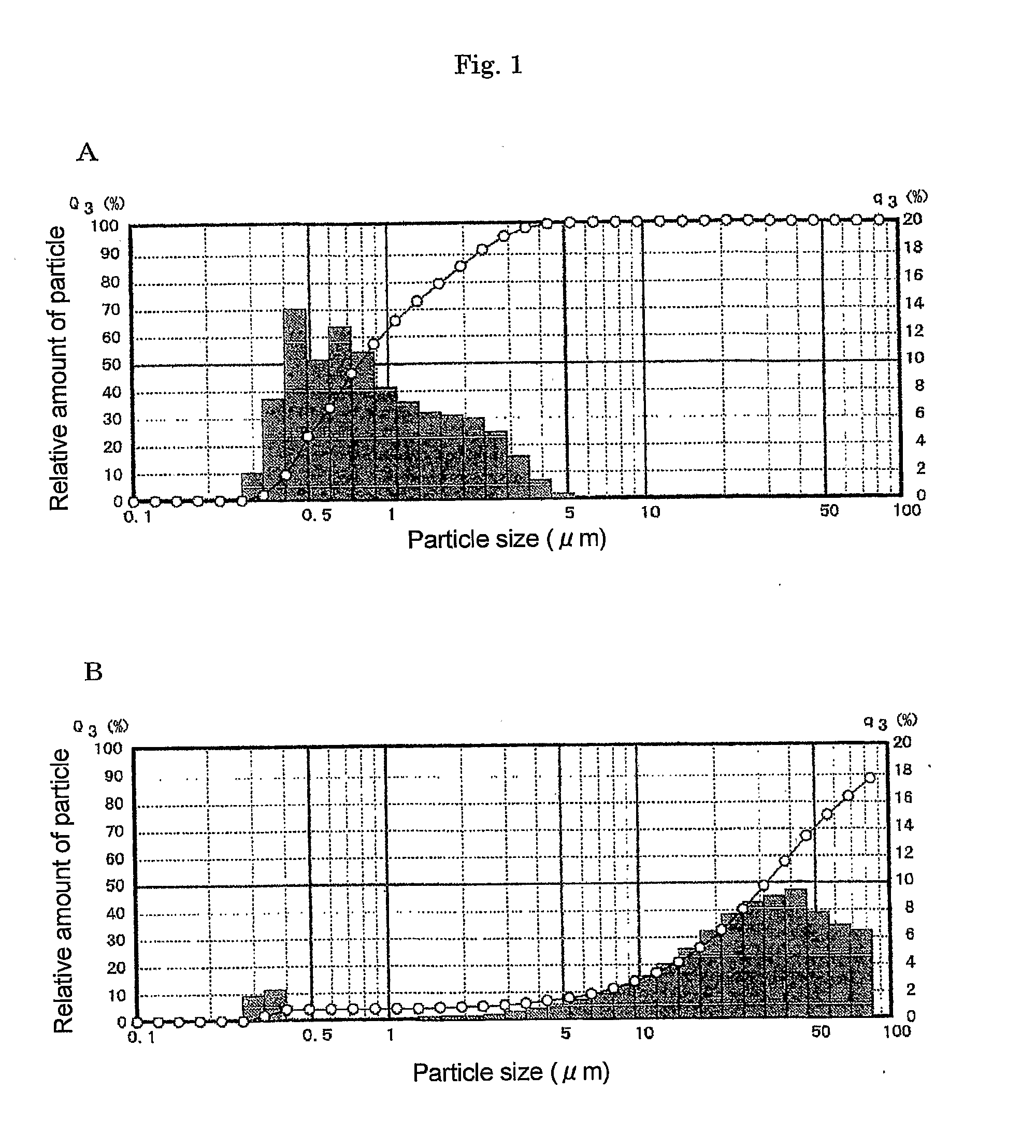
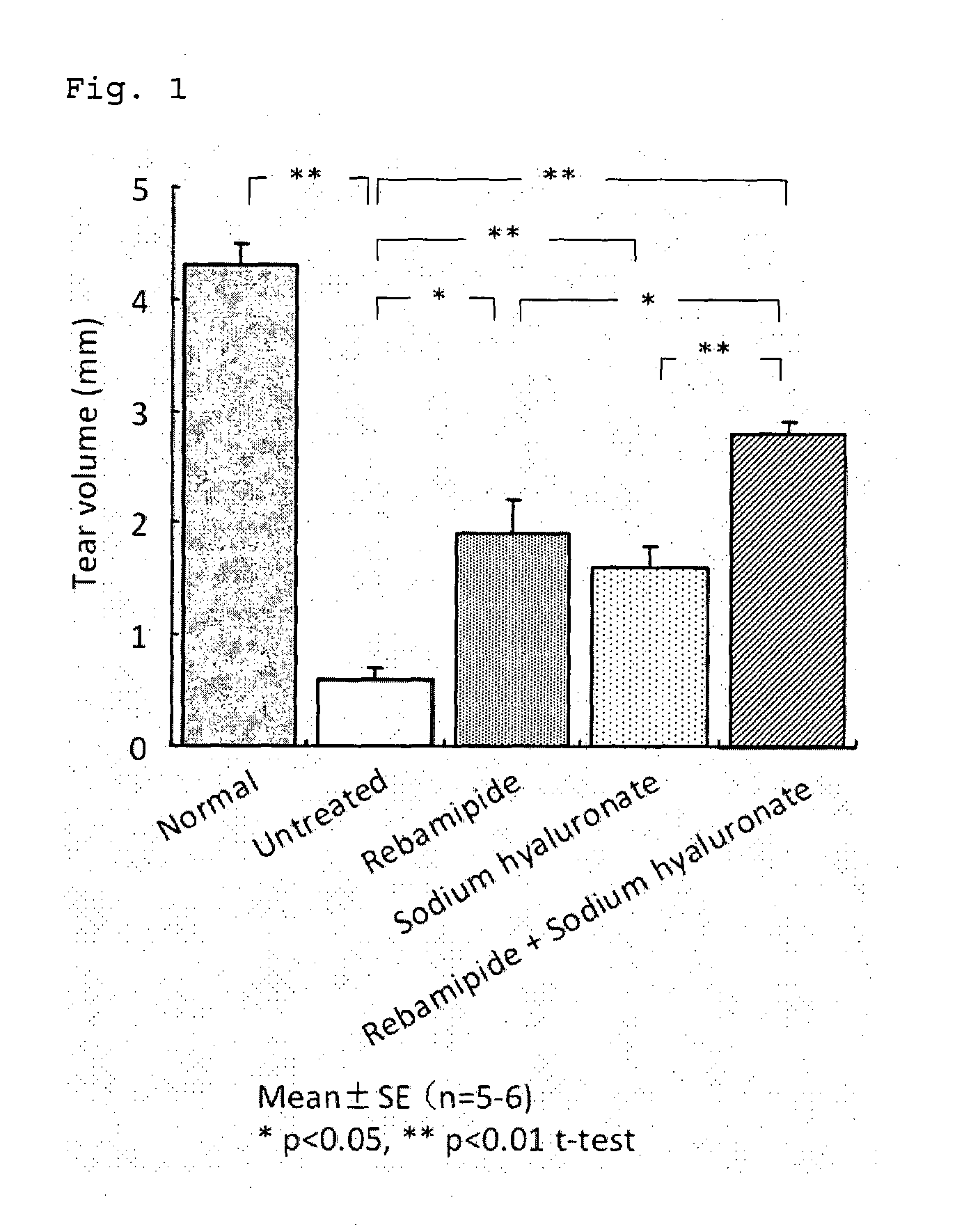
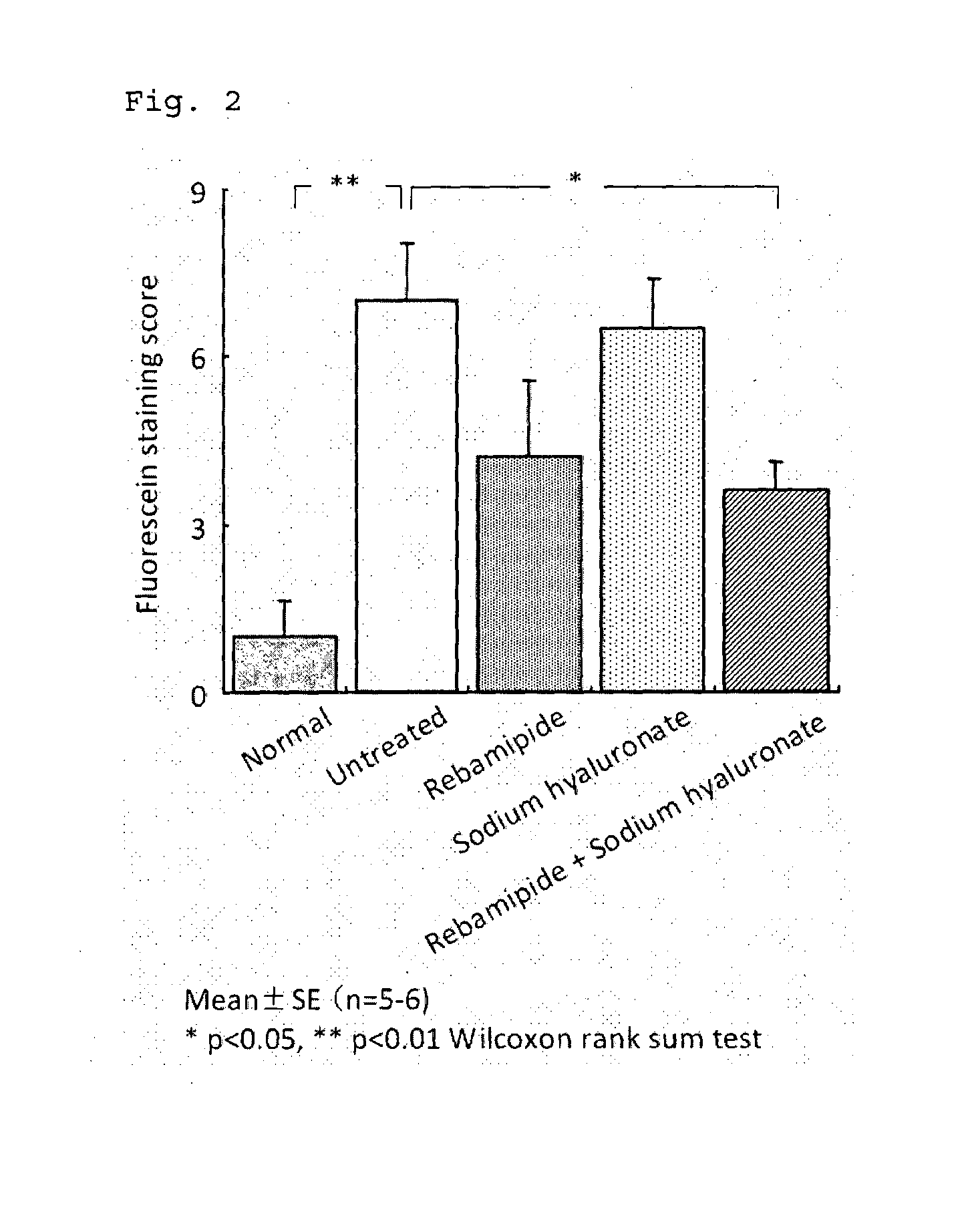
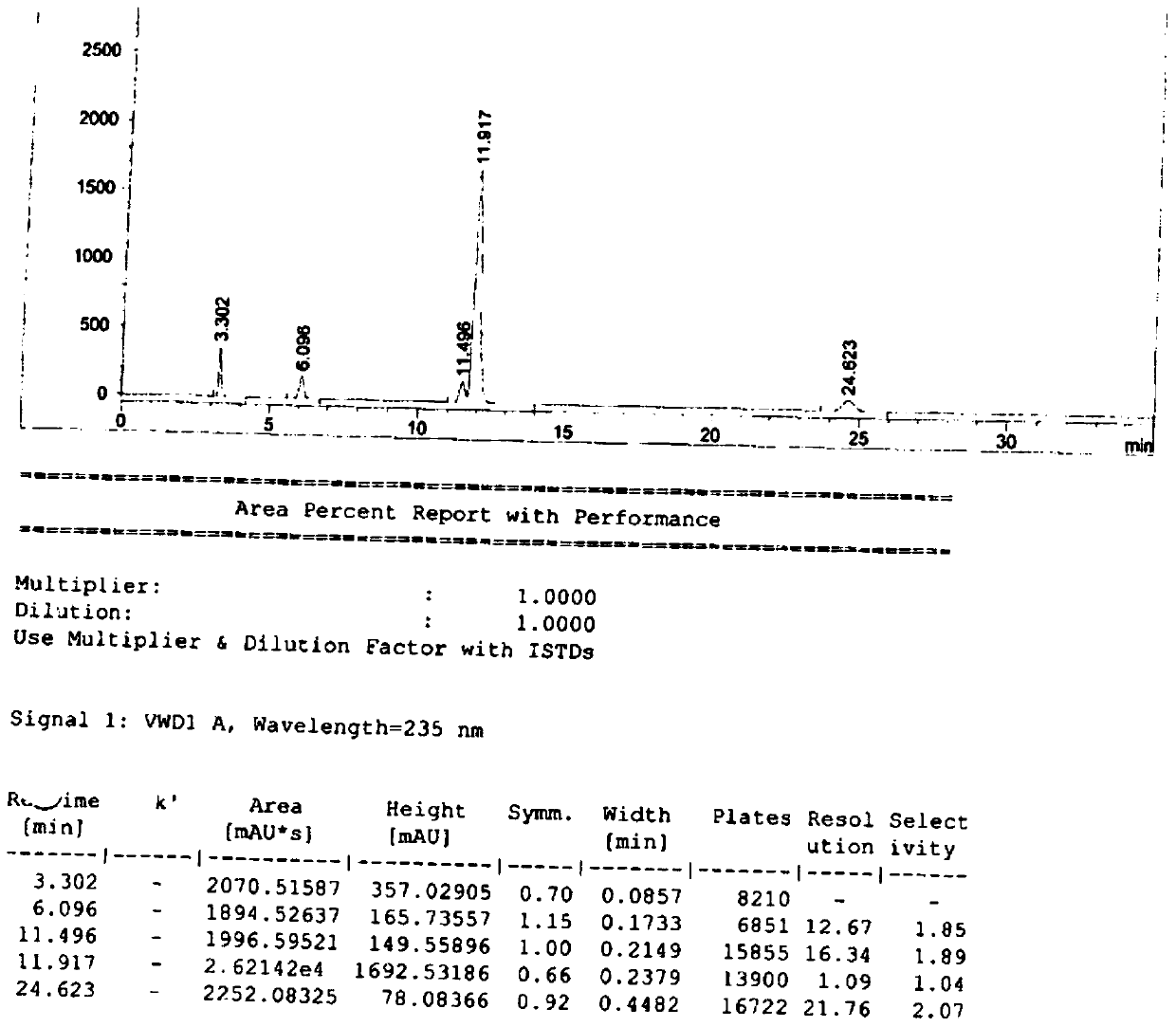
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
68 results about "Rebamipide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
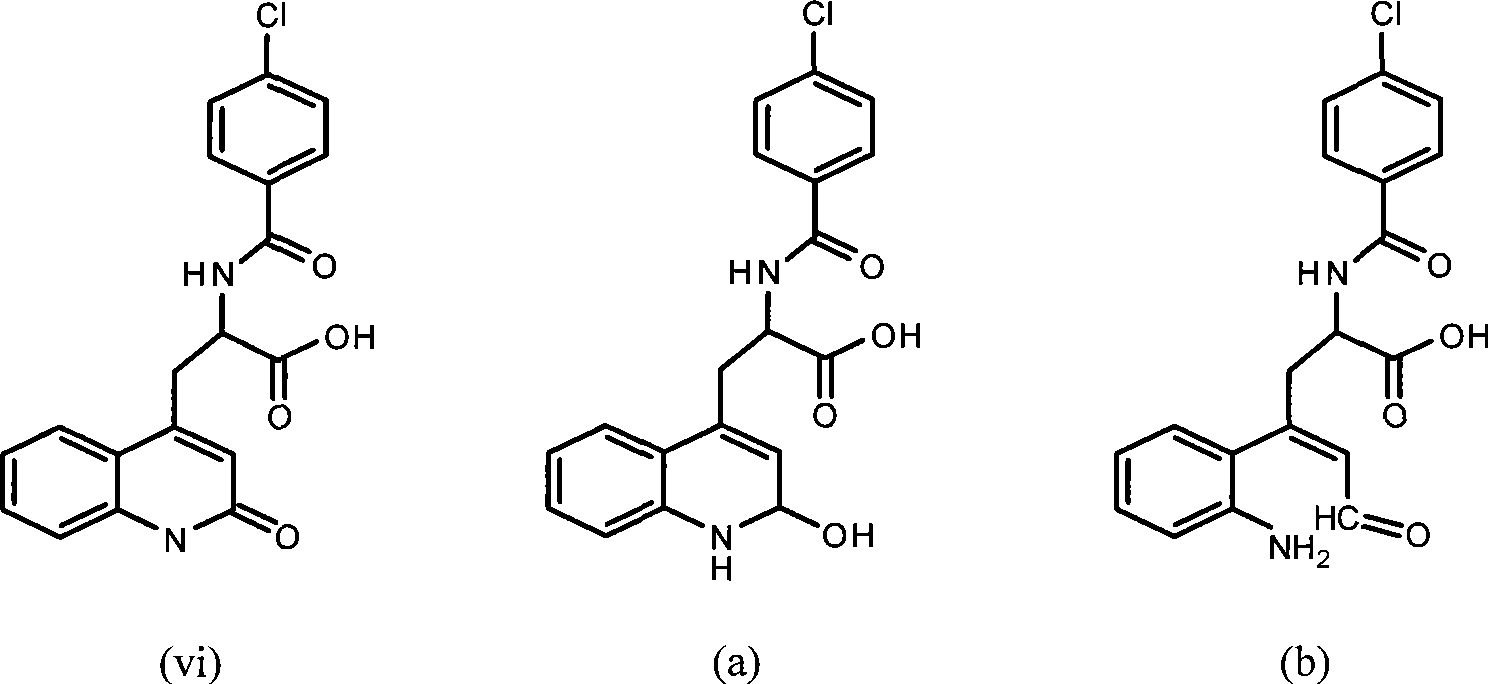
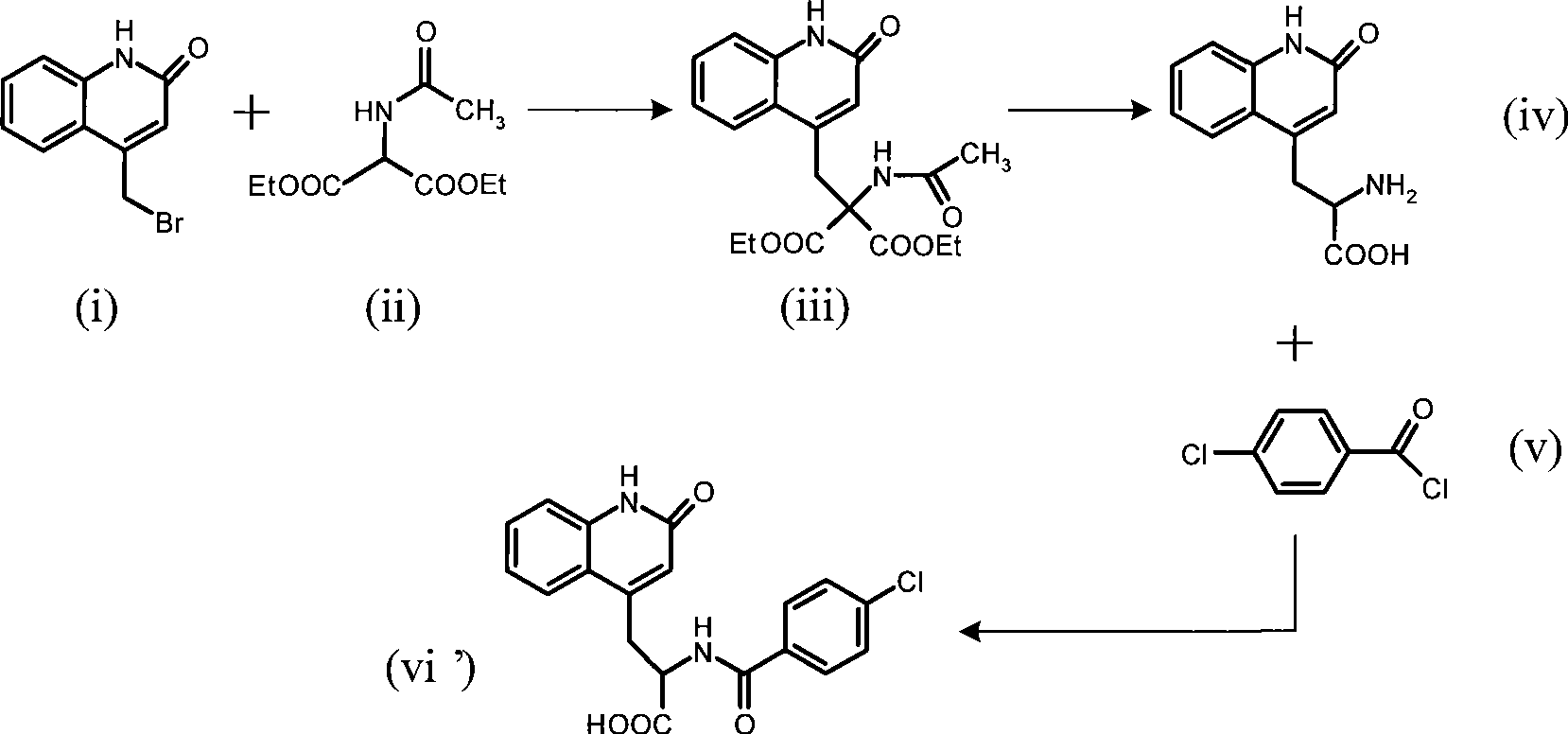
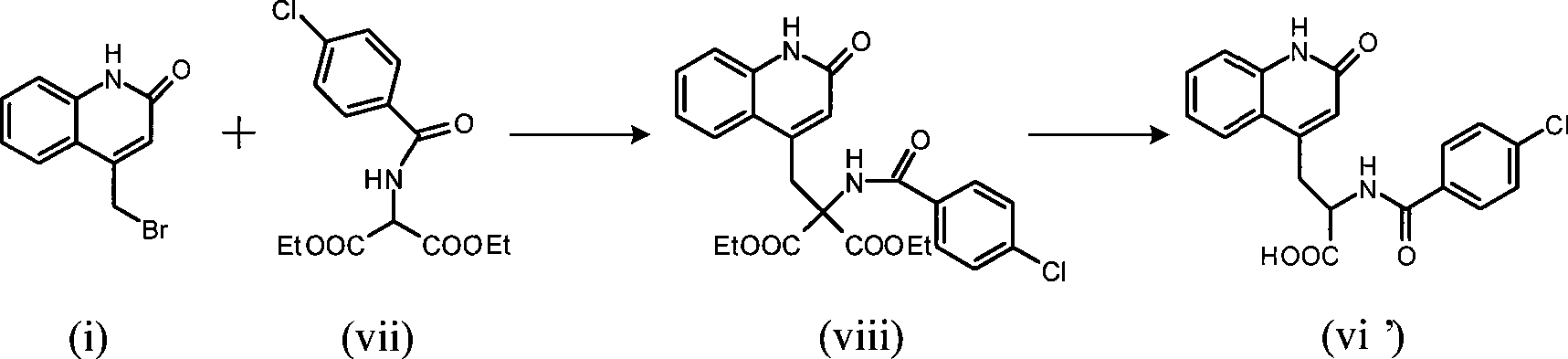
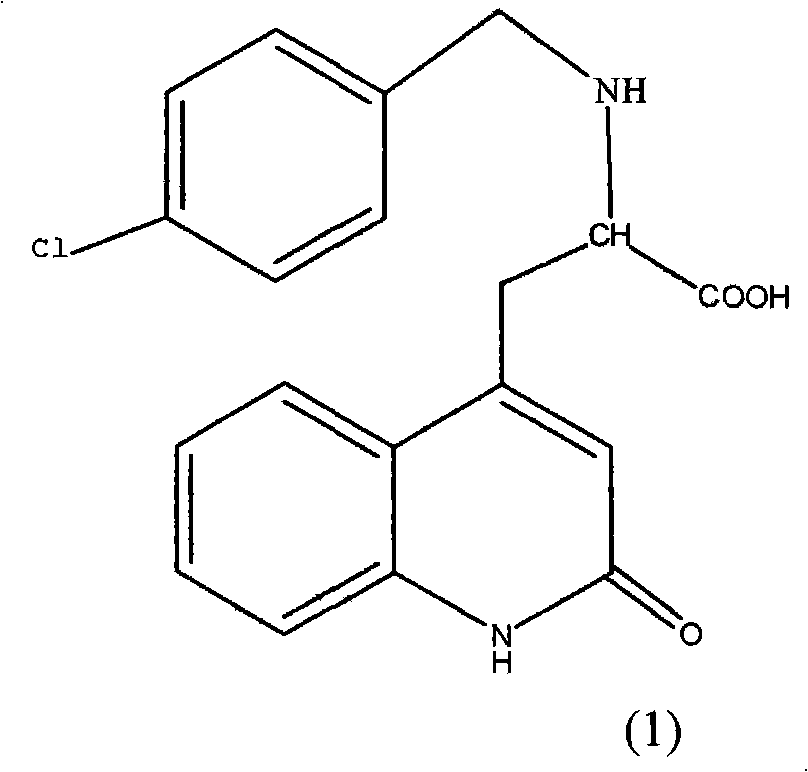
Rebamipide, an amino acid derivative of 2-(1H)-quinolinone, is used for mucosal protection, healing of gastroduodenal ulcers, and treatment of gastritis. It works by enhancing mucosal defense, scavenging free radicals, and temporarily activating genes encoding cyclooxygenase-2.
Method for purifying rebamipide crude product
InactiveCN101463005AImprove applicationInhibition of ring opening reactionOrganic chemistryDigestive systemActivated carbonProtective drugs
The invention relates to a purifying method for a Rebamipide crude product. Alkaline is added in alcohol-water mixed solvent to dissolve the Rebamipide crude product. Then, activated carbon is added for