Favipiravir L-arginine frozen-dried preparation for injection
A technology of freeze-dried preparations and arginine salts, which is applied in the field of medicine, can solve problems such as difficult reconstitution, unsatisfactory satisfaction, and limited solubilization effect, and achieve uniform color, high unit loading dose, and good pharmaceutical characteristics Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
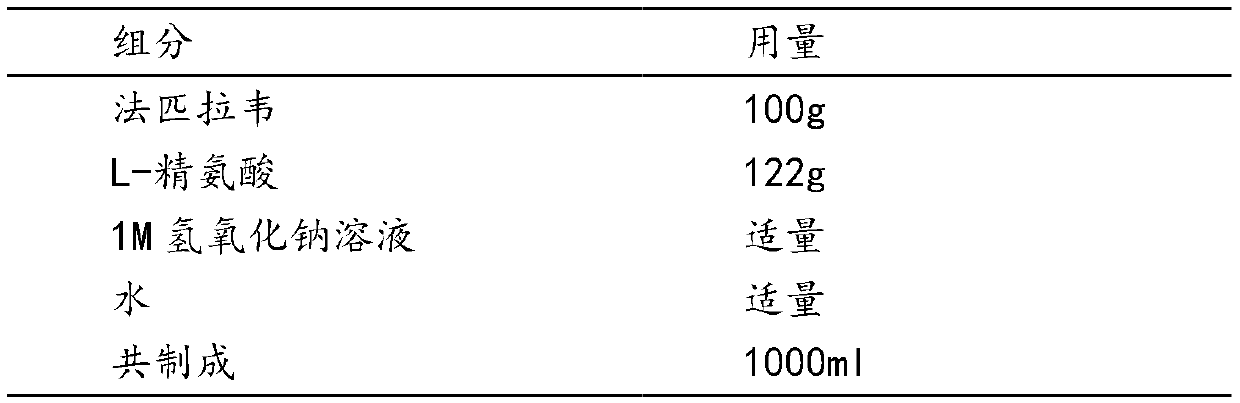
[0030] 100 prescriptions
[0031]
[0032] Preparation
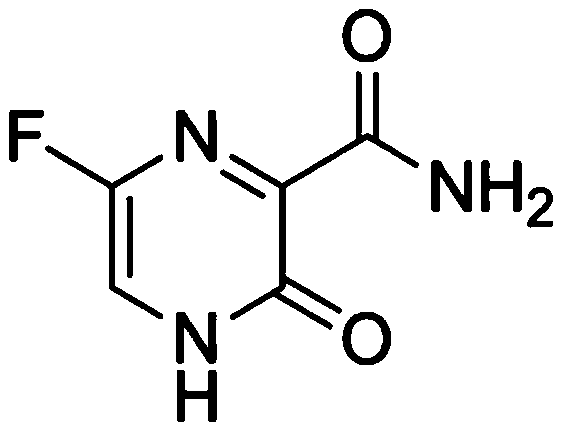
[0033] (1) Preparation of favipiravir L-arginine salt solution: take 80% of the recipe water for injection, heat to a temperature between 60 and 70 ° C, add L-arginine, stir to dissolve; then add method Pilavir, stir for 1-2 hours to obtain a clear solution, measure the pH between 7.2-7.6, then add 1M sodium hydroxide solution, adjust the pH to between 7.8-8.2, add water to the full amount, filter with a 0.22 μm membrane, the filtrate Filled in 20ml vials, each bottle contains 10ml.
[0034] (2) Freeze-drying: the above-mentioned favipiravir L-arginine salt solution is rapidly cooled (the cooling rate is greater than 20°C / h), so that the temperature of the sample is reduced to -35~-60°C and maintained for 1~4h; turn on the vacuum pump Vacuum, after the vacuum degree of the front box is lower than 200Pa, adjust the temperature of the partition to -15~-5℃, maintain the vacuum pressure at 25~150Pa, and keep it for 8~20...
