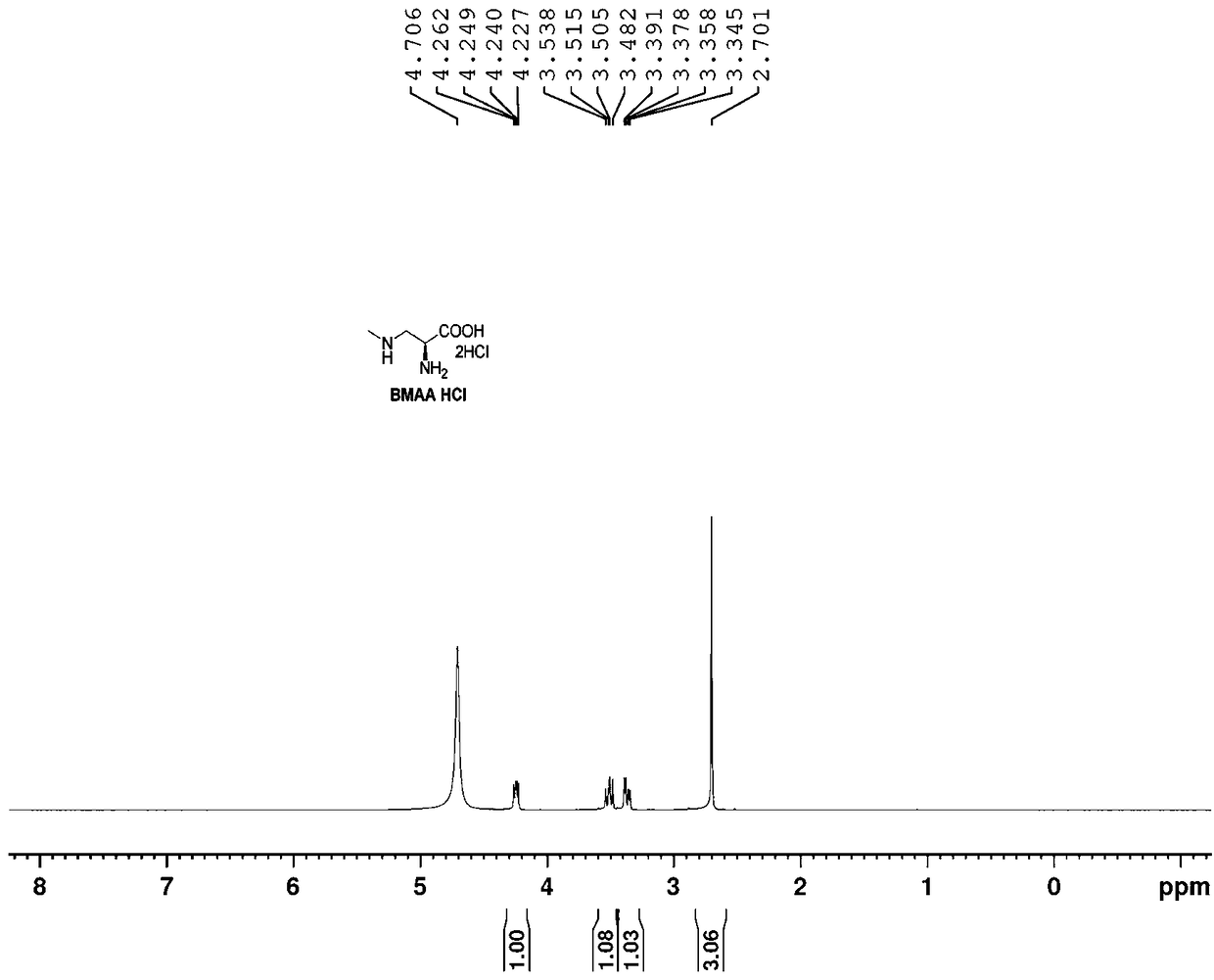
Method for preparing beta-N-methylamino-L-alanine
A technology of alanine and methylamino, which is applied in the field of preparation of β-N-methylamino-L-alanine (BMAA), can solve problems such as low yield, unstable performance of intermediates, and difficult separation of products. Achieve the effect of simple process operation and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
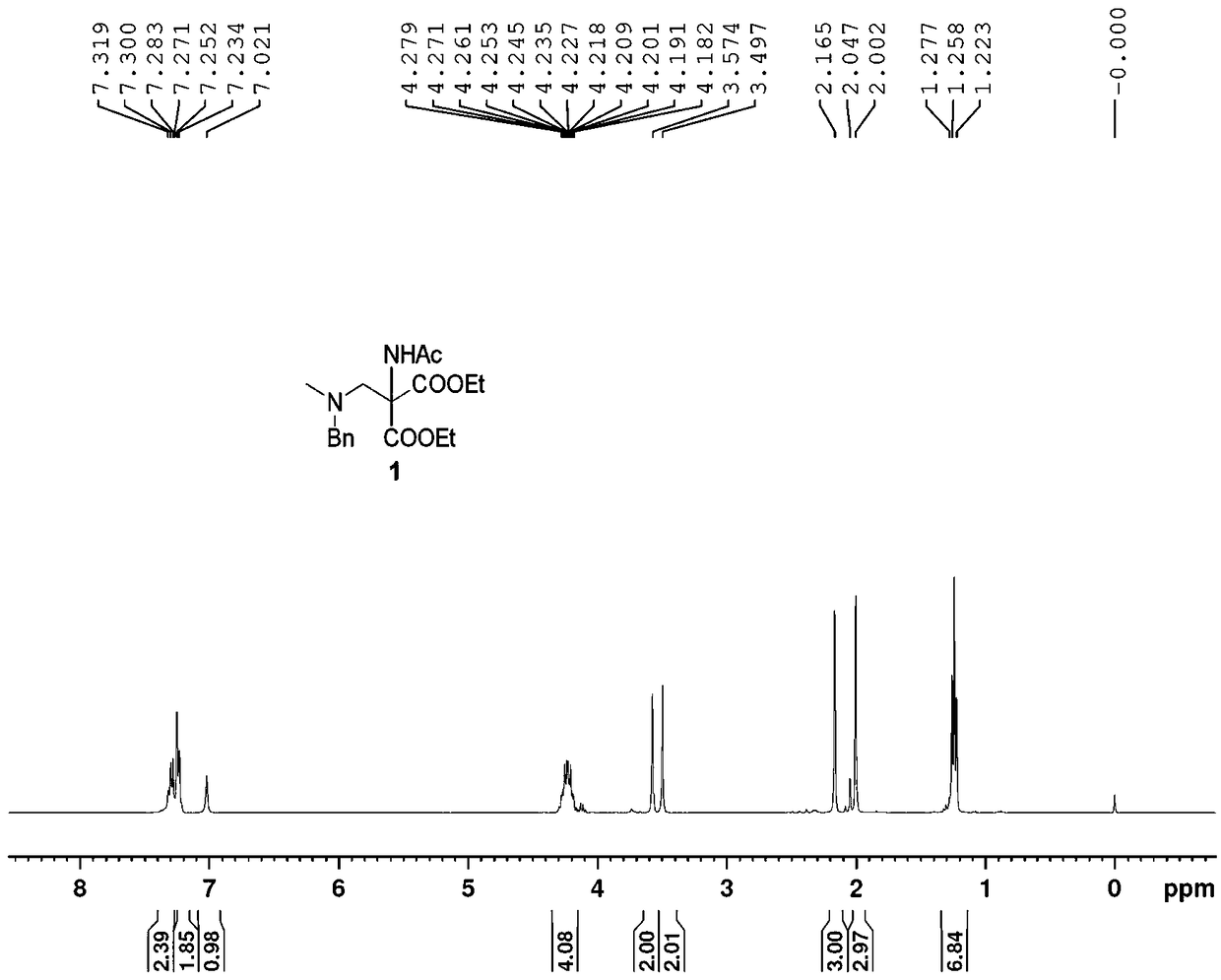
[0040] (1): Add 108.5 g (0.5 mol) N-acetylaminomalonate diethyl ester, (72.6 g, 0.6 mol) N-methylbenzylamine and 48.8 mL (0.65 mol) mass hundred 37% aqueous formaldehyde solution was added and stirred at room temperature for 4 hours. 100 mL of water was added to quench the reaction, and then extracted three times with 600 mL of ethyl acetate, the organic phase was washed three times with water, saturated sodium bicarbonate solution and saturated brine successively, dried over anhydrous sodium sulfate, and concentrated to obtain 183 g of intermediate 1. figure 1 , colorless oil, yield ~100%, directly to the next step reaction.
