Pyrrolecarboxylic acid derivative synthesis method
A technology for pyrrole carboxylic acid and synthesis method, which is applied in the field of synthesis of pyrrole carboxylic acid derivatives, can solve the problems of many by-products, unsuitability for scale-up production, and low yield, and achieve easy scale-up production, good operability, and yield high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] A kind of synthetic method of pyrrole carboxylic acid derivative, the steps are as follows:
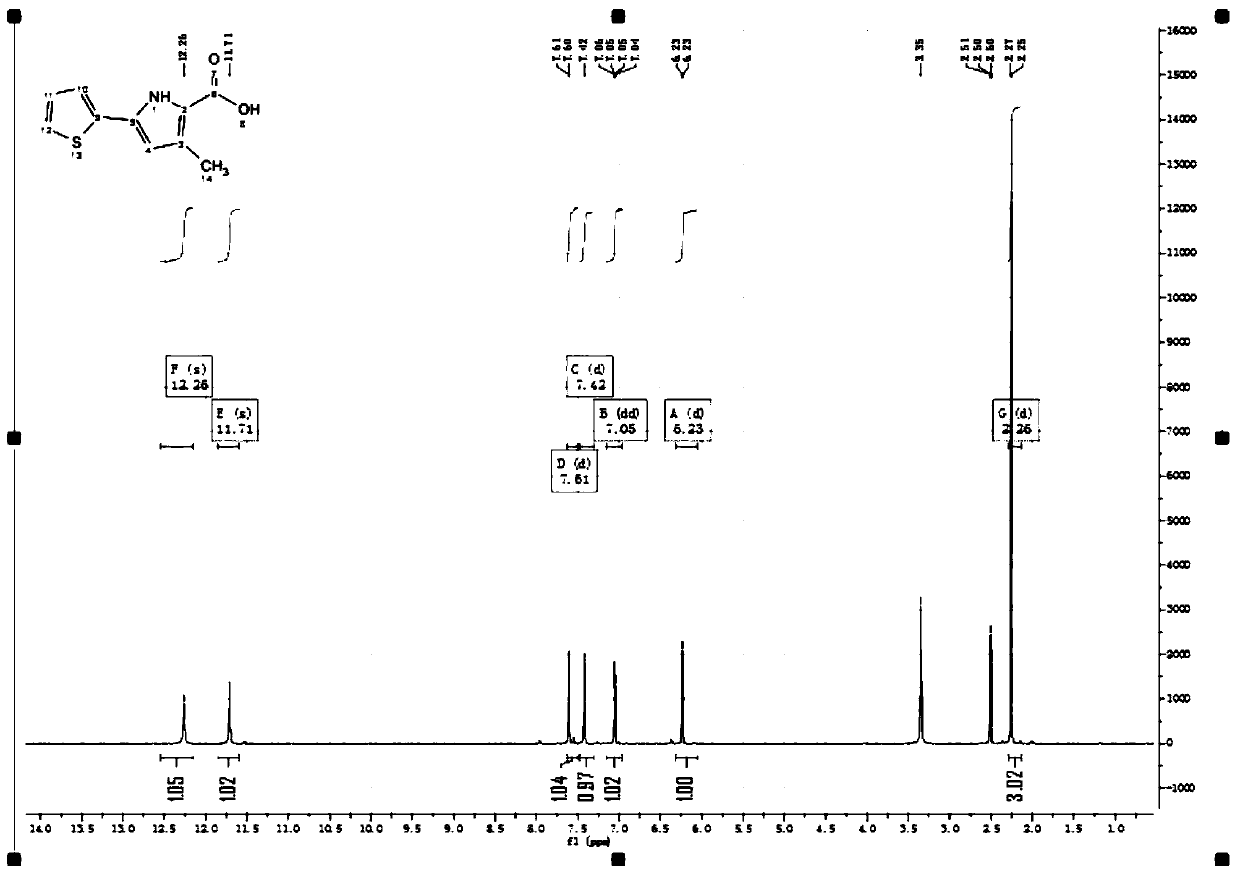
[0065] Step 1: Under the protection of argon, add 10 g of 2-acetylthiophene (1-1) into anhydrous tetrahydrofuran (100 ml), control the temperature below 10 degrees Celsius, and slowly add sodium hydride (60%, 6.3 g , 2eq), after the addition, the temperature was raised to 40 degrees Celsius, and ethyl acetate (14g, 2eq) was slowly added dropwise, and the drop was completed, kept at 40 degrees Celsius, stirred and reacted for 1h, after TLC detected that the reaction was complete, slowly added ice water (200ml) and ethyl acetate Ester (100ml), separated, the aqueous phase was extracted with ethyl acetate (100ml*5), all organic phases were combined, washed with saturated brine (200ml), concentrated under reduced pressure until almost no liquid flowed out, and a brown liquid 1 was obtained -(2-Thienyl)-1,3-butanedione (1-2) (17 g, crude).