decolorizing. Concentrated hydrochloric acid is added dropwise, pH is regulated to 2-3, solids are precipitated, and purified Rebamipide is obtained after filtering and drying. During the process, the mol ratio of the added amount of alkaline to the Rebamipide is controlled to be less than 3 to 1. And the operational temperature during the alkaline adding for dissolution and the acid adding for crystallization is controlled at 150 DEG C; therefore; the ring cleavage reaction of quinolone is inhabited and the generation of two impurities which are similar in structure with Rebamipide is reduced. Rebamipide with the purity of higher than 99.5 percent is obtained. Conditions are provided for improving the application degree of Rebamipide in pharmaceutical aspects of gastric mucosal protective drugs, etc.
Owner:苏州天马医药集团天吉生物制药有限公司
Aqueous Ophthalmic Suspension of Crystalline Rebamipide
InactiveUS20070287729A1High transparencySignificant contributionAntibacterial agentsBiocideCompound (substance)Water soluble
The invention provides an ophthalmic product containing rebamipide, which has a transparency enough to be agreeable feeling on using it and has neutral to weakly acidic pH not to injury of the keratoconjunctiva of a patient suffering from dry eye. An aqueous suspension of crystalline rebamipide which has an improved transparency is provided by adding an aqueous solution of rebamipide dissolved by a base such as sodium hydroxide or an aqueous solution of a salt of rebamipide to an aqueous acidic solution such as hydrochloric acid containing at least one of the compounds selected from water-soluble polymers and surfactants, and mixing them.
Owner:OTSUKA PHARM CO LTD
Pharmaceutical composition comprising rebamipide
InactiveUS20110124682A1Sufficient transparencyHigh transparencyBiocideSenses disorderAmino sugarDrug
An object of the present invention is to provide a pharmaceutical composition containing rebamipide, which is unnecessary to be re-dispersed, has an enough transparency, and exhibits neutral to weakly acidic pH not to injure the keratoconjunctiva of a patient suffering from dry eye. The present pharmaceutical composition comprises (1) rebamipide, (2) an amino sugar, and (3) an buffer agent, which has no inorganic cation.
