Preparation method of high-purity rebamipide
A rebamipide, high-purity technology, applied in the field of preparation of high-purity rebamipide, can solve problems such as difficult filtration, lower production efficiency, lower product yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
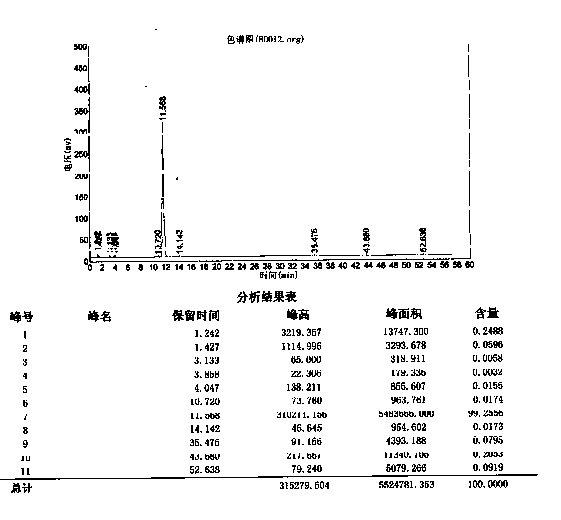
Image
Examples
Embodiment 1
[0017] Add 50g (0.215mol) of 2-amino-3-[2(1H)-quinolinone-4-methylpropionic acid], compound (5), into 250ml of acetone, and dropwise add the Chlorobenzoyl chloride 40g (0.228mol) and 12% NaOH solution 250ml, dropwise, keep the temperature for 1.5 hours. Cool to 0-5 ° C and stir for crystallization for 2 hours. After filtering, the filter cake was dried under normal pressure at 50-60°C for 8 hours, and the crude product of Rebamipide sodium salt was 78.8g (HPLC purity 99.63%).
Embodiment 2
[0019] Add 60g KOH (1.07mol) into 250ml water and 250ml tetrahydrofuran mixed solvent, add 50g (0.215mol) of compound (5) under stirring, add 40g (0.228mol) p-chlorobenzoyl chloride dropwise at 0-15°C, and drop , continue the reaction for 2.5 hours, cool the reaction solution to 0-5°C for crystallization for 2 hours, filter, and dry the filter cake at 50-60°C under normal pressure for 8 hours, yielding 72.2 g of crude product of Rebamipide salt (HPLC 99.55 %).
Embodiment 3
[0021] Mg(OH) 2 Add 50g (0.86mol) into 250ml of water and 500ml of acetonitrile mixed solvent, add 50g (0.215mol) of compound (5) under stirring, add 100g (0.571mol) of p-chlorobenzoyl chloride dropwise at 20-30°C, dropwise, keep React for 3 hours, cool the reaction solution to 0-5°C and crystallize for 2 hours, filter, and dry the filter cake under normal pressure at 50-60°C for 8 hours to obtain 70.5 g of crude product of rebamipide salt (HPLC purity 99.58%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com