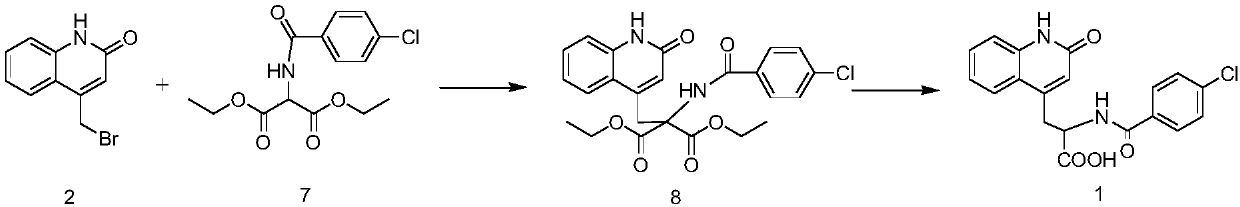
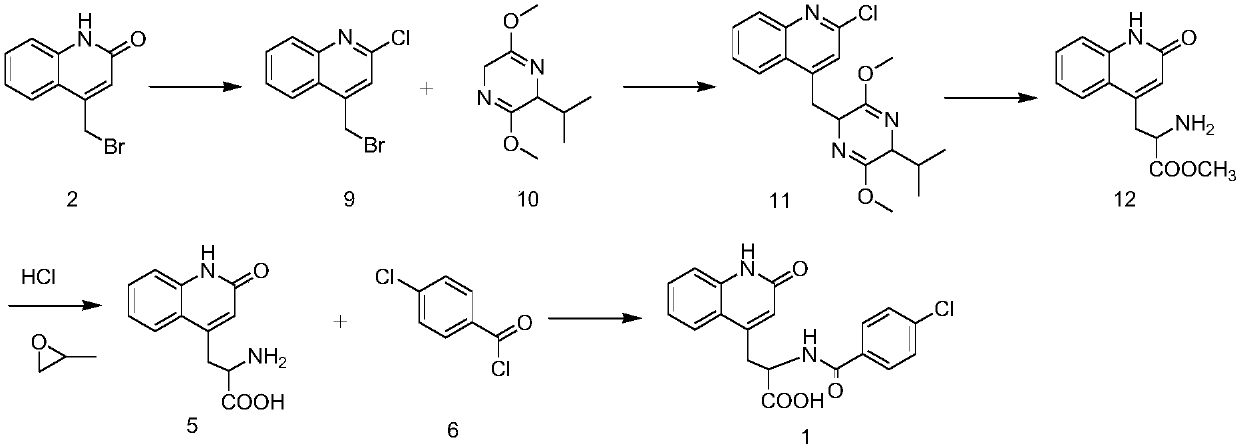
Method for synthesizing rebamipide
A synthetic method, the technology of rebamipide, applied in the field of synthesis of rebamipide, can solve problems such as complex production process, expensive starting materials, and difficulty in industrialization, so as to simplify the synthesis process, avoid incomplete reaction, and process conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1) Synthesis of p-chlorobenzoyl glycine methyl ester (compound 14):
[0044] Add 12.5g (100mmol) of glycine methyl ester hydrochloride into a reaction flask containing 125ml of dichloromethane, stir and dissolve thoroughly, then add 30.3g of triethylamine, then cool at 0-5°C, and pour into the above reaction solution 18.4g (105mmol) p-chlorobenzoyl chloride was added dropwise for acylation reaction, and after the addition was completed, the temperature was raised to 25°C for 4 hours, and TLC monitored that the reactant was completely converted into the product (the developing solvent was dichloromethane). After completion of the reaction, add 30 mL of water and stir, separate the organic layer, add 30 mL of dichloromethane to the water layer to extract twice, combine the organic layers, wash with water until neutral, add 20 g of anhydrous sodium sulfate to dry, filter off the desiccant, and concentrate under reduced pressure to Until there is no slip-out, 21.5 g of soli...
Embodiment 2
[0052] 1) p-chlorobenzoyl glycine methyl ester (compound 14):
[0053] Add 12.5g (100mmol) of glycine methyl ester hydrochloride into a reaction flask containing 125ml of diethyl ether, stir well to dissolve, then add 25.2g (300mmol) of sodium bicarbonate, then cool at 0-5°C, and drop 18.4g (105 mmol) p-chlorobenzoyl chloride, after the addition, the temperature was raised to 25° C. for 4 h, and TLC monitored that the reactant was completely converted into the product (the developing solvent was dichloromethane). After the reaction is complete, add 30 mL of water and stir, separate the organic layer, add 30 mL of dichloromethane to the water layer to extract twice, combine the organic layer, add 50 mL of water to the organic layer, wash 3 times until neutral, add anhydrous sodium sulfate to dry, filter off Desiccant, concentrated under reduced pressure until there is no slip-out, to obtain 20.6g of solid p-chlorobenzoylglycine methyl ester, the yield is 91%, which is directly ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com