Owner:OTSUKA PHARM CO LTD
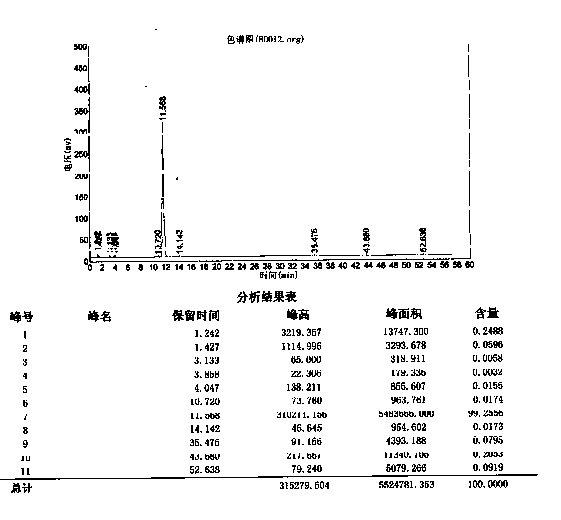
Analytic method for related substance examination of rebamipide
ActiveCN103076421AQuality improvementHigh selectivityComponent separationColumn temperatureChromatographic column
The invention relates to an analytic method for related substance examination of rebamipide, in particular to a method for mass analysis of rebamipide. The method comprises the step of adopting the high-performance liquid chromatography to achieve mass analysis of rebamipide drug substances or pharmaceutical preparations containing rebamipide. A chromatographic column used in the method is a C18 chromatographic column with the column temperature of 30 DEG C-40 DEG C. The analytic method effectively separate rebamipide and all impurities of rebamipide under a certain chromatographic condition, and can accurately measure the quantities of all the impurities in rebamipide.
Owner:北京元延医药科技股份有限公司
Synthesizing method of rebamipide
The invention provides an improved method for preparing rebamipide from 2-amino-3-(1.2-dihydro-2-oxo-4-quinolyl) propionic acid hydrochloride (abbreviated as amino acid salt) through a one-step reaction. By utilizing the method, the rebamipide with high purity can be prepared. After activated carbon is added to the amino acid salt to carry out adsorption decoloration under the alkaline condition, the amino acid salt reacts for 1 to 4 hours in alkaline alcohol liquid to obtain the finished product, wherein the yield is from 80% to 95%; and the product purity is more than 99.5%. According to the method, the yield of the rebamipide product is improved; few impurities are generated; the environment pollution is small; and the production cost is lowered. Therefore, the method provided by the invention is suitable for the large-scale production mode with the high yield.
Owner:ZHEJIANG YUANLIJIAN PHARMA
Green and efficient preparation method of rebamipide
ActiveCN108440409ALow priceEase of industrial productionOrganic chemistryBenzoic acidSynthesis methods
The invention belongs to the technical field of medical synthesis and in particular relates to a green and efficient preparation method of rebamipide. The synthesis method provided by the invention has a green and simple route and the price of raw materials is low, so that industrial production is easy to realize. The invention further discloses a novel method for preparing aniline from benzoic acid.
Owner:JINAN ASIA PHARMA TECH
Novel process for synthesizing rebamipide
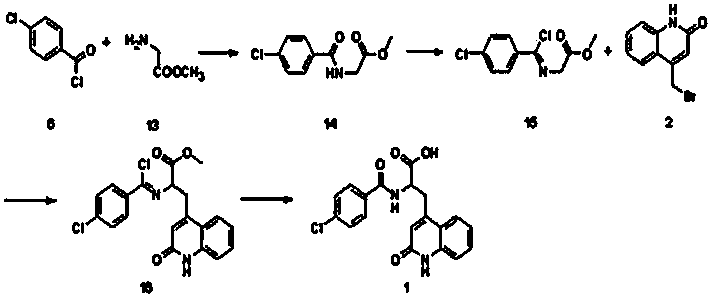
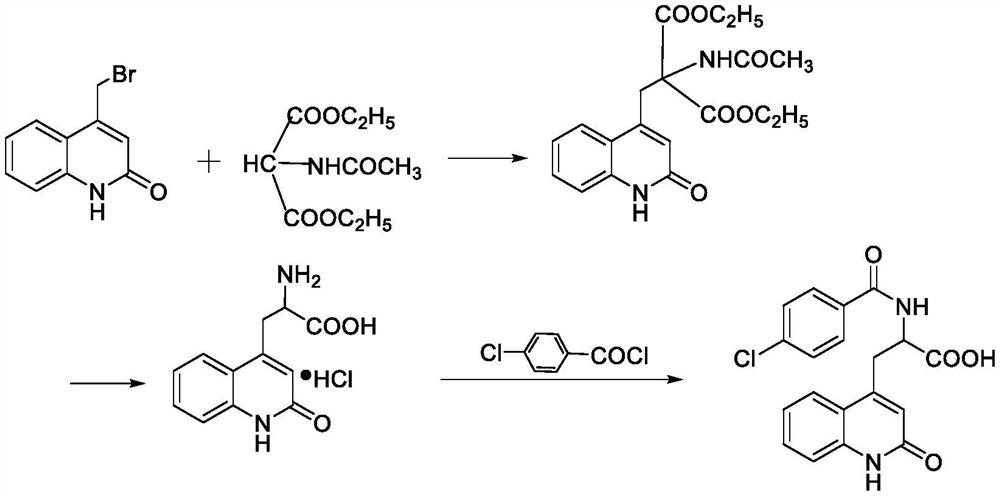
The invention discloses a novel process for synthesizing rebamipide. The novel process comprises the following steps: adopting glycine methyl ester as a starting raw material, then performing amidation and chlorination to obtain chloroimide intermediate, enabling the chloroimide intermediate to react with bromomethylquinolone, and hydrolyzing to obtain the rebamipide. The novel process has the advantages that the starting raw material is low in price and easy to obtain, the reaction yield is high, the industrialization is easy to realize and the like.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD
Medicinal composition as well as preparation and application thereof
InactiveCN103920138APromote secretionGood treatment effectOrganic active ingredientsSenses disorderXerophthalmiaTreatment effect
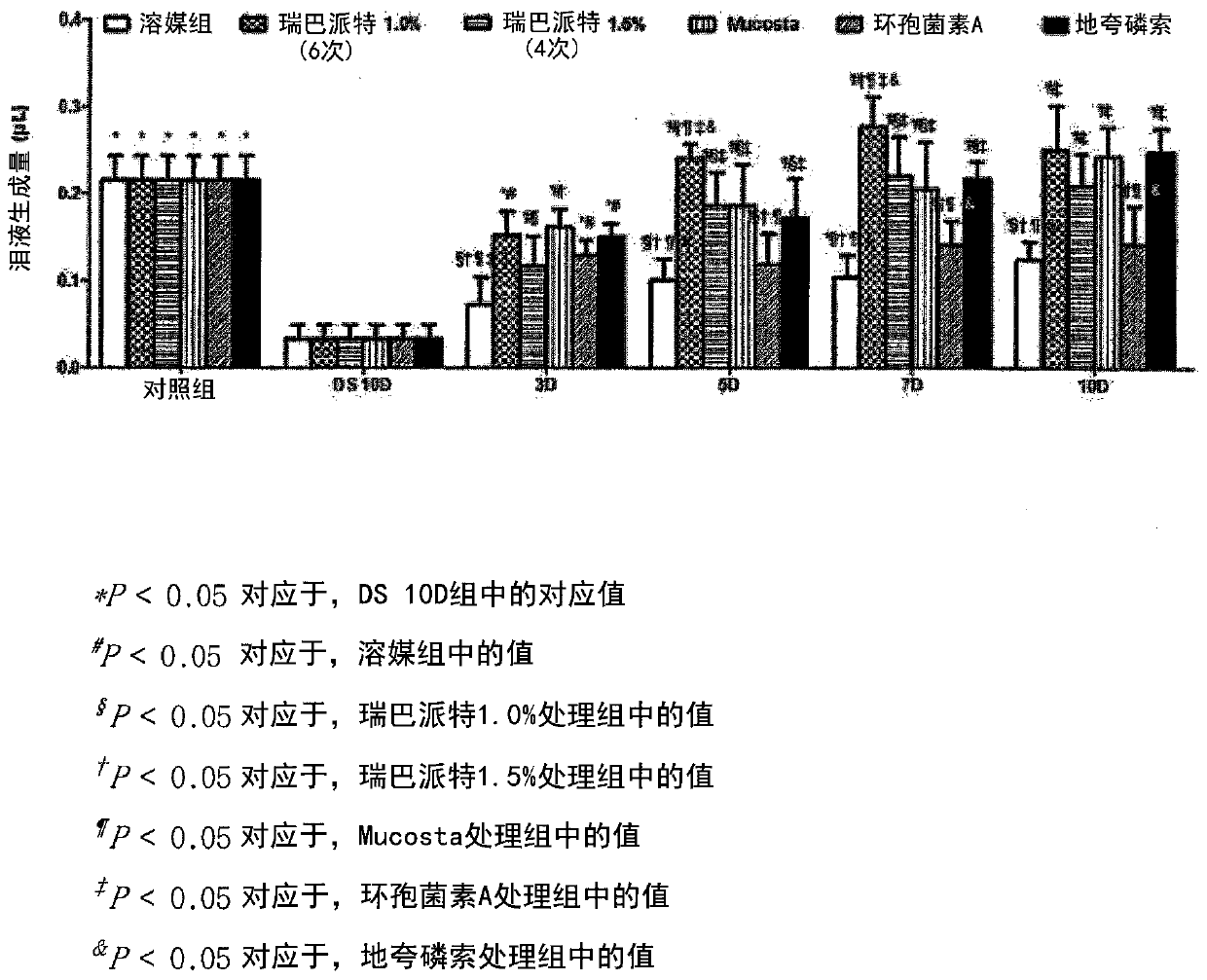
The invention relates to the technical field of medicines, and in particular relates to a medicinal composition as well as a preparation and application thereof. The medicinal composition comprises a component a and a component b; the component a is rebamipide, pharmaceutically acceptable salt or isomeride thereof; and the component b is ciclosporin A, pharmaceutically acceptable salt or isomeride thereof. Experiments prove that a synergistic effect can be achieved when the rebamipide and the ciclosporin A are used together. The composition significantly improves the effects of promoting lacrimal secretion and eliminating inflammation compared with the effects achieved when the rebamipide and the ciclosporin A are used separately (P<0.05). Therefore, when the rebamipide and the ciclosporin A are compounded to prepare the medicament for treating xerophthalmia, the treatment effect can be improved.
