic agents and processes of making and using the same
In its several embodiments, this invention discloses a pharmaceutical formulation comprising at least one antineoplastic agent or a pharmaceutically acceptable salt thereof, and at least one dissolution enhancing agent sufficient to substantially dissolve said at least one antineoplastic agent in at least one aqueous diluent, wherein said dissolution enhancing agent is urea, L-histidine, L-threonine, L-asparagine, L-serine, L-glutamine or mixtures thereof; a lyophilized powder comprising said pharmaceutical formulation, and articles of manufacture thereof.
Owner:MERCK SHARP & DOHME LLC
Method for preparing L-tryptophan by enzymatic conversion
The invention belongs to the technical field of medicine and biology and particularly relates to a method for preparing L-tryptophan by enzymatic conversion. The method, of which L-tryptophan is prepared from mixed amino acids containing L-serine, comprises the following steps: mixing the high-activity genetic engineering bacteria of L-tryptophan synthetase or L-tryptophanase with the conversion solution of the mixed amino acid containing L-serine; carrying out the enzymatic reaction at the temperature of 30 to 45 DEG C; and then, separating the converted products by the isoelectric point crystallization method or the method combining isoelectric point crystallization and ion exchange resin to obtain high-purity L-tryptophan. The method solves the problems of the separation and overall development of L-serine in the mixed amino acids by obtaining L-serine products with wider applicable range and higher added value, therefore, the invention has the advantages of wide material availability, low price, convenient operation, short conversion time, low production cost and the like.
Owner:NANJING UNIV +1
Technique for preparing phosphatidyl serine rich in highly-unsaturated fatty acid
The invention relates to a technique for preparing diacylglyceryl-phosphorylserine rich in unsaturated acid, which is characterized in that a reactor is preheated to 30 to 60 DEG C, squid lecithin and L-serine at the mass ratio of 1:1-15 is added, phosphatidase D2-15U / g is also added as reaction substrate, gas R134a is pumped to lead the pressure of the reactor to reach 4-6MPa, the pressure is reduced after agitating reaction for 2 to 8 hours, R134a is recycled after the reaction mixture comes into a separation pot which is communicated with the reactor under the temperature of 30 to 60 DEG C, reaction mixture is collected from the a sample receiving port of the separation pot, at last, the reaction mixture is washed by using acetone and centrifuged for 5 to 10 min to separate the solid phase for further purification. The diacylglyceryl-phosphorylserine prepared by the invention rich in high unsaturated fatty acid as the medium in catalytic reaction of phosphatidase D has the advantages of simple technique, low equipment input, short reaction time, high content of unsaturated fatty acid and high yield and the neglecting of organic solvent. Furthermore, R134a can be completely recycled after reaction, which is beneficial to the separation of products and repeated use of enzyme.
Owner:OCEAN UNIV OF CHINA
Preparation method of phosphatidylserine
The invention relates to a preparation method of phosphatidylserine. The preparation method comprises the steps of carrying out enzymic catalytic reaction on natural lecithin and L-serine in the presence of phospholipase D enzyme liquid, and carrying out a simple purification process after the reaction, so as to obtain phosphatidylserine with the yield exceeding 60%. The preparation method has the beneficial effects that the operation is simple and convenient, the yield is high, the cost is low, the product quality is stable, and the process is environmentally friendly.
