Rebamipide tablet and preparation method thereof
A technology of rebamipide and rebamipide, which is applied in the field of medicine, can solve the problems of insecure clinical effectiveness and safety, difficulty in disintegration and dissolution, and small bulk density, and achieve stable clinical effectiveness and safety, The effect of ensuring the quality of the drug and the simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
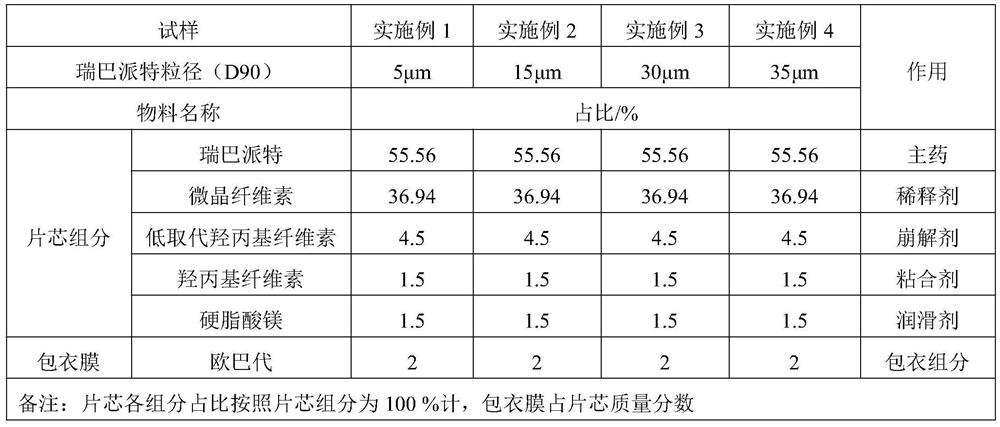
[0036] The present invention will be further described below in conjunction with specific examples, so that those skilled in the art can better understand the present invention and implement it, but the given examples are not intended to limit the present invention.
[0037] In the present invention, unless otherwise specified, the proportion range and replacement scheme of each component in the rebamipide tablet can be combined with each other to form a new formulation scheme.
[0038] In the present invention, unless otherwise specified, the preparation process of rebamipide tablets is carried out sequentially.
[0039] In the present invention, unless otherwise specified, the percentage (%) in the prescription refers to the mass percentage relative to the core of the rebamipide tablet, and the sum of the proportions of each component of the core in the examples is 100%, including The dosage of the tablet refers to the dosage of the tablet core.
[0040] In the present inve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com