Owner:ZHAOKE PHARMA GUANGZHOU
Aqueous pharmaceutical suspensions containing rebamipide and manufacturing process thereof
InactiveUS20100029714A1Promote recoverySimple processBiocideSenses disorderPolyvinyl alcoholSodium salt
The invention provides a rebamipide-containing aqueous pharmaceutical suspension which can be prepared by a simple process and keep the dispersed fine-particle state of rebamipide stable without having the fine particle agglutinated. The rebamipide-containing aqueous pharmaceutical suspension of the invention is prepared by mixing polyvinyl alcohol and additionally a sodium salt compound with rebamipide.
Owner:OTSUKA PHARM CO LTD
Medicament for treating anterior eye disease comprising rebamipide and a tear-retaining agent
The present invention provides a combination of rebamipide and a tear-retaining agent as a medicament for the treatment of anterior eye diseases.
Owner:OTSUKA PHARM CO LTD
Pharmaceutical composition for treating disease in oral cavity comprising rebamipide
The present invention is directed to a pharmaceutical composition comprising rebamipide having a mean particle size of less than 500 nm, a dispersing agent, and a viscosity enhancing agent wherein the viscosity enhancing agent has no aggregative action for the rebamipide particles, which is used as a gargle or a liquid preparation for swish and swallow comprising rebamipide for preventing and / or treating stomatitis caused by radiotherapy.
Owner:OTSUKA PHARM CO LTD
Analysis method for determining rebamipide related substances by using HPLC
ActiveCN111595985AEfficient separationMeet separation requirementsComponent separationSilanesGradient elution
The invention relates to the technical field of analytical chemistry, and discloses an analysis method for determining rebamipide related substances by using HPLC. According to the analysis method, octadecylsilane chemically bonded silica is used as a reversed phase chromatographic column of a filler, a buffer salt solution is used as a mobile phase A, the buffer salt solution, methanol and tetrahydrofuran are used as a mobile phase B, gradient elution is carried out on a rebamipide sample solution, and HPLC analysis is carried out. The analysis method provided by the invention can effectivelyseparate rebamipide and related substances thereof, so that impurity peaks and rebamipide peaks are not overlapped, the peak shape is good, the separation requirement is met, and the method is suitable for controlling related substances and researching impurities.
Owner:苏州正济药业有限公司
Preparation method of high-purity rebamipide
ActiveCN104230798AHigh purityEfficient removalOrganic chemistryBiochemical engineeringCombinatorial chemistry
The invention discloses a preparation method of high-purity rebamipide. During synthesis process of a rebamipide crude product, the rebamipide crude product is precipitated out, in the form of a rebamipide salt solid, directly from a reaction solution. Thus, effective separation of impurities from a product is realized. A rebamipide salt crude product has good quality and high purity. Without further refining, dissociating is carried out directly by an acid-alkali method so as to obtain a high-purity rebamipide finished product. By the above method to obtain rebamipide, operational steps and refining frequency are minimized, and production efficiency is raised remarkably. In addition, a solvent used is cheap and easily available, and it is beneficial to realize industrial production.
Owner:CHONGQING SHENGHUAXI PHARMA CO LTD +2
Process for Preparing Rebamipide
InactiveUS20070249835A1High yieldHigh purityOrganic active ingredientsOrganic chemistryReduction treatmentHydrogen
The invention provides an improved process for preparing rebamipide that is useful as a medicament, which makes it possible to prepare rebamipide with high purity and high yield. The invention is an improved process for preparing rebamipide of the formula (1), comprising subjecting a carbostyril amino acid compound of the formula (5) or a salt thereof containing a compound of the formula (11) as an impurity to a reduction treatment in the presence of hydrogen and a catalyst in a basic aqueous solution, thereby selectively reducing the impurity compound (11) to transform into the carbostyril amino acid compound (5); and then acylating the compound (5) in a basic aqueous solution to give rebamipide (1).
Owner:OTSUKA PHARM CO LTD
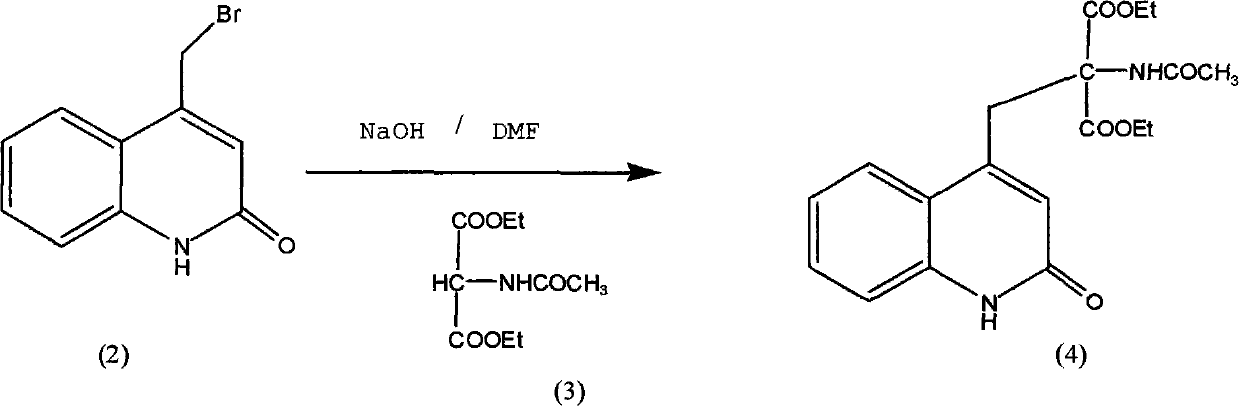
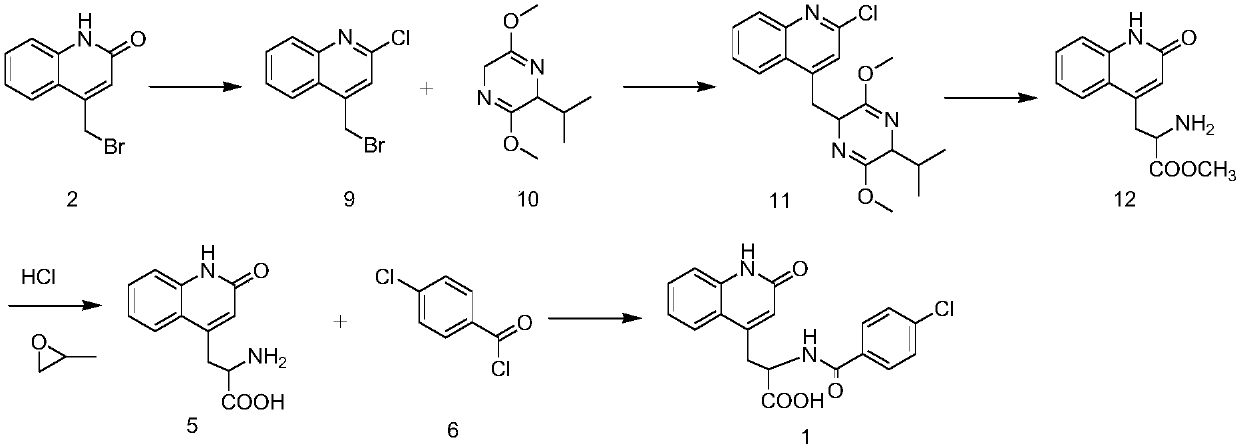
Improved preparation method of rebamipide intermediate
InactiveCN101870674AHigh purityMild reaction conditionsOrganic chemistryMedicinal chemistryCondensation reaction
The invention provides an improved preparation method of a rebamipide intermediate. An intermediate (4) is obtained by condensation reaction of an intermediate (2) and an intermediate (3) in DMF (Dimethyl Formamide) / NaOH, wherein the reaction time is 5-7h, the yield is 85-92 percent, and the product purity is above 98.5 percent. The method has mild reaction condition as well as simple and convenient operation, can remarkably improve the operability on the product yield and industry and is beneficial to industrial production.
Owner:SHAANXI DASHENG PHARMA TECH
Oral pharmaceutical composition for preventing or treating dry eye syndrome comprising rebamipide or a prodrug thereof
ActiveUS20160081922A1Employed safely and convenientlyBiocideSenses disorderBULK ACTIVE INGREDIENTActive ingredient
Disclosed is an oral pharmaceutical composition for preventing or treating dry eye syndrome, which comprises rebamipide or a prodrug thereof, or a pharmaceutically acceptable salt thereof, as an active ingredient. The compounds can treat dry eye syndrome via oral route, and can be thus employed safely and conveniently compared to conventional eye drops.
Owner:SAMJIN PHARMA +1
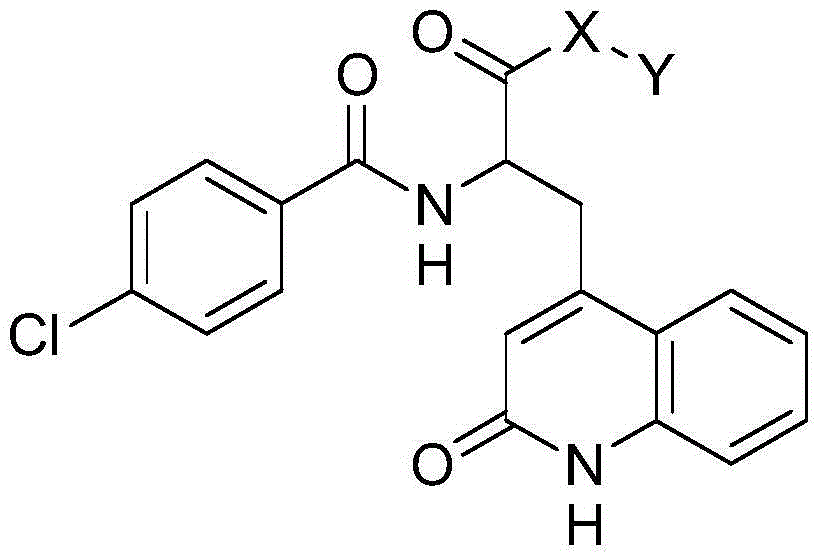
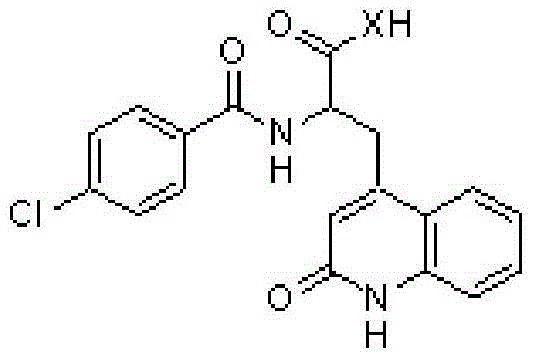
Novel rebamipide prodrug, method for producing same, and usage thereof
ActiveCN104662004AImproved absorption in the bodyImprove absorption in the bodyOrganic active ingredientsSenses disorderMedicineBULK ACTIVE INGREDIENT
Disclosed are a novel rebamipide prodrug, a method for preparing the same, and use thereof. Also, a pharmaceutical composition comprising the novel rebamipide prodrug as an active ingredient is provided. The rebamipide prodrug is increased 25-fold in absorption rate compared to rebamipide itself, and can be applied to the prophylaxis or therapy of gastric ulcer, acute gastritis, chronic gastritis, xerophthalmia, cancer, osteoarthritis, rheumatoid arthritis, or obesity.