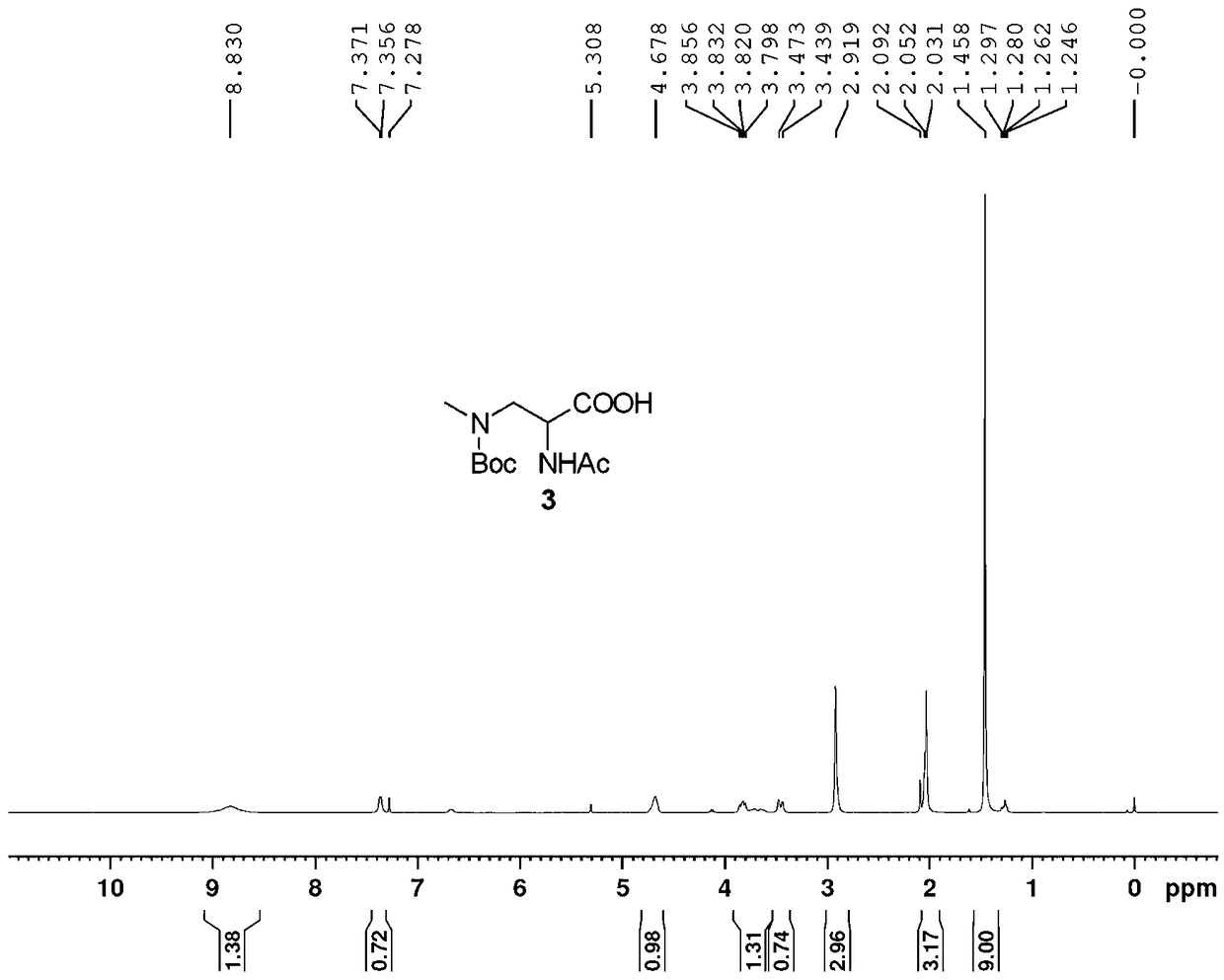
[0041] (2): Dissolve 183g (0.5 mol) of intermediate 1 in 300 mL of ethanol, add a solution made of 22 g (0.55 mol) of sodium hydroxide and 1L of water, stir and heat under reflux for 12 hours. After cooling, the reaction solution was washed three times with ethyl acetate, acidified with 1N dilute hydrochloric acid to Ph=4, concentrated to dryness to ob...
Embodiment 2
[0045] Example 2: The molar ratio of diethyl acetamidomalonate and N-methylbenzylamine used in step (1) is 1:1, and the rest are the same as in Example 1. Steps (1), (2) and (3) are combined Yield 33%.
Embodiment 3
[0046] Example 3: The sodium hydroxide used in step (2) is 2eq, and the rest are the same as in Example 1. The combined yield of steps (1), (2) and (3) is 38%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com