Method for detecting related substance in diethyl 2-(4-chlorobenzamido)malonate sample
A technology of diethyl chlorobenzoylaminomalonate and a detection method is applied in the field of detection of related substances in a sample of diethyl p-chlorobenzoylaminomalonate, can solve problems such as short synthesis process, and achieves The effect of reproducible and efficient detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] High performance liquid chromatography: Agilent 1260;
[0058] Mobile phase: Phase A: 0.01M potassium dihydrogen phosphate aqueous solution, pH=2.5;
[0059] Phase B: acetonitrile;
[0060] Elution gradient:
[0061] Table 1 Gradient elution program
[0062] Elution time Phase A (%) Phase B (%) 0min 60 40 3min 60 40 13min 50 50 33min 20 80 38min 20 80 43min 60 40 50min 60 40
[0063] Chromatographic column: Inertsustain C18 4.6×250mm, 5.0μm;
[0064] Detection wavelength: 220nm;
[0065] Flow rate: 1.0mL / min;
[0066] Column temperature: 40°C;
[0067] Injection volume: 10μL;
[0068] Mixed solution preparation: Accurately weigh the appropriate amount of the sample and impurities, place it in a 25mL measuring bottle, first add 5.0mL DMF to dissolve, and then dilute to the mark with acetonitrile. (sample concentration: 1.0mg / mL)
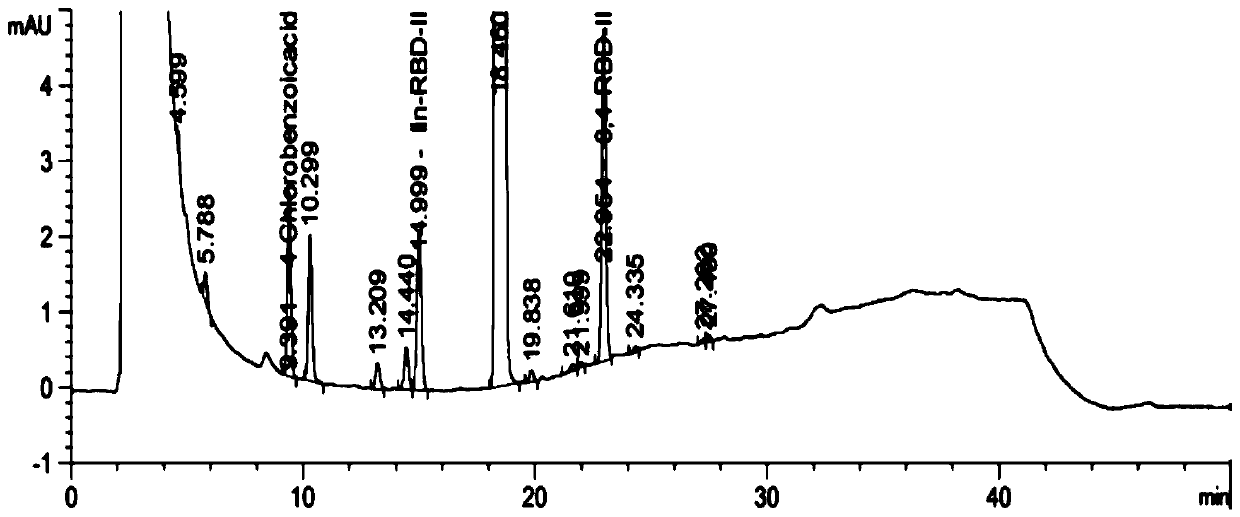
[0069] Test results: See the attached figure 1 , so it can be seen ...
Embodiment 2
[0071] High performance liquid chromatography: Agilent 1260;
[0072] Mobile phase: Phase A: 0.01M potassium dihydrogen phosphate aqueous solution, pH=2.5;
[0073] Phase B: acetonitrile;
[0074] Elution gradient:
[0075] Table 2 Gradient elution program
[0076] Elution time Phase A (%) Phase B (%) 0min 50 50 13min 50 50 25min 20 80 35min 20 80 40min 50 50 45min 50 50