Owner:SAMJIN PHARMA +1
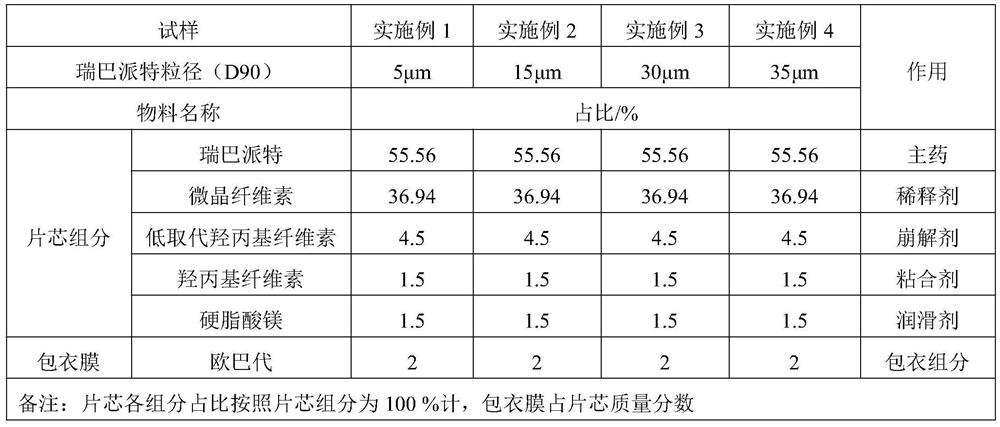
Rebamipide tablet and preparation method thereof
PendingCN114053242AIncrease surface areaLarge porosityOrganic active ingredientsDigestive systemCelluloseCross-link
The invention relates to a rebamipide tablet and a preparation method thereof, and relates to the technical field of medicines. The rebamipide tablet provided by the invention contains rebamipide and other pharmaceutically available inactive components, and the particle size D90 of the rebamipide is 3-30 [mu]m. The other pharmaceutically available inactive components comprise a diluent, a disintegrating agent, an adhesive and a lubricant. The disintegrating agent is one or two of cross linked sodium carboxymethyl cellulose, low-substituted hydroxypropyl cellulose, polyvinylpolypyrrolidone and sodium carboxymethyl starch. The dissolution curve of the rebamipide tablet is not obviously different from that of a product produced by Otsuka Pharmaceutical Co., LTD in Japan, which is an original research factory. The rebamipide tablet granulation process provided by the invention can improve the problems of difficult granulation and poor fluidity, and the preparation process is simple and controllable.
Owner:苏州天马医药集团天吉生物制药有限公司
Method for synthesizing rebamipide
InactiveCN107674023AThe synthesis process is simpleLow costOrganic chemistryHydrolysisGlycine methyl ester
The invention discloses a method for synthesizing rebamipide. The method includes carrying out acylation and chlorination on glycine methyl ester to obtain chlorimide intermediates; carrying out substitution reaction on the chlorimide intermediates and bromomethyl quinolinone; carrying out hydrolysis to obtain the rebamipide. The glycine methyl ester is used as a starting material. The method hasthe advantages that the starting material is low in cost and is easily available, the method is high in reaction yield, industrialization can be facilitated, and the like.
Owner:CHONGQING UNIV OF TECH
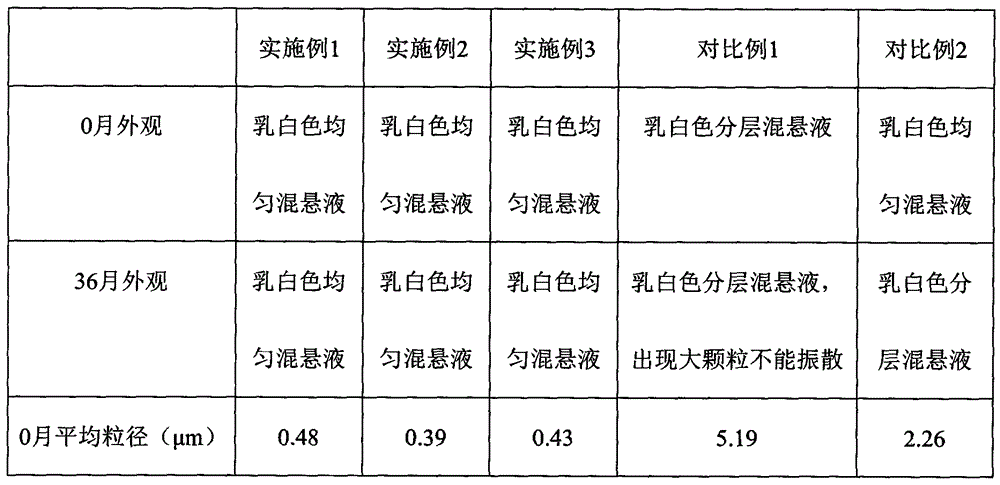
Preparation method of rebamipide aqueous suspension
ActiveCN105878245ALittle change in particle sizeParticle size does not increaseOrganic active ingredientsSenses disorderSterile environmentAdjuvant
The invention discloses a preparation method of a rebamipide aqueous suspension. The preparation method comprises the following steps: (1) dissolving all adjuvant materials in injection water, so that an adjuvant material aqueous solution is obtained; (2) adding rebamipide to the adjuvant material aqueous solution obtained in the step (1), performing crushing and dispersing, so that primary liquid of the rebamipide aqueous suspension is obtained; (3) homogenizing the primary liquid of the rebamipide aqueous suspension under high pressure, so that the aqueous suspension containing the rebamipide is finally obtained; and (4) sterilizing the rebamipide aqueous suspension obtained in the step (3), and sub-packaging the rebamipide aqueous suspension in a sterile environment in containers for clinical use. Compared with the prior art, the preparation method has the advantages that the average grain size of the rebamipide in the rebamipide aqueous suspension prepared by the rebamipide aqueous suspension preparation method disclosed by the invention is less than 1[micron]m; and the grain size cannot change much after high-temperature sterilization and cannot increase after long-term storage.
Owner:ZHUHAI ESSEX BIO PHARMA
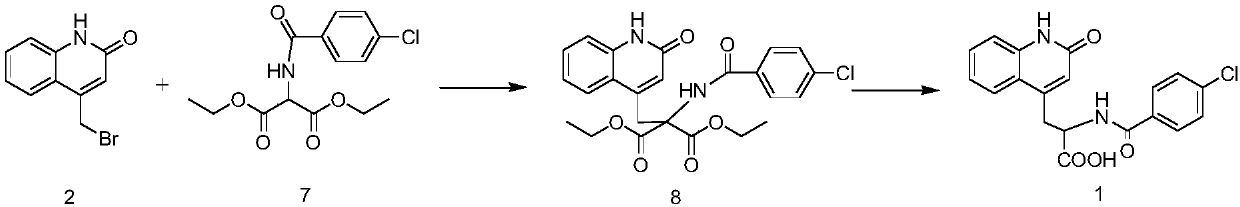
Preparation method of rebamipide bulk drug
PendingCN113248429AHigh purityReduce manufacturing costOrganic chemistryChlorobenzeneMethyl palmoxirate
The invention discloses a preparation method of a rebamipide bulk drug. The preparation method comprises the following steps: 1, preparing a crude product of the rebamipide bulk drug: preparing the crude product of the rebamipide bulk drug by utilizing a compound III and 4-chlorobenzoyl chloride; and 2, purifying the rebamipide bulk drug. The compound III is prepared from a compound II, deionized water and concentrated hydrochloric acid, the compound II is prepared from a compound I, glacial acetic acid and concentrated hydrochloric acid, and the compound I is prepared from diethyl acetamidomalonate and 4-bromomethylquinolone. A process control method is utilized, impurities in the synthesized compound I, impurities in the synthesized compound II and impurities in the synthesized compound III are respectively subjected to impurity removal and purification by a common purification method, and the purity of the rebamipide bulk drug is improved in combination with a method for crystallizing and purifying the crude product of the rebamipide bulk drug.