Owner:SINPHAR TIAN LI PHARMA
Normal temperature and sub-normal temperature in vitro heart preserving perfusate
InactiveCN1930961AMorphologically normalRich in granulesDead animal preservationPantothenic acidValine
The normal temperature and sub-normal temperature in vitro heart preserving perfusate as one kind of organ preserving matter consists of basic electrolyte, energy matter and protecting matter. Each liter of the heart preserving perfusate contains NaCl 5.0-7.0 g, KCl 0.3-0.5 g, NaH2PO4 .H2O 100-150 mg, MgSO4 50-100 mg, NaHCO3 3.0-5.0 g, L-argine 70-100 mg, histidine 30-60 mg, L-cystine 50-70 mg, L-methionine 10-50 mg, levo glutamine 500-700 mg, L-lysine 100-170 mg, glycine 10-50 mg, L-isoleucine 70-150 mg, L-serine 30-50 mg, L-leucine 70-150 mg, L-tryptophane 10-20 mg, L-tyramine 80-150 mg, L-valine 70-150 mg, L-threonine 80-150 mg, choline chloride 3-8 mg, vitamin B2 0.5-3 mg, pantothenic acid 3-g mg, folic acid 3-8 mg, pyridoxal 3-10 mg, nicotinamide 3-10 mg, etc. The present invention has the effects of preserving in vitro heart efficiency, reducing rejection reaction of organ and prolonging the survival period of organ.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Escherichia coli engineering bacteria for producing L-serine with high yield and fermentation method for engineering bacteria
InactiveCN102703371APromote accumulationBacteriaMicroorganism based processesEscherichia coliL serine
The invention relates to escherichia coli engineering bacteria for producing L-serine with high yield and a fermentation method for the engineering bacteria. The bacteria are escherichia coli Q3 with the collection number CGMCC 6213, and the genotype of the bacteria is MG1655 delta sdaA delta sdaB delta tdcG:: Cm. By using wild escherichia coli as a contrast, the culture results show that in a fermentation medium (containing 10 g / L of peptone, 5 g / L of yeast powder, 10 g / L of sodium chloride and 10 g / L of glucose), under the fermentation culture conditions that the pH value is 7.0, the temperature is 37+ / -1 DEG C, the revolving speed of a shaker is 200+ / -20 revolutions per minute and the fermentation time is 48+ / -3 hours, the accumulation quantity of the L-serine produced by fermenting the engineering bacteria is improved by about 40 percent, a foundation is laid for constructing high-yield strains of phosphatidylserine, and the engineering bacteria have important application prospect and value.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Method for preparing phosphatidylserine under catalysis of immobilized phospholipase D
InactiveCN103966277AHigh immobilization rateIncrease temperatureChemical industryOn/in organic carrierSodium acetrizoateSodium acetate
The invention discloses a method for preparing phosphatidylserine under catalysis of immobilized phospholipase D. The method comprises the following steps: (1) with chitin as a vector and glutaraldehyde as a cross-linking reagent, immobilizing free phospholipase D to obtain immobilized enzyme of phospholipase D; (2) with the immobilized enzyme of the phospholipase D prepared in the step (1) as a catalyst, n-butyl acetate as an organic phase solvent, and 50mM of sodium acetate buffer solution of which the pH is 5.5 as a water phase solvent, reacting L-serine to react with soyabean lecithin at 20-60 DEG C according to the molar ratio of (10-100):1, thus obtaining phosphatidylserine; and at the end of reaction, filtering and recovering the phospholipase D for repeated use. By adopting the method disclosed by the invention, the ester transfer rate in the dual-phase catalytic system can be up to 78%, the immobilized enzyme can be repeatedly used, and the method is simple, easy to operate, and low in preparation cost.
Owner:NANJING UNIV OF TECH
Synthetic method of L-serine
InactiveCN102220389AHigh yieldShort processOrganic compound preparationAmino-carboxyl compound preparationL serineUltrafiltration
The invention discloses a synthetic method of L-serine, which comprises the following steps: A) transforming the concentration of a substrate: adding the substrate, namely glycine and serine hydroxymethyltransferase accounting for 3%-8% by weight into a biological reactor; feeding 37% of formaldehyde water solution when the temperature is 40-50 DEG C and getting L-serine transformation liquid by transformation; B) purifying through an ultrafiltration membrane: purifying the L-serine transformation liquid in the A) step through the ultrafiltration membrane under the conditions that the temperature is 25-35 DEG C, the pressure at an inlet is 0.4-0.8MPa and the pressure at an outlet is 0.1-0.3MPa; C) performing adsorption and elution through ion-exchange resin: regulating the pH value of penetrating fluid in the B) step to 4.5-5.5, then exchanging the L-serine transformation liquid onto resin through the ion-exchange resin and further eluting with 0.8-1.5mol / L of hydrochloric acid; D) concentrating through a nanofiltration membrane: concentrating L-serine eluent in the step C) through the nanofiltration membrane under the conditions that the temperature is 25-35 DEG C, the pressure at the inlet is 0.4-0.8MPa and the pressure at the outlet is 0.1-0.3MPa; and getting an L-serine finished product after concentration. The synthetic method of the L-serine is simple in operation, short in production period, low in cost, high in yield and low in environmental protection pressure, and suitable for large-scale industrialized production.
