Preparation method of diethyl aminomalonate hydrochloride
A technology of diethyl aminomalonate and diethyl oximomalonate, which is applied in the field of drug synthesis, can solve the problems of expensive catalysts and easy deactivation, and achieve low cost, easy operation and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
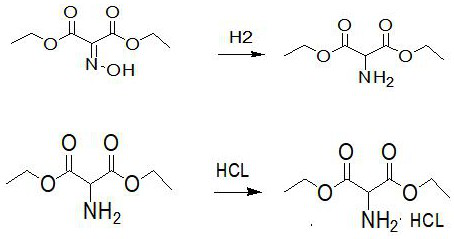
[0048] Add 80 grams of diethyl malonate, 400 milliliters of ethyl acetate and 90 grams of glacial acetic acid into a 1000 milliliter four-necked bottle, stir for 30 minutes, cool to 5 ° C, dropwise add sodium nitrite solution (69 grams of sodium nitrite dissolved in 81 grams of water) 150 grams, the temperature is controlled at 0-10°C during the dropwise addition, the dropwise addition time is 2 hours, stirred after the dropwise addition is completed, kept at 15-25°C for 20 hours, standing for stratification, and using 200ml of water for the lower layer Extract once with ethyl acetate, discard the water layer, wash the ethyl acetate layer with 200 ml of water, let stand to separate the layers, discard the washing water, evaporate the ethyl acetate layer under reduced pressure to obtain the oximino malonate di Ethyl ester 93 grams. The yield is 98.4%. The synthetic reaction formula of diethyl oximino malonate in embodiment 1 is as figure 1 shown.
[0049] The synthesis of di...
Embodiment 2
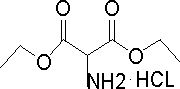
[0051] Put 240g of absolute ethanol into the hydrogenation kettle, add 60g of diethyl oximomalonate, 3.0g of AlNiFe three-way catalyst, close the hydrogenation kettle, replace the system with nitrogen, keep the hydrogen pressure at 1.0-2.0MPa, and the system temperature at 40 At ~50°C, the reaction was stirred for 6 hours. Cool, depressurize, and filter to remove catalyst. The filtrate was added into a 500 ml four-neck flask, stirred, and cooled. Add 50 g of 35% hydrogen chloride ethanol dropwise at 0-5°C, drop it in 1 hour, and stir for 1 hour. Ethanol was distilled off under reduced pressure, and 200 ml of acetone was added to the residue, stirred for 1 hour, and cooled to 5-10°C. Filter, wash the filter cake with acetone, and dry at 60°C to obtain 61 grams of the target product, diethyl aminomalonate hydrochloride. The yield is 91%. The content is 99.7%. The synthetic reaction formula of diethyl aminomalonate hydrochloride in embodiment 2 is as figure 2 shown.
Embodiment 3
[0053] Put 240g of absolute ethanol into the hydrogenation kettle, add 60g of diethyl oximomalonate, 3.0g of aluminum-nickel-molybdenum three-way catalyst, close the hydrogenation kettle, replace the system with nitrogen, keep the hydrogen pressure at 1.0-2.0MPa, and the system temperature at 40 At ~50°C, the reaction was stirred for 6 hours. Cool, depressurize, and filter to remove catalyst. The filtrate was added into a 500 ml four-neck flask, stirred, and cooled. Add 50 g of 35% hydrogen chloride ethanol dropwise at 0-5°C, drop it in 1 hour, and stir for 1 hour. Ethanol was distilled off under reduced pressure, and 200 ml of acetone was added to the residue, stirred for 1 hour, and cooled to 5-10°C. Filter, wash the filter cake with acetone, and dry at 60°C to obtain 59 grams of the target product, diethyl aminomalonate hydrochloride. Yield 88%. The content is 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com