Preparation method of rebamipide aqueous suspension
A technology of rebamipide and water suspension, which is applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, liquid delivery, etc., which can solve the problem of sandy feeling, irritation, and discomfort of patients and other problems, to achieve the effect that the particle size will not increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
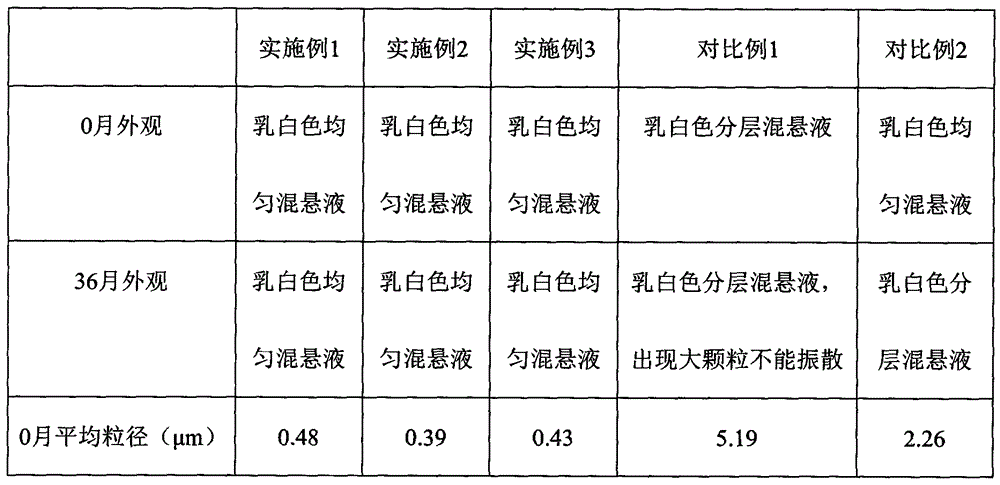
Examples
Embodiment 1
[0044] The preparation method of the rebamipide aqueous suspension provided in this embodiment comprises the following steps:
[0045] (1) According to the prescription requirements, weigh all the excipients with an electronic balance, and dissolve all the excipients in purified water through the liquid mixing tank to obtain an aqueous solution of the excipients;
[0046] (2) Insert the cutting head of the high-speed shearing machine into the auxiliary material aqueous solution, turn on the shearing machine, adjust the rotation speed to 12000-16000rmp, add rebamipide while cutting, and then cut 5 -10 minutes to obtain the initial liquid of rebamipide aqueous suspension;
[0047] (3) After the initial solution of rebamipide aqueous suspension is constant in volume and pH is adjusted, add a high-pressure homogenizer, homogenize 2-3 times at a low pressure of 8-12MPa, and homogenize at a high pressure of 50-55MPa for 10-15 times to obtain Rebamipide Pat water suspension; ...
Embodiment 2
[0051] The preparation method of the rebamipide aqueous suspension provided in this embodiment comprises the following steps:
[0052] (1) According to the prescription requirements, weigh all the excipients with an electronic balance, and dissolve all the excipients in purified water through the liquid mixing tank to obtain an aqueous solution of the excipients;
[0053] (2) Insert the cutting head of the high-speed shearing machine into the auxiliary material aqueous solution, turn on the shearing machine, adjust the rotation speed to 12000-16000rmp, add rebamipide while cutting, and then cut 5 -10 minutes to obtain the initial liquid of rebamipide aqueous suspension;
[0054] (3) After the initial solution of rebamipide aqueous suspension is constant to volume and pH is adjusted, add a high-pressure homogenizer, homogenize 2-3 times at a low pressure of 8-12MPa, and homogenize at a high pressure of 56-60MPa for 10-15 times to obtain Rebamipide Pat water suspension; ...
Embodiment 3
[0057] Rebamipex
[0058] Sodium citrate
[0059] Prepare with reference to the method of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Osmotic pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com