Owner:HENGDIAN GRP JIAYUAN CHEM +1
Process for preparing non-proteinogenic L-amino acids
InactiveUS20020177196A1Avoid isolationProcess economyHydrolasesFermentationL serineDrug biotransformation
Process is provided for preparing a non-proteinogenic L-amino acid by means of an enzymic biotransformation in which O-acetyl-L-serine is reacted with a nucleophilic compound, while using an O-Acetyl-L-serine sulfhydrylase as catalyst, to give a non-proteinogenic L-amino acid. The process is carried out at a pH in the range between pH 5.0 and 7.4.
Owner:WACKER CHEM GMBH
Novel HIV integrase inhibitors and HIV therapy based on drug combinations including integrase inhibitors
InactiveUS20050049242A1Strong synergyBiocideAnimal repellantsCombination drug therapyResistant virus
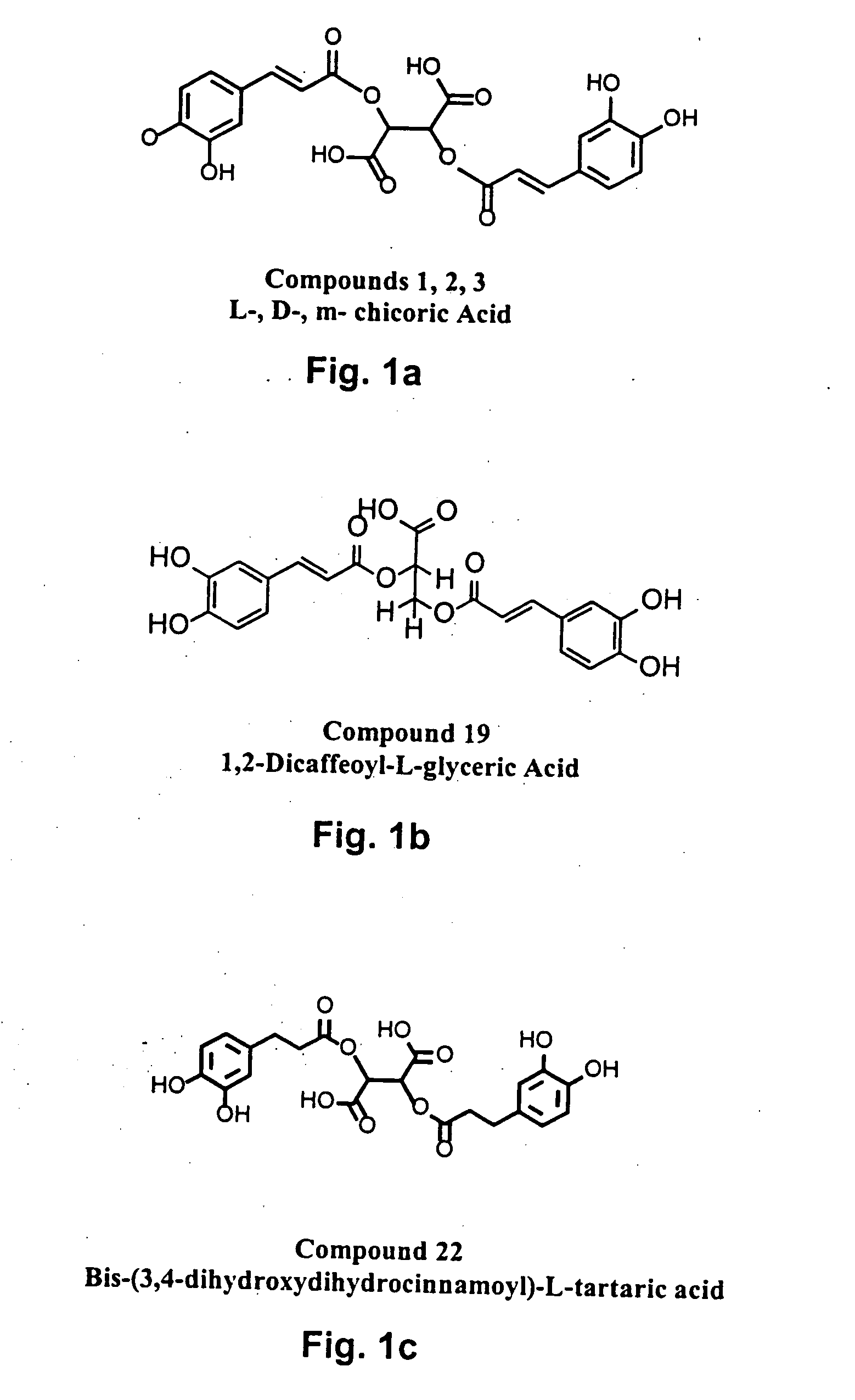
The present invention includes a group of novel compounds that are demonstrated to potently and selectively inhibit HIV integrase (IN) activity in vitro and to potently inhibit HIV replication in live, cultured cells at non-toxic concentrations. The novel compounds disclosed include 2,3-di(3,4-dihydroxy-dihydroxydihydrocinnamoyl)-L-tartaric acid, 2,3-di-(3,4-dihydroxybenzoyl)-L-tartaric acid, 2,3-di-(3,4-dihydroxyphenylacetyl)-L-tartaric acid, 2,3-di-(3,4,5-trihydroxybenzoyl-L-tartaric acid, 2,3-dicaffeoyldiamidopropionic acid, 1,2,-dicaffeoyl-L-glyceric acid, bis,-3,4-dicaffeoyldiamidobenzoic acid, di-3,4-dihydroxybenzylidene succinic acid, di-3,4-dihydrodihydroxybenzylidine succinic acid, 2,3-dicaffeoyl-L-serine, bis-dicaffeoyl-L-isoserine and 1,4-dicaffeoyl-L-lysine. Tests of integrase inhibitors with 2′,3′-dideoxycytidine, zidovudine and nelfinavir (protease inhibitor) indicated a potent synergy against reverse transcriptase inhibitor resistant virus. The potential benefit from the addition of integrase inhibitors to combination drug therapies is significant.
Owner:ROBINSON W EDWARD JR +2
Recombination methyl nourishment bacillus and application thereof
