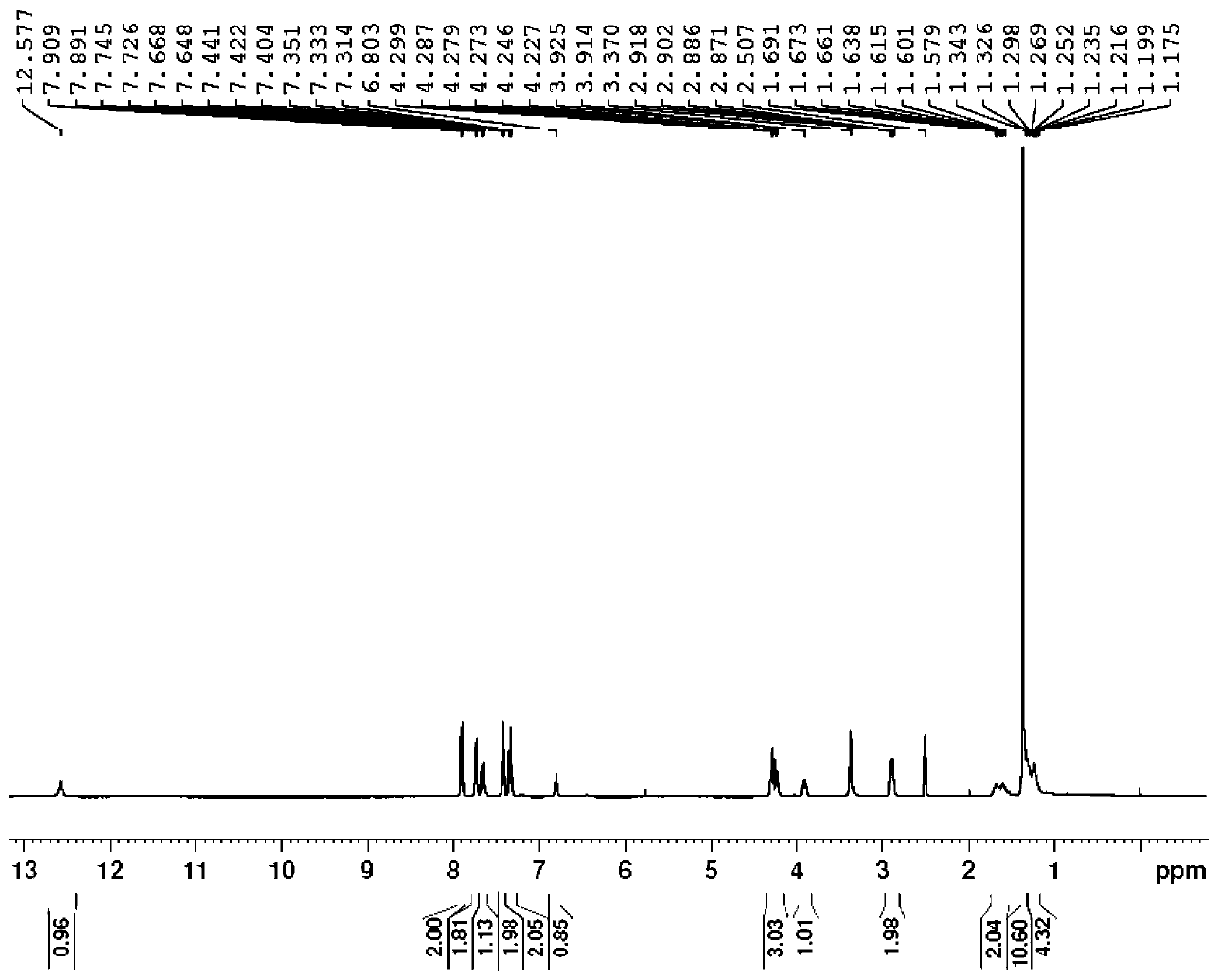
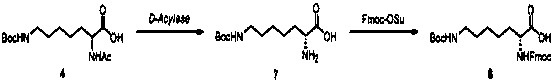
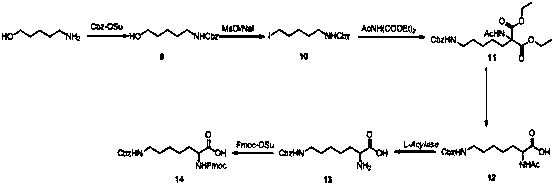
Synthesis method of double different protected amino acids
A synthesis method and amino acid technology are applied in the preparation of carbamate derivatives, chemical instruments and methods, preparation of organic compounds, etc., which can solve the problems of unsuitability for scale-up production, harsh and dangerous reaction conditions, etc., and achieve strong versatility and post-processing. Simple, high chiral purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: N-fluorenylmethoxycarbonyl-N'-tert-butoxycarbonyl-homolysine
[0039]
[0040] Step 1.1:
[0041] Add 6-amino-1-hexanol (100.0 g, 0.97 mol) into a 2000 ml three-necked flask, then add acetone (600 mL) and water (600 mL); slowly add Boc 2 O (211.4 g, 0.97 mol) and triethylamine (107.7 g, 1.06 mol). After stirring at room temperature for 4 hours, the solvent was evaporated by a rotary evaporator, 1500 mL of ethyl acetate was added, the organic phase was separated, washed with water (1000 mL) and saturated brine (1000 mL), and dried over anhydrous sodium sulfate. The filtrate was spin-dried to obtain a colorless oil, compound 1 (1-Boc amino-5-pentanol, 197.1 g, yield 100%), which was directly used in the next reaction;
[0042] Step 1.2:
[0043] Compound 1 (197.1 g, 0.97 mol) was added to a 2000 ml three-necked flask, followed by dichloromethane (1000 mL), and methanesulfonyl chloride (116.2 g, 1.02 mol) and triethylamine (108.1 g, 1.07 mol). After the ...
Embodiment 2
[0052] Example 2: N-fluorenylmethoxycarbonyl-N'-tert-butoxycarbonyl-D-homolysine
[0053]
[0054] Step 2.1:
[0055] Add compound 4 (100 g, 0.33 mol) to a three-necked flask, adjust the pH to 8.0 with pure water (4 L) with 1 N NaOH, add D-acetylase (0.5 g, Sigma-Aldrich), and the reaction solution is heated at 38 °C Stir for 24 hours. After chiral HPLC detection, it was confirmed that the reaction was complete; the reaction solution was cooled to room temperature, filtered, the filtrate was adjusted to pH 7.2 with dilute hydrochloric acid, extracted with ethyl acetate (1500 mLх2), the aqueous phase was concentrated to 500 mL, and a solid precipitated. Filter and dry to obtain a white solid, compound 7 [(R)-2-amino-7-7-(Boc-amino)heptanoic acid, 37.5 g, yield 43%, ee:99%], directly used in the next step reaction;
[0056] Step 2.2:
[0057] Add compound 7 (37.5 g, 0.144 mol), acetone (300 mL), water (300 mL), and then potassium carbonate (23.8 g, 0.17 mol) and Fmoc-OSu ...
Embodiment 3
[0058] Embodiment 3: N-fluorenylmethoxycarbonyl-N'-benzyloxycarbonyl-homolysine
[0059]
[0060] Step 3.1:
[0061] Add 6-amino-1-hexanol (10.0 g, 97 mmol) to a 200 mL three-necked flask, then add acetone (60 mL) and water (60 mL); add Cbz-OSu (24.9 g, 0.1 mol) and Sodium (4.8 g, 0.12 mol). After stirring at room temperature for 4 hours, the solvent was evaporated by a rotary evaporator, 200 mL of ethyl acetate was added, the organic phase was separated, washed with water (100 mL) and saturated brine (100 mL), and dried over anhydrous sodium sulfate. The filtrate was spin-dried to obtain a colorless oil, compound 9 (1-Cbz amino-5-pentanol, 22.3g, yield 100%), which was directly used in the next step;
[0062] Step 3.2 was the same as Step 1.2 of Example 1 to obtain compound 10 (1-Cbz-amino-5-iodopentane, yield 85%);
[0063] Step 3.3 was the same as Step 1.3 of Example 1 to obtain compound 11 [2-acetylamino-2-(7-Cbz-aminopentyl)diethyl malonate, yield 75%];
[0064] St...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com