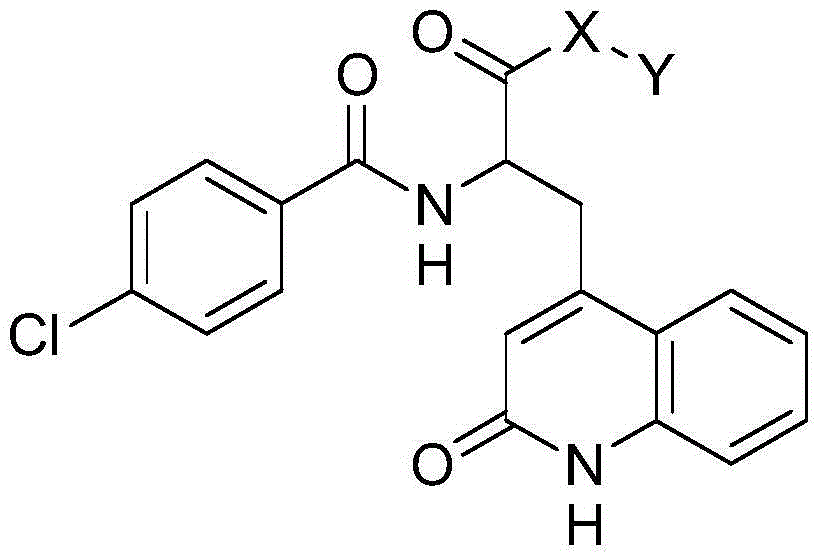
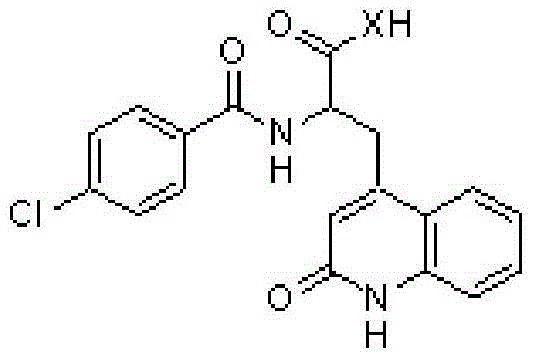
Novel rebamipide prodrug, method for producing same, and usage thereof
一种药学、烷基的技术,应用在新型瑞巴派特前药领域,达到吸收性能改善、体内吸收性能增强的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment approach A
[0251]
[0252] The compound of chemical formula II was dissolved in DMF (dimethylformamide, 8-10 times the volume of the compound of chemical formula II) at high temperature, and then quenched to 0°C. To this mixture were added DCC (dicyclohexylcarbodiimide, 1-1.5 equivalents) and DMAP (dimethylaminopyridine, 0.1-0.3 equivalents). When the internal temperature became stable, alcohol or amine (1-1.2 equivalents) was slowly added. The resulting mixture was stirred at room temperature for 4 to 24 hours. The product thus formed was obtained by filtration followed by removal of DMF in vacuo. Subsequently, the residue was subjected to column chromatography using dichloromethane:methanol (9:1, v / v) to obtain the compound of formula I as a solid. Recrystallization was performed when necessary.
experiment approach B
[0254]
[0255] The compound of chemical formula II was dissolved in DMF (dimethylformamide, 8-10 times the volume of the compound of chemical formula II) at high temperature, and then quenched to 0°C. Add EDCI (1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride, 1 to 3 equivalents) and HOBT (1-hydroxybenzotriazole, 1 to 3 equivalents). When the internal temperature became stable, alcohol or amine (1-1.5 equivalents) was slowly added. The resulting mixture was stirred at room temperature for 4 to 24 hours. The product thus formed was obtained by filtration followed by removal of DMF in vacuo. Subsequently, the residue was subjected to column chromatography using dichloromethane:methanol (9:1, v / v) to obtain the compound of formula I as a solid. Recrystallization was performed when necessary.
experiment approach C
[0257]
[0258] In the compound of chemical formula II, add DMF (dimethylformamide, 8~10 times of the volume of the compound of chemical formula II), halogen compound (1~1.5 equivalent) and inorganic salt (1~2 equivalent) successively, and make gained The mixture is reacted at 20-80° C. for 1-24 hours. After completion of the reaction, the product was obtained by filtration followed by removal of DMF in vacuo. The residue was subjected to column chromatography using dichloromethane:methanol (9:1, v / v) to afford the compound of formula I as a solid. Recrystallization was performed when necessary.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com