The present invention discloses one kind of recombinant methylotrophic bacillus and its application. The recombinant methylotrophic bacillus MB202 is obtained through inserting glyA into the polyclonal site of pLAFR3 to obtain recombinant vector pLAFRg, and the subsequent three parent crossbreeding to introduce pLAFRg into methylotrophic bacillus MB200 to obtain the recombinant methylotrophic bacillus MB202. Through shaking table culture of MB202 at 32 deg.c in 50mM Tris-HCl culture medium with glycine in 10 mg / ml, methanol in 50 mg / ml, and biomass of 10<8>-10<9> CFU / ml, in pH 8.5 for 48 hr, L-serine yield may reach 11.5mg / ml; and through fermentation culture of MB202 at 32 deg.c for 12 hr, the activity of serine hydroxymethlase may reach 115 U. The recombinant methylotrophic bacillus MB202 has broad application foreground in producing L-serine and treating methanol waste water.
Owner:GUANGXI UNIV
Preparation method of phosphatidylserine
The invention discloses a preparation method of phosphatidylserine. The preparation method comprises the following steps: (1) mixing soya bean lecithin and water, thus preparing a mixture M1; (2) adding L-serine and phospholipase D into the mixture M1, and mixing L-serine, phospholipase D and the M1, thus preparing a phosphatidylserine crude product; (3) adding a solvent into the prepared phosphatidylserine crude product for extraction, thus preparing a phosphatidylserine semi-finished product; and (4) enabling the prepared phosphatidylserine semi-finished product to sequentially pass through a silicagel column and an ion exchange resin, thus preparing phosphatidylserine, wherein the solvent is composed of chloroform, ethanol, diethyl ether and water. According to the design, soya bean lecithin is mixed with water firstly, then L-serine and phospholipase D are added for carrying out reaction, then the solvent containing chloroform, ethanol, diethyl ether and water is adopted for carrying out extraction, and the obtained phosphatidylserine semi-finished product is filtered by the silicagel column and the ion exchange resin, the operation is simple and convenient, the prepared phosphatidylserine is high in purity, and the application range of phosphatidylserine is greatly expanded.
