Analytic method for related substance examination of rebamipide
A technology of rebamipide and mass analysis, applied in the direction of analyzing materials, material separation, measuring devices, etc., can solve unreliable problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
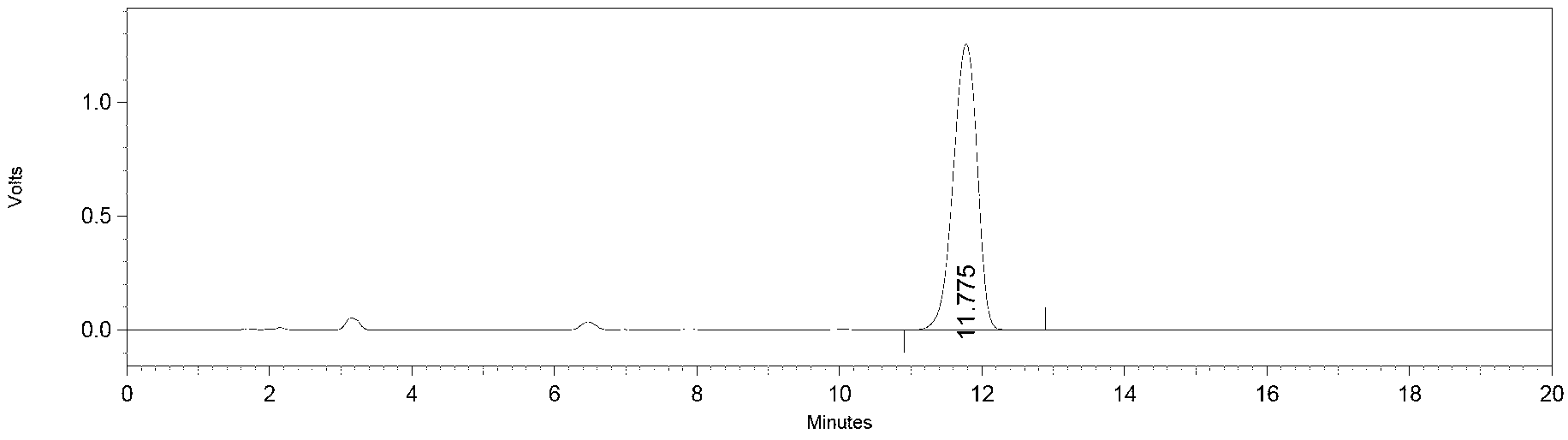
Image
Examples
Embodiment 1
[0060] Instruments and conditions:
[0061] Shimadzu 2010AH all-in-one high-performance liquid chromatography and Shimadzu's LC-solution workstation; octadecylsilane-bonded silica gel as a filler column (Unilmate, XB-C18, 4.6mm×15cm×5μm, month Asahi); with methanol as mobile phase A, with phosphate buffer (take potassium dihydrogen phosphate 6.8g, add 0.1mol / L sodium hydroxide solution 152ml, add 10% tetrabutylammonium hydroxide solution 10ml, add water to 1000ml, use Phosphoric acid to adjust the pH to 6.5, shake well, to get) is mobile phase C, mobile phase is mobile phase A methanol and mobile phase C phosphate buffer according to the volume ratio of 45:55; detection wavelength is 235nm; column temperature is 35 ℃. Mobile phase flow rate: 1.0ml / min, liquid phase analysis injection volume: 20μl, automatic injection.
[0062] experiment procedure:
[0063] Weigh an appropriate amount of 4-(bromomethyl)-2-quinolinone, intermediate 1, intermediate 2, ortho-chloro isomer and ...
Embodiment 2
[0073] Instruments and conditions:
[0074] Shimadzu 2010AH all-in-one high-performance liquid chromatography and Shimadzu's LC-solution workstation; octadecylsilane-bonded silica gel as a filler column (Unilmate, XB-C18, 4.6mm×15cm×5μm, month Asahi); with methanol as mobile phase A, with phosphate buffer (get 6.8g of potassium dihydrogen phosphate, add 0.1mol / L sodium hydroxide solution 152ml, add 10% tetrabutylammonium hydroxide solution 10ml, add water to 1000ml, use Phosphoric acid to adjust the pH to 6.5, shake well, to get) is mobile phase C, mobile phase is mobile phase A methanol and mobile phase C phosphate buffer according to the volume ratio of 45:55; detection wavelength is 235nm; column temperature is 35 ℃. Mobile phase flow rate: 1.0ml / min, liquid phase analysis injection volume: 20μl, automatic injection.
[0075] experiment procedure:
[0076] Get rebamipide (crude drug) in an appropriate amount, dissolve with mobile phase, and be mixed with the need testing...
Embodiment 3
[0080] Instruments and conditions:
[0081] Shimadzu 2010AH all-in-one high-performance liquid chromatography and Shimadzu's LC-solution workstation; octadecylsilane-bonded silica gel as a filler column (Unilmate, XB-C18, 4.6mm×15cm×5μm, month Asahi); with methanol as mobile phase A, with phosphate buffer (get 6.8g of potassium dihydrogen phosphate, add 0.1mol / L sodium hydroxide solution 152ml, add 10% tetrabutylammonium hydroxide solution 10ml, add water to 1000ml, use Phosphoric acid to adjust the pH to 6.5, shake well, to get) is mobile phase C, mobile phase is mobile phase A methanol and mobile phase C phosphate buffer according to the volume ratio of 45:55; detection wavelength is 235nm; column temperature is 35 ℃. Mobile phase flow rate: 1.0ml / min, liquid phase analysis injection volume: 20μl, automatic injection.
[0082] experiment procedure:
[0083] Get the rebamipide tablet, dissolve it with the mobile phase, and be prepared into a test solution approximately equ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com