[0066] The synthetic route is as follows...
Embodiment 2
[0078] A kind of synthetic method of pyrrole carboxylic acid derivative, the steps are as follows:
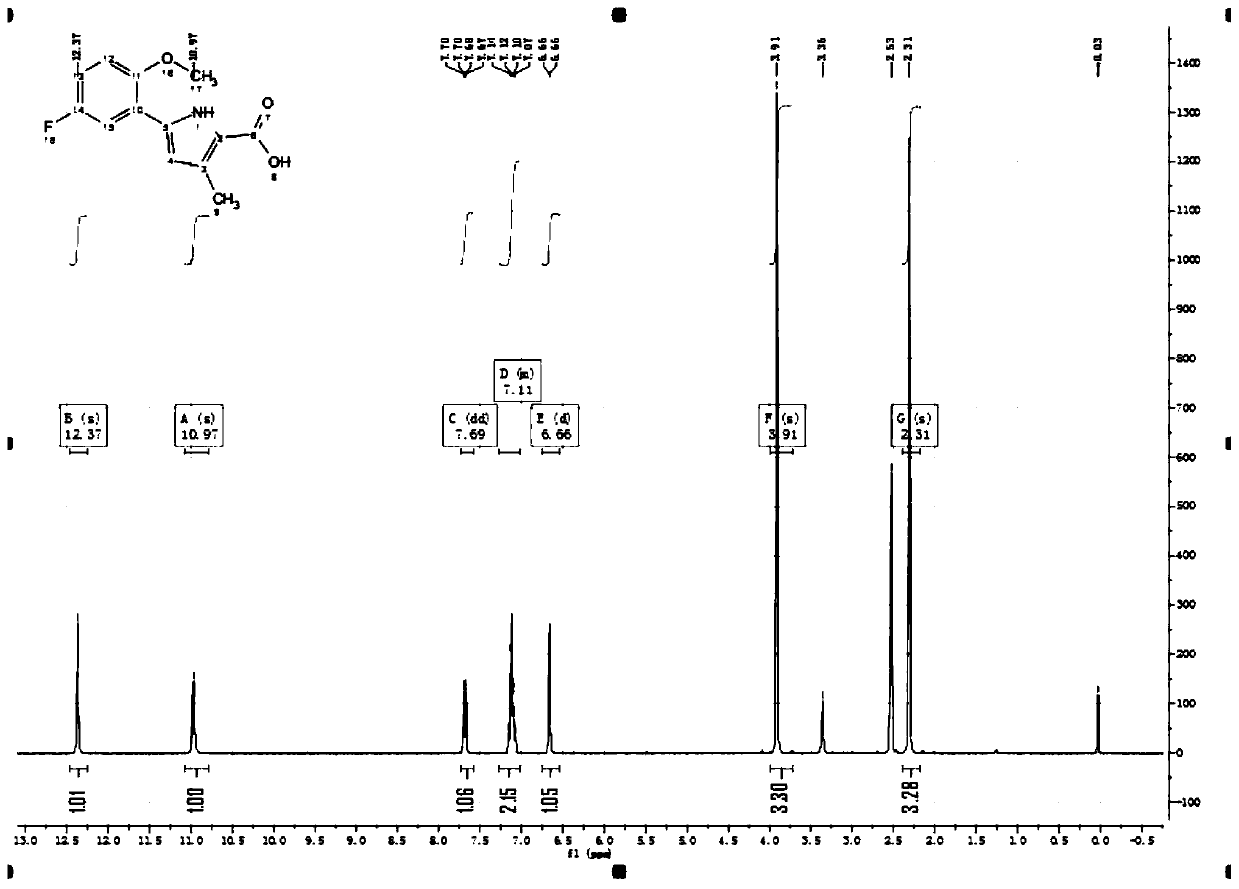
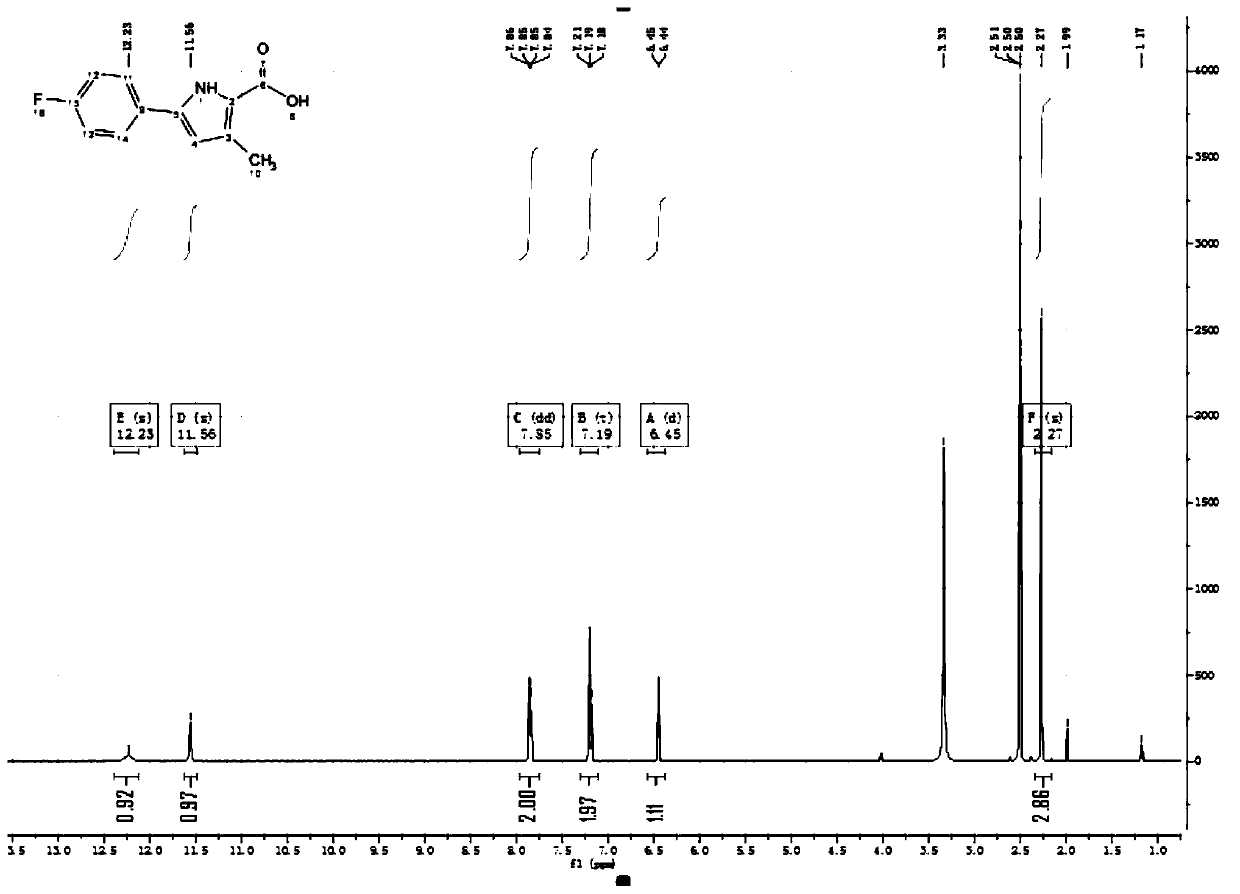
[0079] Step 1: Add 1-(4-fluoro-phenyl)-butane-1,3-dione (2-1) (26g) to glacial acetic acid (250ml), add diethyl aminomalonate Acetate (85.5g, 2.4eq) and sodium acetate (112.4g, 5eq) were heated and refluxed for overnight reaction. After TLC detected that the reaction was complete, ice water (400ml) and ethyl acetate (200ml) were added, and then adjusted with solid sodium carbonate. pH value to 8-9. After the adjustment, stir for 30min and separate layers. The aqueous layer was extracted with ethyl acetate (200ml*3). All organic layers were combined and washed with saturated brine (500ml). Concentrate under reduced pressure until almost no liquid flows out, add silica gel to spin dry, and obtain white solid compound 5-(4-fluorophenyl)-3-methyl- 1H-Pyrrole-2-carboxylic acid ethyl ester (2-2) (24.6 g, yield 67%, UPLC purity: 99%).
[0080] The synthetic route is as follows: ...
Embodiment 3
[0089] A kind of synthetic method of pyrrole carboxylic acid derivative, the steps are as follows:
[0090] Step 1: Add acetylcyclohexane (3-1) (30g) into tetrahydrofuran (300ml), slowly add sodium hydrogen (19.02g, 2eq) at 10°C, drop ethyl acetate (41.9g, 2eq), reacted at 40°C for 1h, after the completion of the reaction as detected by TLC, poured into ice water, extracted the aqueous layer with ethyl acetate (200ml*3), combined all organic layers, and washed with saturated brine (500ml). Concentrate under reduced pressure until almost no liquid flows out, add silica gel to spin dry, and pass column chromatography (petroleum ether: ethyl acetate = 20:1) to obtain the white solid compound 1-cyclohexylbutane-1,3-dione (3 -2) (36.2 g, yield: 90.5%, UPLC purity: 99%).
[0091] The synthetic route is as follows:
[0092]
[0093] Step 2: Add 1-cyclohexylbutane-1,3-dione (3-2) (36.2g) to glacial acetic acid (360ml), add diethyl aminomalonate hydrochloride (109.3g, 2.4eq) and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com