Owner:WUHU FOMAN BIOPHARMA CO LTD
Culture medium for tissue engineering skin convey and formulating method thereof
InactiveCN101486995AEffective maintenance of structureEffective maintenance functionArtificial cell constructsVertebrate cellsBiotechnologyPenicillin
The invention discloses a culture medium that is used in transportation of tissue engineered skin. A basic culture fluid is added with additional hydrocortisone, epidermal growth factors, isoproterenol, insulin, transferrin, triiodothyronine, adenine, vitamin C, cholera toxin, L-serine, L-novain, palmic acid, linoleic acid, arachidonic acid, bovine pituitary extract, bovine serum albumin, penicillin, streptomycin and fungizone B. The obtained culture medium has strong stability and long shelf life of about 12 months, completely meet the demands of large-range transportation of the tissue engineered skin products, can effectively maintain the structure and functions of the tissue engineered skin in the transportation process, becomes solid after being cooled, and consequently has convenient carry in the transportation process.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
3-phosphoglycerate dehydrogenase variants whose inhibition by L-serine is reduced, and genes encoding them
A 3-phosphoglycerate dehydrogenase (PGD) which exhibits a susceptibility to inhibition by serine which is reduced a as compared with that of an Escherichia coli wild-type PGD and which possesses an amino acid sequence which differs from the amino acid sequence of the Escherichia coli wild-type PGD (SEQ ID NO: 2) in that an amino acid apart from glycine is present at position 249 or an amino acid apart from threonine is present at position 372.
Owner:WACKER CHEM GMBH
L-tyrosine preparation method through enzymatic conversion
ActiveCN103224972AHigh catalytic efficiencyHigh implementabilityMicroorganism based processesFermentationTyrosinasePyridoxine phosphate
The invention provides an L-tyrosine preparation method through enzymatic conversion. The method comprises the following steps: 1, respectively culturing strains having the activities of serine deaminase and strains having the activities of tyrosine phenol-lyase in a culture medium; 2, mixing two cells of the serine deaminase and the tyrosine phenol-lyase with an L-serine-containing amino acid material liquid, adding an aqueous phenol solution having a concentration