Synthetic method of monoamino inhibitor intermediate monoethyl 2-acetylamino-2-benzylmalonate
A technology of benzyl monoethyl malonate and monoethyl malonate, which is applied in the field of synthesis of compound 2-acetylamino-2-benzyl monoethyl malonate, can solve the problems of poor economy, 2-acetyl Amino-2-benzylmalonate monoethyl ester has the problems of high production cost and expensive starting materials, and achieves the effects of low cost, strong operability and simple reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
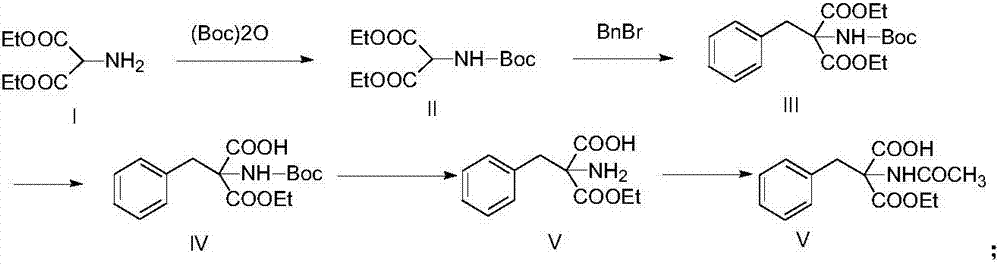
[0028] Under ice-cooling, diethyl aminomalonate (17.5g, 0.1mol) and triethylamine (20g, 0.2mol), 250ml of dichloromethane, di-tert-butyl dicarbonate (26g, 0.12mol) were added to the three-neck In the bottle, it was gradually raised to room temperature and the reaction was stirred at this temperature, and the reaction was monitored by TLC. After the reaction was completed, the solvent was evaporated under reduced pressure, dichloromethane was added to the residue for extraction (200ml×2), and the obtained organic phase was dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain diethyl 2-Boc-aminopropanediol as a colorless oil Acid 27g (yield 98%).
[0029] Under ice-cooling, diethyl 2-Boc-aminomalonic acid (27.5g, 0.1mol) and DMF (200ml) were added into a three-necked flask, and then sodium hydride (4.8g, 0.12mol) was added in batches under stirring, Then the mixed solution was raised to room temperature, benzyl bromide (19g, 0.11mol) was added, the react...
Embodiment 2
[0034] Under ice-cooling, add diethyl aminomalonate (17.5g, 0.1mol) and triethylamine (20g, 0.2mol), 250ml methanol, di-tert-butyl dicarbonate (26g, 0.12mol) into a three-necked flask , was gradually raised to room temperature and the reaction was stirred at this temperature, and the reaction was monitored by TLC. After the reaction was completed, the solvent was evaporated under reduced pressure, dichloromethane was added to the residue for extraction (200ml×2), and the obtained organic phase was dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain diethyl 2-Boc-aminopropanediol as a colorless oil Acid 26.7g.
[0035] Under ice-cooling, diethyl 2-Boc-aminomalonic acid (27.5g, 0.1mol) and NMP (200ml) were added into a three-necked flask, and then sodium hydride (4.8g, 0.12mol) was added in batches under stirring, Then the mixed solution was raised to room temperature, benzyl bromide (19g, 0.11mol) was added, the reaction solution was stirred at room te...
Embodiment 3
[0040] Under ice bath, diethyl aminomalonate (17.5g, 0.1mol) and N-ethyldiisopropylamine (25.8g, 0.2mol), 200ml ethanol, di-tert-butyl dicarbonate (26g, 0.12mol) Add it into a three-necked flask, gradually rise to room temperature and stir the reaction at this temperature, and monitor the end of the reaction by TLC. After the reaction was completed, the solvent was evaporated under reduced pressure, dichloromethane was added to the residue for extraction (200ml×2), and the obtained organic phase was dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain diethyl 2-Boc-aminopropanediol as a colorless oil Acid 26.5g.
[0041] Under ice-cooling, diethyl 2-Boc-aminomalonic acid (27.5g, 0.1mol) and methanol (200ml) were added into a three-necked flask, and then sodium methoxide (6.48g, 0.12mol) was added in batches under stirring, Then the mixed solution was raised to room temperature, added benzyl bromide (19g, 0.11mol), the reaction solution was stirred at r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com