Preparation method of favipiravir intermediate 6-bromo-3-hydroxypyrazine-2-formamide
A technology of hydroxypyrazine and favipiravir, which is applied in the direction of organic chemistry, can solve the problems of high risk and severe reaction conditions, and achieve the effects of reducing risk, improving product purity and yield, and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of 6-bromo-3-hydroxypyrazine-2-carboxamide
[0031] (1) Add 560mL of acetonitrile into a 1000mL three-necked flask, add 80g of sodium (3-oxo-3,4-dihydropyrazine-2-carbonyl)amide under stirring, and protect the reaction solution under nitrogen; control the reaction solution at 10-20°C, add 13g of acetic acid, the reaction solution pH=6 at this time;
[0032] (2) 86g of liquid bromine was added dropwise, and the temperature of the reaction solution was controlled to be 5 to 25°C during the dropwise addition;
[0033] (3) After the dropwise addition is completed, heat-preserve and react. After 1 hour, HPLC detects that the remaining intermediate II is less than 2%, stop the reaction, filter the reaction solution, beat the filter cake with water until the filtrate pH=6~7, and obtain a wet product;
[0034] (4) The wet product was dried in an oven at 65°C to obtain 87.3 g of 6-bromo-3-hydroxypyrazine-2-carboxamide, yield: 80.6%.
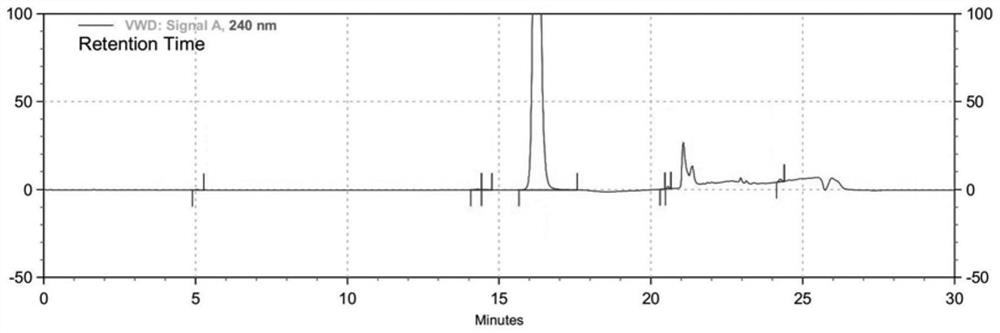
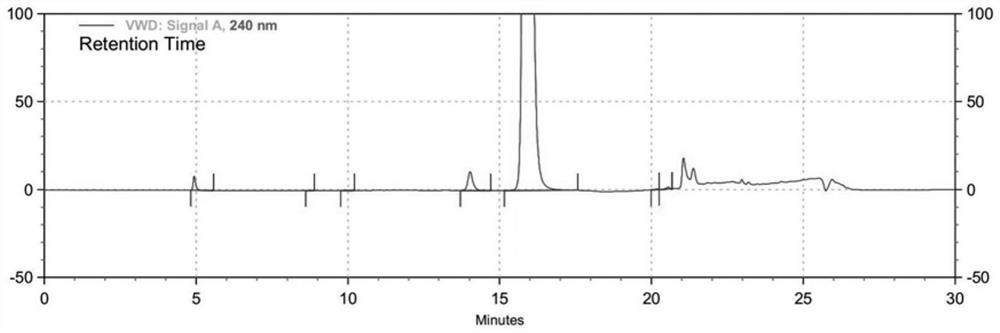
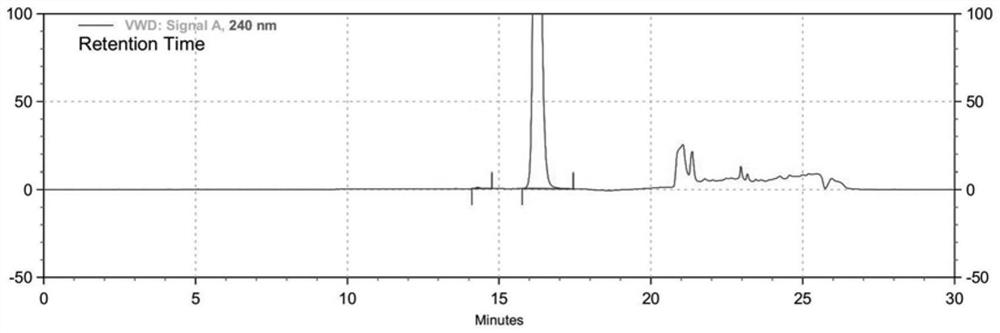
[0035] The 6-bromo-3-hydroxypy...
Embodiment 2
[0039] Preparation of 6-bromo-3-hydroxypyrazine-2-carboxamide
[0040] (1) Add 560mL of acetonitrile into a 1000mL three-necked flask, add 80g of sodium (3-oxo-3,4-dihydropyrazine-2-carbonyl)amide under stirring, and protect the reaction solution under nitrogen; control the reaction solution at 10-20°C, add Phosphoric acid 9g, at this time the pH of the reaction solution=6;
[0041] (2) 86g of liquid bromine was added dropwise, and the temperature of the reaction solution was controlled to be 5 to 25°C during the dropwise addition;
[0042] (3) After the dropwise addition is completed, heat-preserve and react. After 1 hour, HPLC detects that the remaining intermediate II is less than 2%, stop the reaction, filter the reaction solution, beat the filter cake with water until the filtrate pH=6~7, and obtain a wet product;
[0043] (4) The wet product was dried in an oven at 65°C to obtain 86.0 g of 6-bromo-3-hydroxypyrazine-2-carboxamide, yield: 79.4%.
[0044] The 6-bromo-3-hy...
Embodiment 3
[0049] Preparation of 6-bromo-3-hydroxypyrazine-2-carboxamide
[0050] (1) Add 56L of acetonitrile into a 100L reaction kettle, add 8000g of sodium (3-oxo-3,4-dihydropyrazine-2-carbonyl)amide under stirring, and protect it under nitrogen; control the reaction liquid at 10-20°C, Then slowly add 1.3kg of acetic acid, and the reaction solution pH=5~6 at this time;
[0051] (2) Slowly add 8600 g of liquid bromine, and control the temperature of the reaction solution during the dropping process to be 5 to 25° C.;
[0052] (3) After the dropwise addition is completed, heat preservation reaction, HPLC detection after 2h, intermediate II remains less than 2%, stop the reaction, filter the reaction solution, and beat the filter cake with water until the filtrate pH=6~7 to obtain a wet product;
[0053] (4) The wet product was dried in an oven at 65°C to obtain 8911 g of 6-bromo-3-hydroxypyrazine-2-carboxamide, yield: 82.3%.
[0054] The 6-bromo-3-hydroxypyrazine-2-carboxamide prepare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com