of 500g / L, pyridoxal phosphate having a concentration of 0.2g / L and a surfactant having a concentration of 0.01-1.0g / L, adjusting the pH value to 6-11 by using ammonia water having a volume percentage of 25%, and carrying out an enzymatic reaction for 6-35h; and 3, centrifuging the obtained conversion liquid for 15min in 4000r / min, collecting the obtained mixture, dissolving the mixture by using 200-7000ml of pure water, adding 10mol / L sodium hydroxide in a dropwise manner to adjust the pH value to 12-13, heating to 80DEG C while stirring, adjusting the pH value of the obtained filtrate to 6 by using 6mol / L hydrochloric acid, and drying to obtain required L-tyrosine.
Owner:湖北新生源生物工程有限公司
Monkey-bread tree moisturizing cream
ActiveCN103520042AIncrease elasticityGood moisturizing effectCosmetic preparationsToilet preparationsAdditive ingredientIrritation
The invention discloses monkey-bread tree moisturizing cream. The monkey-bread tree moistening cream comprises the following ingredients in parts by weight: 3-7 parts of ANANSODNIA DIGITATA seed oil, 2-6 parts of diacetyl tartaric acid ester of mono-and diglycerides, 10-20 parts of lanolin, 0.1-0.5 parts of L-serine, 8-12 parts of a moisturizing agent, and 50-60 parts of water. The monkey-bread tree moisturizing cream is natural and mild, nearly has no irritation, can be used for nourishing the skin and moisturizing for a long time, has better water supplementing and moisturizing effects on dry skin, sensitive skin and the like, and can be used for effectively improving the skin color and increasing the skin resiliency.
Owner:清远市望莎生物科技有限公司
Process for the synthesis of lacosamide
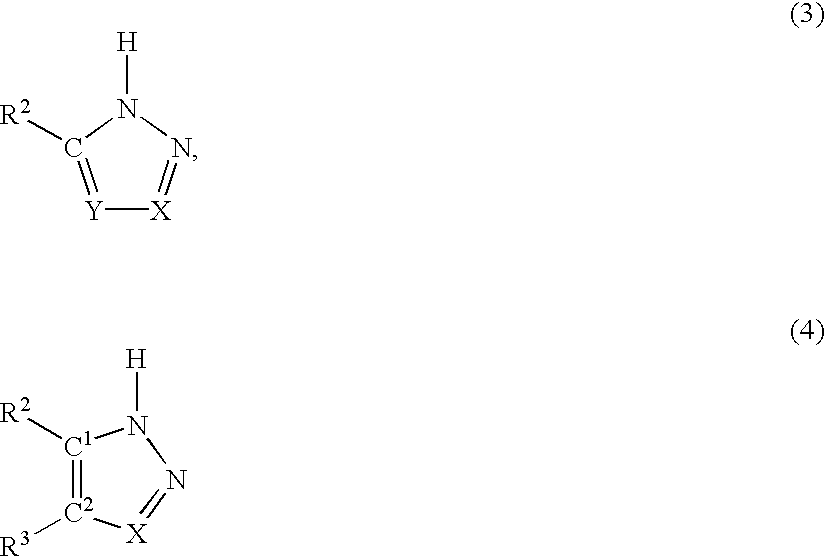
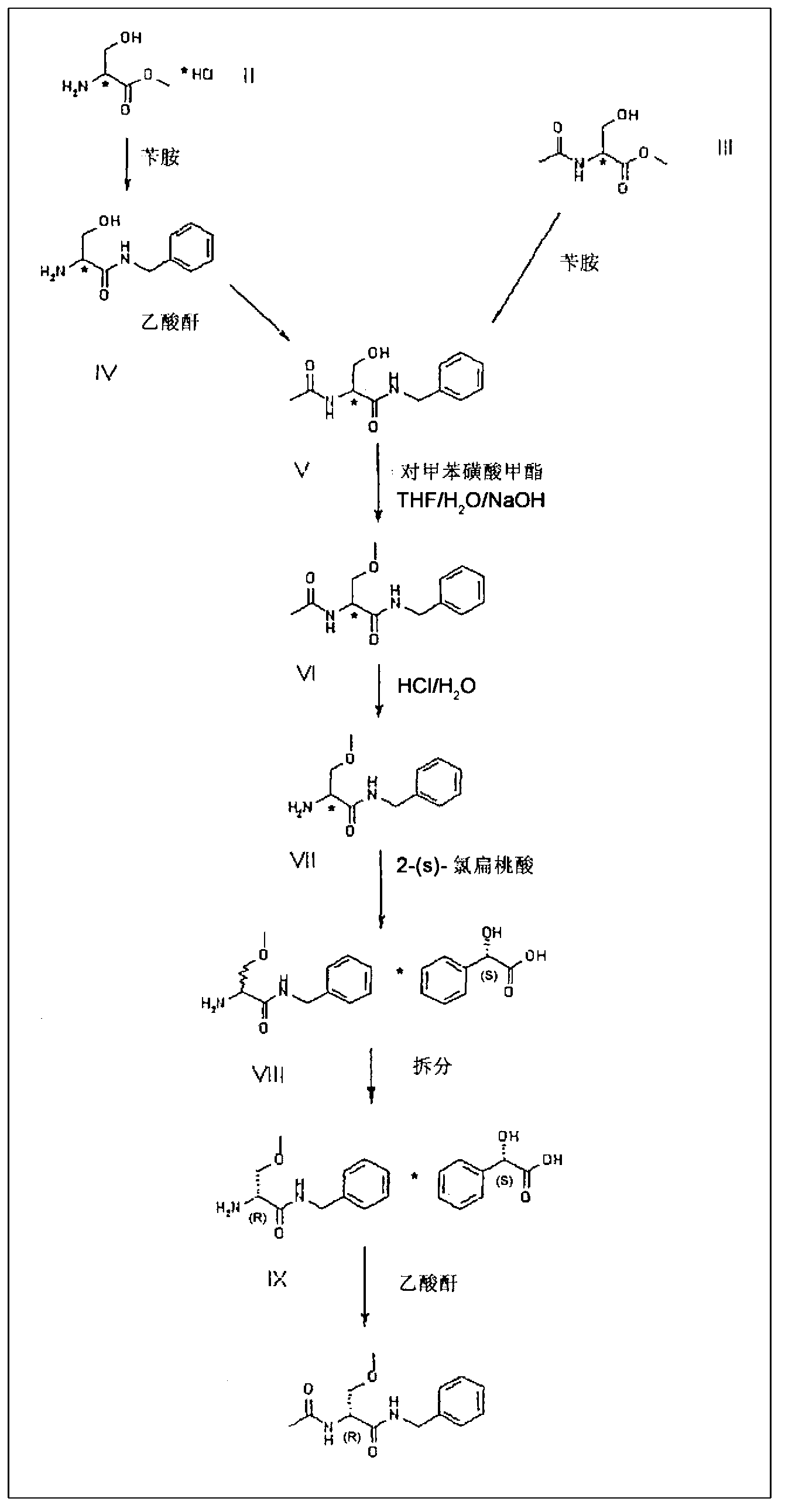
A novel process for the synthesis of Lacosamide using D,L-serine as starting material is described, where the methylation reaction of hydroxyl is carried out using an inexpensive base such as NaOH and an inexpensive alkylating agent, non-toxic and non-carcinogenic, such as methyl p-toluenesulfonate; the R enantiomer is isolated from the racemic mixture of Lacosamide after selective hydrolysis of the acetamide, salification of the racemic mixture with a chiral acid (HX*) in an organic solvent, resolution of the diastereoisomeric mixture, preferably by precipitation of the R enantiomer, and subsequent acetylation of the optically pure intermediate.