Owner:千辉药业(安徽)有限责任公司
Synthesis technology of rebamipide
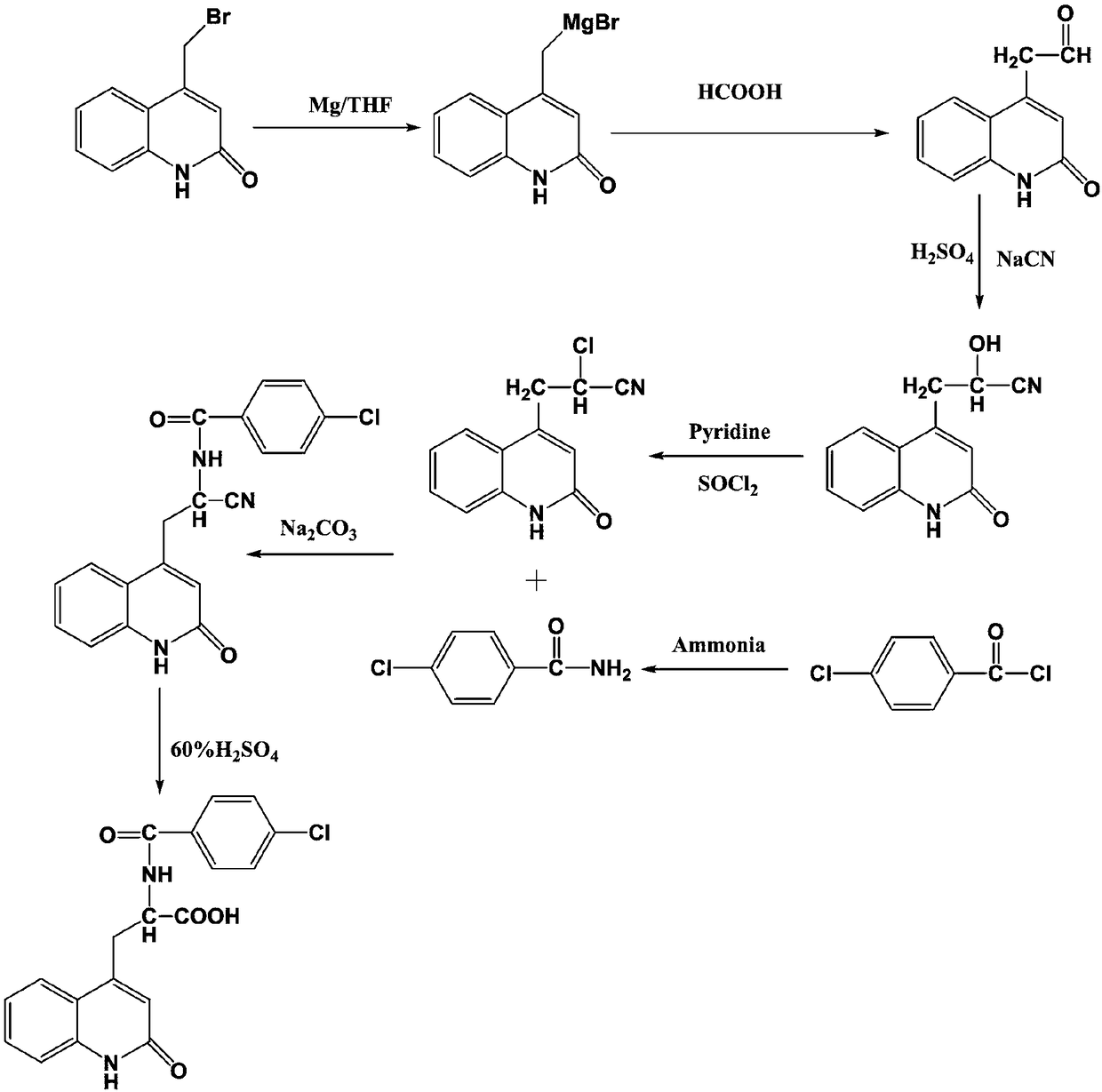
The invention discloses a synthesis technology of rebamipide. A specific synthesis process comprises the following steps: adding ammonia water into a reaction container; then dropwise adding 4-chlorobenzoyl chloride into the reaction container to obtain 4-chlorobenzamide; after carrying out Grignard reaction, reacting with formic acid to prepare a product 1; carrying out addition reaction on the product 1 to prepare a product 2; after carrying out chlorination on the product 2, taking the product 2 and the 4-chlorobenzamide to react to obtain a product 3; adding the product 3 into the reactioncontainer and adding a sulfuric acid solution with the mass percent of 60 percent into the reaction container; heating to 80 DEG C and stirring to react for 3h to obtain a product 2-(4-chlorobenzoylamino)-3-[2(1H)-quinolinone-4]propionic acid. In a synthesis process, the rebamipide is prepared through the Grignard reaction, nucleophilic addition and substitution reaction; the production cost is low and the yield of the product is high; synthesis conditions are simple and the synthesis technology can be applicable to large-scale production.
Owner:千辉药业(安徽)有限责任公司
Rebamipide for prevention and treatment of Crohn's disease
PendingCN114340628AControl disease symptomsOrganic active ingredientsDigestive systemDiseaseSmall intestine permeability
The present invention provides rebamipide for use in a method of preventing and / or treating Crohn's disease. In particular, rebamipide is used to prevent and / or treat Crohn's disease in a person suffering from or at risk of increased intestinal permeability.
Owner:方电力有限公司
Rebamipide monohydrate crystal form and preparation method thereof
The invention relates to a rebamipide monohydrate crystal form and a preparation method thereof. The invention further relates to a pharmaceutical composition taking a rebamipide monohydrate containing the crystal form as an active compound and application of the pharmaceutical composition in manufacturing of medicaments for treating diseases related to gastric ulcer, acute gastritis, chronic gastritis and gastric mucosal injuries caused by acute exacerbation.
Owner:CHONGQING HUIZHI PHARMA RES INST +1
Drug for treating oral diseases and preparation method thereof
InactiveCN105327359AGood viscosity enhancement effectHigh inhibition rateOrganic active ingredientsDigestive systemOral diseaseDisease
The invention relates to drug for treating oral diseases and a preparation method thereof. The drug is a liquid preparation and comprises rebamipide serving as an active ingredient, a dispersant and a viscosity enhancer, a viscosity range of the liquid preparation is 10mPa.s-500mPa.s, concentration of rebamipide is 10mg / mL-50mg / mL, and average grain size of rebamipide is smaller than 500 micrometers, and the viscosity enhancer is a mixture of hydroxy propyl cellulose, polyvinyl alcohol and sodium carboxymethylcellulose. The mixture of hydroxy propyl cellulose, polyvinyl alcohol and sodium carboxymethylcellulose is used as the viscosity enhancer, so that viscosity of the drug can be enhanced better and better treatment effect can be realized.
Owner:WUXI CHENGBO SCI & TECH DEV
Water-soluble multi-use eyedrops composition for treatment of dry eye syndrome containing rebamipide and method for solubilization and stabilization thereof
ActiveCN110638748AOutstanding stabilityOutstanding preservation abilityOrganic active ingredientsSenses disorderAqueous solubilityOrganic chemistry
The present invention relates to a water-soluble multi-dose eyedrop composition for treating dry eye syndrome comprising rebamipide, and a method for solubilizing and stabilizing the same, and relatesto an eyedrop composition for treating dry eye syndrome which, by including a preservative and a chelating agent in a disposable water-soluble rebamipide-containing eyedrop, can remain stable withoutforming precipitates during storage, and is characterized by comprising ingredients that are safe to be used as a multi-dose eyedrop; and to a preparation method of the same.
Owner:DAEWOO PHARMA IND
Aqueous ophthalmic suspension of crystalline rebamipide
InactiveUS9211254B2High transparencySignificant contributionAntibacterial agentsBiocideOphthalmic ProductSURFACTANT BLEND
The invention provides an ophthalmic product containing rebamipide, which has a transparency enough to be agreeable feeling on using it and has neutral to weakly acidic pH not to injury of the keratoconjunctiva of a patient suffering from dry eye. An aqueous suspension of crystalline rebamipide which has an improved transparency is provided by adding an aqueous solution of rebamipide dissolved by a base such as sodium hydroxide or an aqueous solution of a salt of rebamipide to an aqueous acidic solution such as hydrochloric acid containing at least one of the compounds selected from water-soluble polymers and surfactants, and mixing them.
Owner:OTSUKA PHARM CO LTD
Ophthalmic preparation and application thereof in treating presbyopia
PendingCN114588156ADelay long-term progressionLittle side effectsOrganic active ingredientsSenses disorderOphthalmologySphincter Pupillae Muscle
The invention discloses an ophthalmic preparation and application thereof in treatment of presbyopia, the ophthalmic preparation comprises acestodine and rebamipide, it is found and proved for the first time that the acestodine and the rebamipide are combined to have a synergistic effect, the rebamipide can enhance the effect of the acestodine and reduce related side effects, and the ophthalmic preparation can be used for treating presbyopia. Vinegar can effectively generate a synergistic effect with rebamipide to contract the pupil sphincter, and the ophthalmic preparation can effectively improve, relieve or treat presbyopia, has a potential effect of delaying the progress of the aging eye course, and has a very good clinical application prospect.
Owner:THE EYE HOSPITAL OF WENZHOU MEDICAL UNIV
Novel eye drop composition for treating dry eye syndrome containing rebamipide and method for solubilizing and stabilizing same
ActiveCN111107838AImprove bioavailabilityEasy to useOrganic active ingredientsPharmaceutical delivery mechanismBuffering agentAqueous solution
The present invention relates to an eye drop composition for treating dry eye syndrome, characterized in that the eye drop composition remains stable during storage without precipitation occurring andcontains a safe ingredient for use as an eye drop, by solubilizing rebamipide that is a poorly soluble compound, and a method for producing the same. The eye drop composition for treating dry eye syndrome of the present invention includes a base for solubilizing rebamipide, and is characterized by containing a stabilizer for maintaining a supersaturated stabilized state, a specific type of bufferfor maintaining biocompatible pH of 7 to 8 and an osmotic agent. A method for producing the same comprises the steps of: adding a base to rebamipide or a pharmaceutically acceptable salt thereof anda stabilizer and dissolving the same in water to obtain an aqueous eye drop solution of pH of 10 to pH of 11; adding at least one osmotic agent selected from a buffer and a nonionic osmotic agent to the eye drop solution and dissolving the same; and after adjusting the pH to 7 to 8 with an acid, filtering the solution with a syringe filter to make the same sterile. The produced eye drop is in theform of an aqueous solution, is stable when frozen, refrigerated, and at room temperature, and includes a therapeutic agent for dry eye syndrome which is stable even when cross-stored in a refrigerator or at room temperature, or at a refrigerating temperature or room temperature.