Embodiment 2
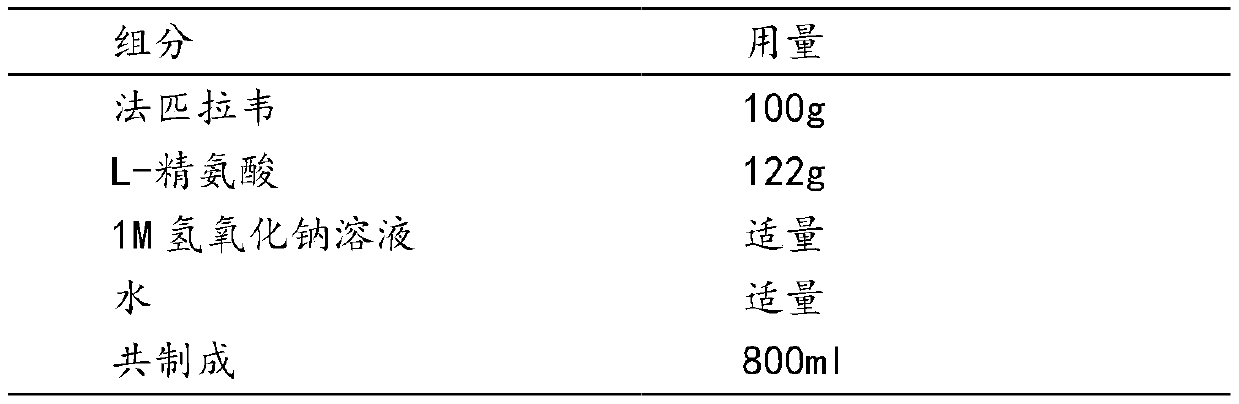
[0036] 100 prescriptions
[0037]
[0038] Preparation
[0039] (1) Preparation of favipiravir L-arginine salt solution: take 80% of the recipe water for injection, heat to a temperature between 60 and 70 ° C, add L-arginine, stir to dissolve; then add method Pilavir, stir for 1-2 hours to obtain a clear solution, measure the pH between 7.2-7.6, then add 1M sodium hydroxide solution, adjust the pH to between 7.8-8.2, replenish water to the full amount, filter with a 0.22 μm membrane, and filter the filtrate. Filled in 20ml vials, each bottle contains 8ml.
[0040] (2) Freeze-drying: the above-mentioned favipiravir L-arginine salt solution was rapidly cooled (the cooling rate was greater than 20°C / h), so that the temperature of the sample was reduced to -35~-60°C and maintained for 1~3h; the vacuum pump was turned on Vacuum, after the vacuum degree of the front box is lower than 200Pa, adjust the temperature of the partition to -15~-5℃, maintain the vacuum pressure at 25~1...
Embodiment 3
[0042] 100 prescriptions
[0043]
[0044] Preparation
[0045] (1) Preparation of favipiravir L-arginine salt solution: take 80% of the recipe water for injection, heat to a temperature between 60 and 70 ° C, add L-arginine, stir to dissolve; then add method Pilavir, stir for 1-2 hours to obtain a clear solution, measure the pH between 7.2-7.6, then add 1M sodium hydroxide solution, adjust the pH to between 7.8-8.2, add water to the full amount, filter with a 0.22 μm membrane, the filtrate Filled in 20ml vials, each bottle contains 12ml.
[0046] (2) Freeze drying: the above-mentioned favipiravir L-arginine salt solution was rapidly cooled (the cooling rate was greater than 20°C / h), so that the temperature of the sample was reduced to -35~-60°C and maintained for 1~6h; turn on the vacuum pump Vacuum, after the vacuum degree of the front box is lower than 200Pa, adjust the temperature of the partition to -15~-5℃, maintain the vacuum pressure at 25~150Pa, and keep it for 10~...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com