Analysis method for determining rebamipide related substances by using HPLC
An analytical method and mobile phase technology used in the field of analytical chemistry to achieve good peak shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1: contrast HPLC analytical method
[0059] High performance liquid chromatography: Shimadzu;
[0060] Chromatographic column: Shiseido CAPCELL PAK UG120 250*4.6mm*5μm;
[0061] Mobile phase: Dissolve 2.44g of sodium 1-decanesulfonate in 1000ml of water, then add 1000ml of methanol and 10ml of phosphoric acid;
[0062] Detection wavelength: 232nm;
[0063] Flow rate: adjust the flow rate so that the retention time of rebamipide is about 12 minutes;
[0064] Column temperature: 25°C;
[0065] Injection volume: 10μl;
[0066] isocratic elution;
[0067] Mixed solution preparation: take appropriate amount of rebamipide, impurity A, impurity B, impurity C, and impurity D, and diluent [water-pH6.0 0.05mol / L phosphate buffer-methanol (7:7:6 )] dissolved and diluted to make a solution containing about 0.4 mg of rebamipide per 1 ml, and 0.04 mg of impurity A, impurity B, impurity C, and impurity D respectively, and shake well.
[0068] Test results: See the at...
Embodiment 2
[0069] Embodiment 2: contrast HPLC analytical method
[0070] High performance liquid chromatography: Shimadzu;
[0071] Chromatographic column: Wakopak Wakosil-II 3C18 4.6mm*150mm;
[0072] Mobile phase A: Phosphate buffer solution of pH 6.2 (weigh 9.08g of potassium dihydrogen phosphate and dissolve in 1000ml of water, weigh 9.46g of anhydrous disodium hydrogen phosphate and dissolve in 1000ml of water. Take 800ml of potassium dihydrogen phosphate solution and add Mix 200ml of disodium hydrogen phosphate solution evenly. If necessary, adjust the pH to 6.2 with potassium dihydrogen phosphate solution or disodium hydrogen phosphate solution) Add 750ml of water to 300ml and mix well;
[0073] Mobile phase B: acetonitrile;
[0074] Detection wavelength: 222nm;
[0075] Column temperature: 25°C;
[0076] Injection volume: 10μl;
[0077] Gradient elution: the flow rate was adjusted so that the retention time of rebamipide was about 20 minutes, see Table 5.
[0078] table 5 ...
Embodiment 3
[0082] Embodiment 3: contrast HPLC analytical method
[0083] High performance liquid chromatography: Agilent 1260;
[0084] Chromatographic column: Kromasil 100-5C18 4.6mm*250mm, 5μm;
[0085] Mobile phase: methanol: phosphate buffer (6.8g of potassium dihydrogen phosphate, add 152ml of 0.1mol / L sodium hydroxide solution, add 20ml of tetrabutylammonium hydroxide 10% aqueous solution, dilute to 1000ml with water, adjust pH to 6.5 with phosphoric acid) = 52:48;
[0086] Detection wavelength: 235nm;
[0087] Flow rate: 0.8ml / min;
[0088] Column temperature: 40°C;
[0089] Injection volume: 20μl;
[0090] isocratic elution;
[0091] Mixed solution preparation: take appropriate amount of rebamipide, impurity A, impurity B, impurity C, and impurity D, dissolve and dilute with mobile phase to make about 0.2 mg of rebamipide, impurity A, and impurity B per 1 ml , Impurity C, and Impurity D are 0.02mg solutions, shake well.
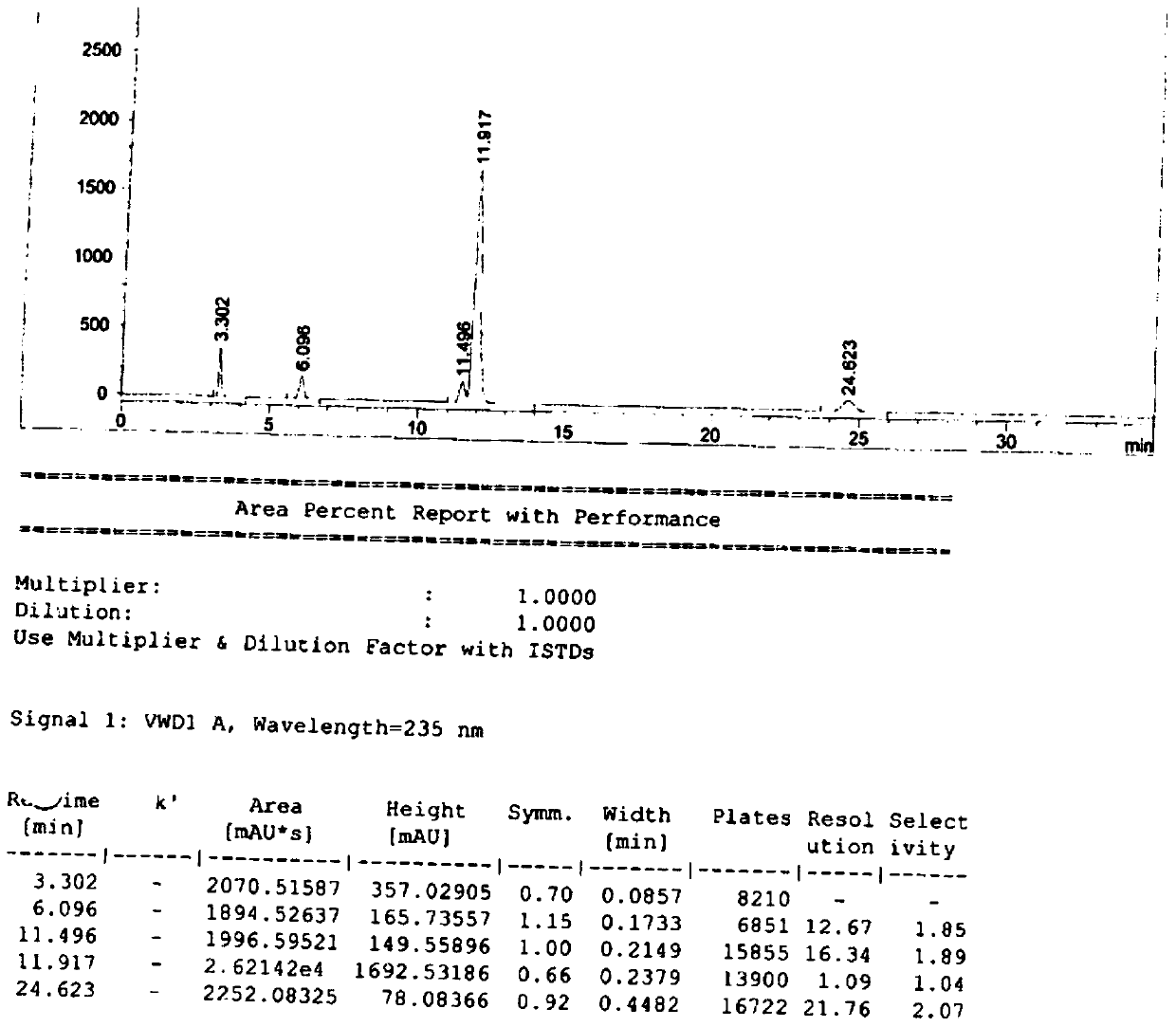
[0092] Test results: See the attached image 3 , ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com