Owner:DAEWOO PHARMA IND
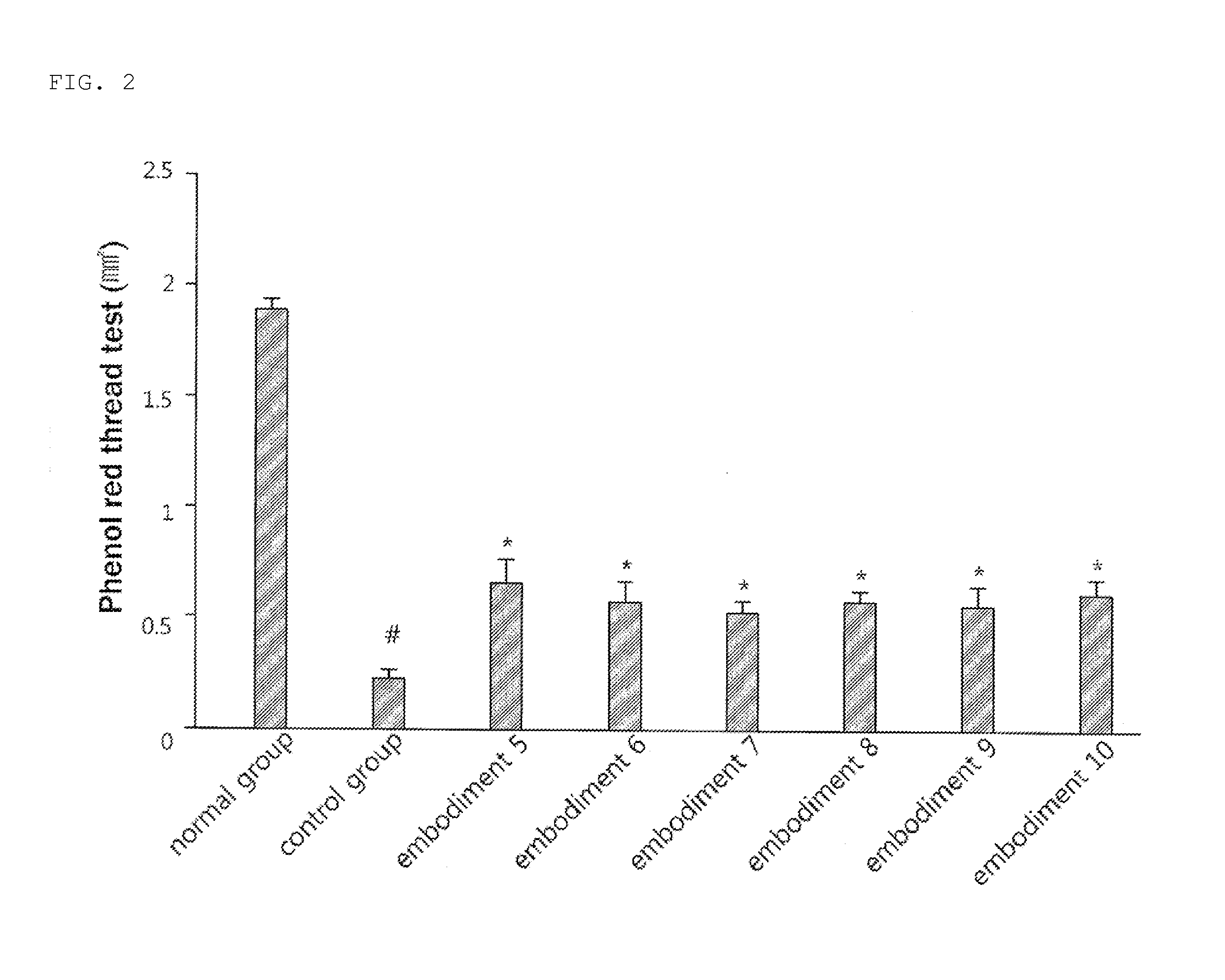
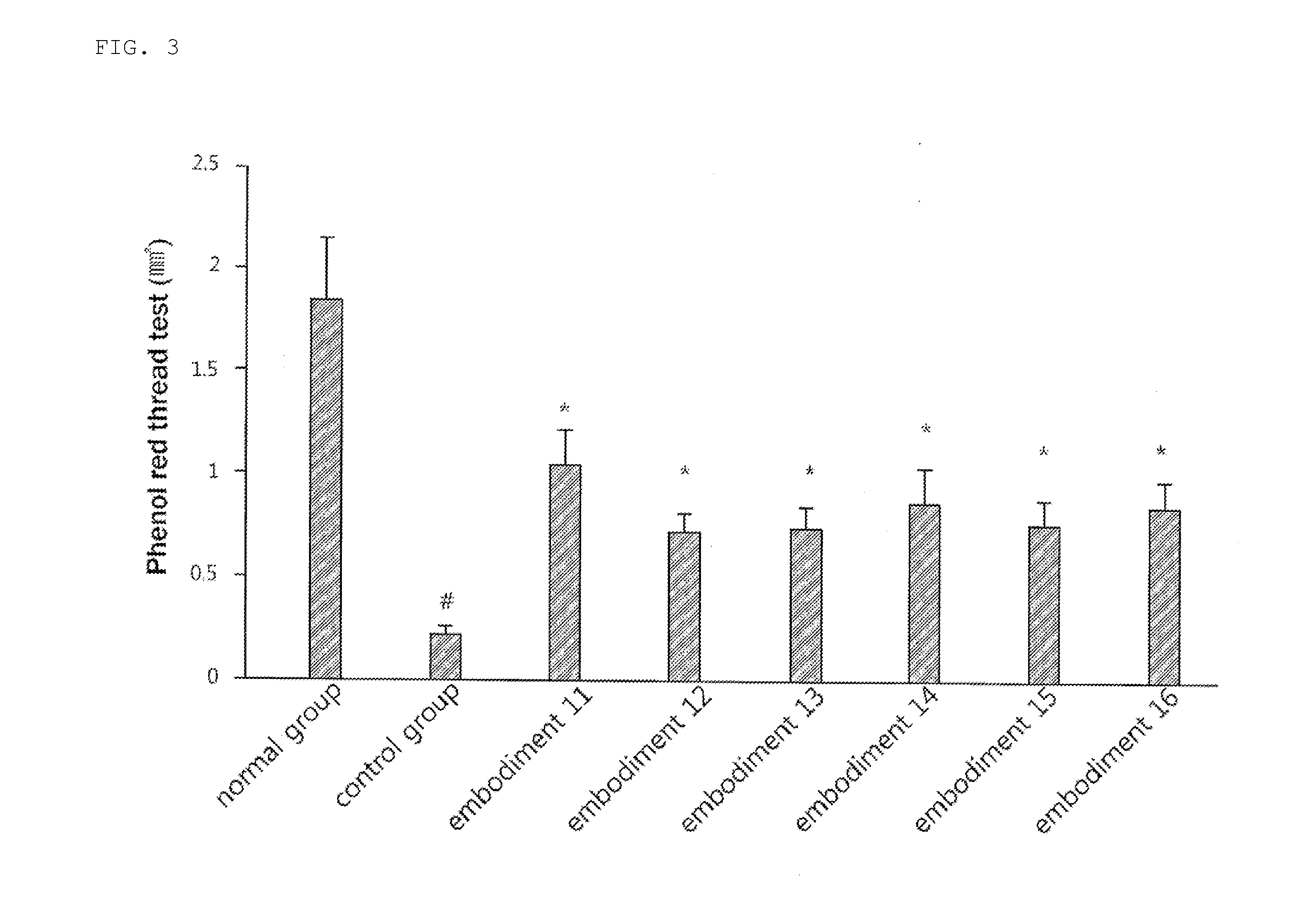
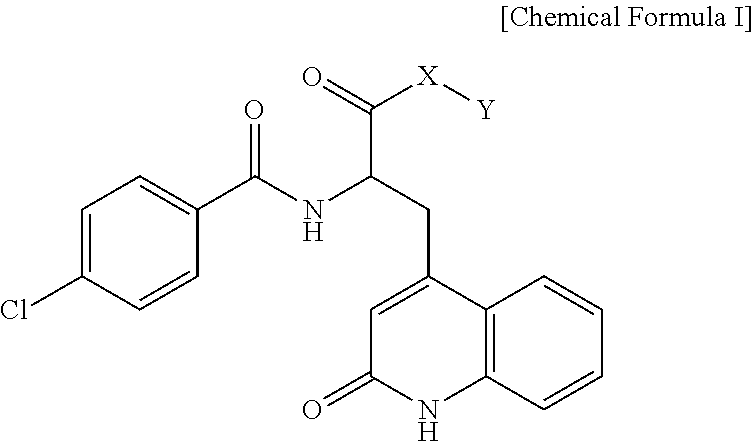
Rebamipide prodrugs, preparation method and use thereof
ActiveUS11420963B2Improve absorption rateImprove performanceOrganic active ingredientsSenses disorderBULK ACTIVE INGREDIENTOsteo arthritis
Disclosed are a novel rebamipide prodrug, a method for preparing the same, and use thereof. Also, a pharmaceutical composition comprising the novel rebamipide prodrug as an active ingredient is provided. The rebamipide prodrug is increased 25-fold in absorption rate compared to rebamipide itself, and can be applied to the prophylaxis or therapy of gastric ulcer, acute gastritis, chronic gastritis, xerophthalmia, cancer, osteoarthritis, rheumatoid arthritis, or obesity.
Owner:SAMJIN PHARMA +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com