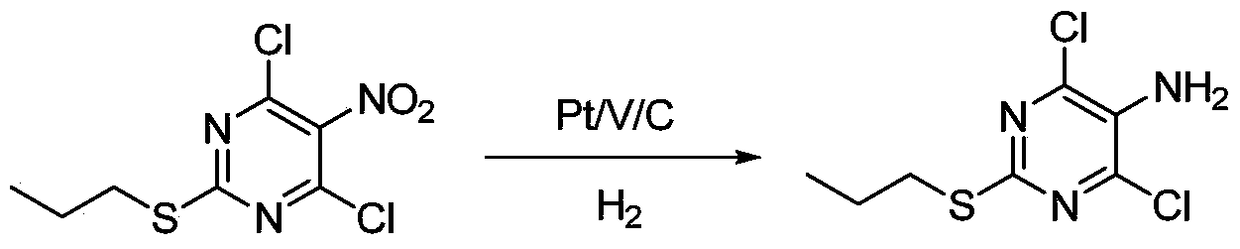
A method for synthesizing 4,6-dichloro-2-(propylthio)-5-aminopyrimidine
A technology of aminopyrimidine and propylthio group, which is applied in the field of synthesizing 4,6-dichloro-2--5-aminopyrimidine, can solve the problems of complicated operation, increased one-step reaction, high price and the like, and achieves simple and simple operation. , the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Synthesis of 2,4,6-trihydroxy-5-acetamidopyrimidine (Ⅳa)
[0043] At room temperature, 50.0 g of diethyl acetamidomalonate (III), 28.8 g of urea, 77.0 g of sodium ethoxide and 750 mL of ethanol were mixed with each other, and refluxed for 15 hours. After the reaction was finished, filter and wash the filter cake with 50 mL of ethanol to collect the filter cake. The obtained solid was dissolved in 1 L of water, and the pH was adjusted to neutral with hydrochloric acid. A solid precipitated out. After the solid was separated and fully dried, 39.3 g of 2,4,6-trihydroxy-5-acetamidopyrimidine (Ⅳa) was obtained, which was directly carried out Next reaction. ESI-MS m / z:186[M+H] + ; 1 HNMR (400MHz, D 2 O) δ2.13(s,3H).
Embodiment 2
[0044] Example 2 Synthesis of 2,4,6-trihydroxy-5-benzamidopyrimidine (Ⅳb)
[0045] At room temperature, 6.43 g of diethyl benzamidomalonate (Ⅲ), 2.9 g of urea, 7.7 g of sodium ethoxide and 75 mL of ethanol were mixed with each other, and refluxed for 13 hours. After the reaction, the solvent was removed under reduced pressure, and 100 mL of water was added. The pH was adjusted to neutral with hydrochloric acid, and the aqueous solution was extracted with 200 mL of chloroform, repeated three times, and the chloroform layers were combined. After drying, the solvent was removed to obtain 5 g of oil containing 2,4,6-trihydroxy-5-benzamidopyrimidine (IVb), which was directly carried out to the next reaction. ESI-MS m / z:248[M+H] + .
Embodiment 3
[0046] Example 3 Synthesis of 2,4,6-trichloro-5-aminopyrimidine (Ⅴ)
[0047] Mix 33.0 g of the crude compound (IVa) obtained in Example 1, 21.2 g of pyridine and 500 mL of toluene, then add 62.2 g of phosphorus oxychloride, and heat to reflux for 24 hours. After the reaction was finished, the solvent and most of the remaining phosphorus oxychloride were removed by concentration. The residue was poured into ice water and extracted twice with 500 mL ethyl acetate. After the organic phases were combined, they were washed once with 300 mL of saturated sodium bicarbonate solution. The organic phase was dried and concentrated to obtain 35.1 g of an oily substance containing 2,4,6-trichloro-5-aminopyrimidine (V). ESI-MS m / z:199[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com