[0077] Chromatographic column: Inertsustain C18 4.6×250mm, 5.0μm;
[0078] Detection wavelength: 240nm;
[0079] Flow rate: 1.0mL / min;
[0080] Column temperature: 40°C;
[0081] Injection volume: 10μL;
[0082] Mixed solution preparation: Accurately weigh the appropriate amount of the sample and impurities, place it in a 25mL measuring bottle, add 5.0mL DMF to dissolve, and then dilute to the mark with acetonitrile. (sample concentration: 1.0mg / mL)
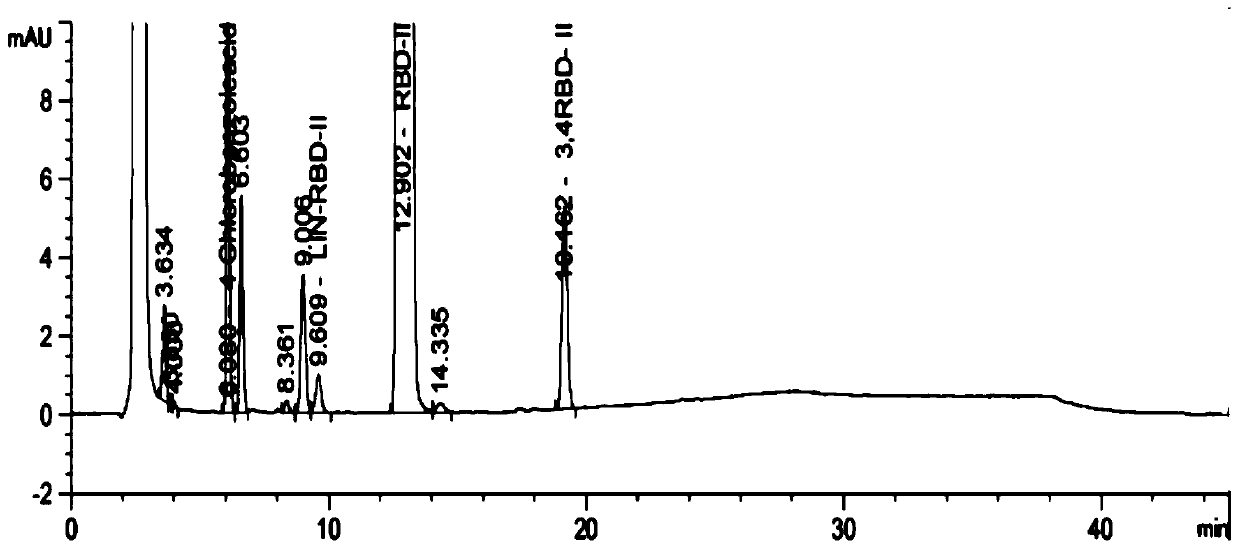
[0083] Test results: See the attached figure 2 , so it can be seen that in this embodimen...
Embodiment 3
[0085] High performance liquid chromatography: Agilent 1260 detector;
[0086] Mobile phase: A: 0.01M potassium dihydrogen phosphate aqueous solution, pH=2.5;
[0087] B: acetonitrile;
[0088] Elution gradient:
[0089] Table 3 Gradient elution program
[0090]
[0091]
[0092] Chromatographic column: Inertsustain C18 4.6×250mm, 5.0μm;
[0093] Detection wavelength: dual wavelength 240nm and 220nm
[0094] Flow rate: 1.0mL / min;
[0095] Column temperature: 40°C;
[0096] Injection volume: 10μL;
[0097] Mixed solution preparation: Weigh the appropriate amount of sample and impurities, place in a 25mL measuring bottle, first add 5.0mL DMF to dissolve, then dissolve with acetonitrile and dilute to the mark, shake well. (sample concentration: 0.5mg / mL)
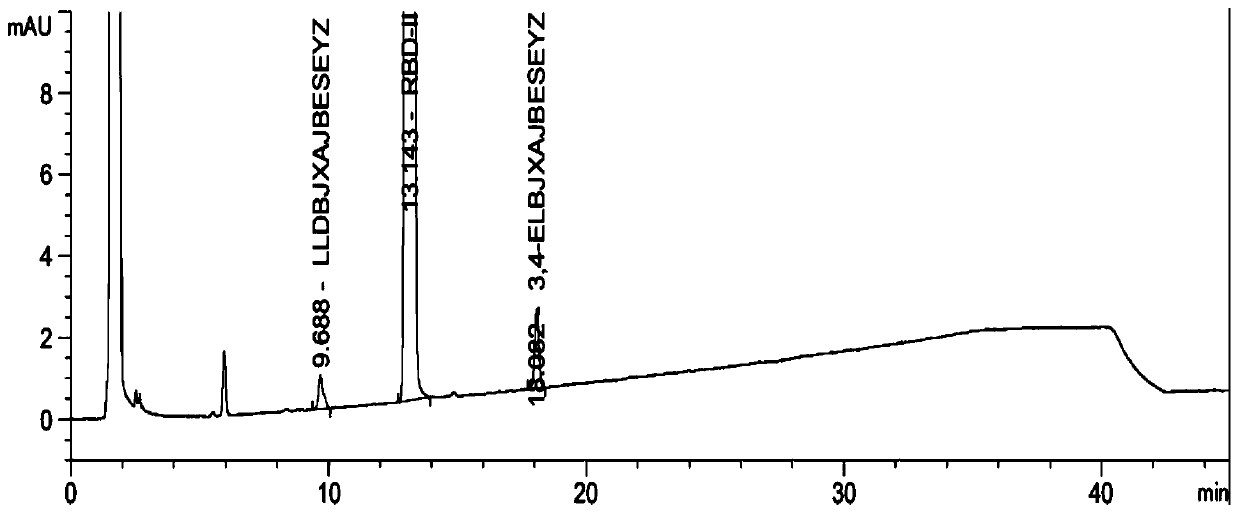
[0098] Test results: See the attached image 3 , wherein, RT=9.1min is the peak of impurity 1, RT=13.4min is the peak of impurity 2, RT=15.7min is the peak of rebamipide raw material, RT=19.0min is the peak of im...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mobile phase a | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com