Owner:EUTICALS
Process of preserving food and food preservative
A process of preserving food which includes adding serine, especially L-serine, to food. According to the present invention, provided is a convenient process of preserving food for a long term by adding serine to food, in particular by performing a heat treatment after the addition of serine to food. Since serine is one kind of amino acids, it does not deteriorate quality of food itself and can preserve food safely.
Owner:F G A LAB FLAVOURENCE CORP
Lipidic Compositions for Induction of Immune Tolerance
InactiveUS20120164189A1Improve the level ofDecrease in ILSSPowder deliverySnake antigen ingredientsL serineTolerability
This invention provides a method for inducing immune tolerance toward an antigen comprising the antigen in lipidic particles or lipidic compositions. The lipidic particles are made up of phosphatidylserine and phosphatidylcholine, or phosphatidylinositol and phosphatidylcholine. The lipidic compositions comprise the antigen and O-phospho-L-serine. Administration of these composition results in inducing immune tolerance to the antigen.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Methods for constructing and fermenting L-serine high-yielding recombinant corynebacterium glutamicum
ActiveCN104561076AIncrease productionImprove conversion rateBacteriaMicroorganism based processesAnserineL serine
The invention provides a strategy combining metabolic engineering reconstruction with a fermentation process. On the one hand, a metabolic flow is changed to flow from a glycolysis route to an L-serine synthetic route by virtue of a metabolic engineering means, so that the sugar-acid conversion efficiency of L-serine is improved; and on the other hand, nutritional ingredients are added to meet requirements of corynebacterium glutamicum on nutrient substances after metabolic reconstruction, so that the problem of slow growth of thalli is solved, and the production strength of L-serine is improved.
Owner:JIANGNAN UNIV +1
Deep whitening and spot removing essence cream and preparation method thereof
InactiveCN106726664AReasonable formulaGood flexibilityCosmetic preparationsToilet preparationsL serinePreservative
The invention belongs to the technical field of cosmetics, and particularly relates to deep whitening and spot removing essence cream and a preparation method thereof. The deep whitening and spot removing essence cream comprises, in weight percentage, 1.5-2.5 % of soybean lecithin, 3-5% of L-serine , 1.5-3% of hydroxyethyl cellulose, 5-7% of alginic acid, 1-3% of dipotassium glycyrrhizinate, 4-6% of L-lactic acid, 0.1-0.3% of licoflavone, 1-2% of glycerinum, 13-16% of base oil, 0.01-0.03% of sweet orange oil, 0.01-0.03% of geranium oil, 0.05-0.1% of silymarin, 0.3-0.5% of preservative and 54.54-69.53% of deionized water. The deep whitening and spot removing essence cream has remarkable whitening and spot removing effect and the advantages that the cream is high in safety, a user cannot generate dependence when the cream is used for a long time and the like.
Owner:安徽檀鑫科技有限公司
Preparation method for L-selenocysteine
InactiveCN105294528ARaw materials are easy to obtainBuy easyOrganic chemistryChemical synthesisL serine
The invention belongs to the field of chemical synthesis, and concretely relates to a synthetic method for L-selenocysteine. The method comprises the following steps: a, chloridizing L-serine hydrochloride to obtain 3-chloro-L-alanine hydrochloride; b, performing seleno-reaction of 3-chloro-L-alanine hydrochloride prepared by step a under alkaline condition to obtain L-selenocystine; and c, performing reduction reaction of L-selenocystine to obtain L-selenocysteine. The method has simple steps, high yield, low cost, and good application prospect.
Owner:CHENGDU BAISHIXING SCI & TECH IND
Alleles of the mqo gene from coryneform bacteria
The invention relates to mutants and alleles of the coryneform bacterium mqo gene which encodes malate quinone oxidoreductases which contain any amino acid apart from L-serine at position 111, or a comparable position, in the amino acid sequence, and to processes for fermentatively preparing amino acids, preferably L-lysine, L-tryptophan and L-proline, using bacteria which comprise these alleles.
Owner:EVONIK DEGUSSA GMBH
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com