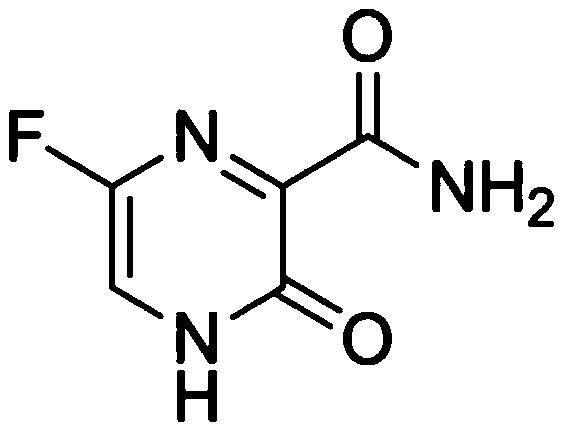
Freeze-dried preparation of favipiravir for injection and preparation method thereof
A technology of freeze-dried preparations and favipiravir, which is applied in the field of medicine, can solve problems such as defects in quality attributes, and achieve the effects of low water content, beautiful properties, and good pharmaceutical characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
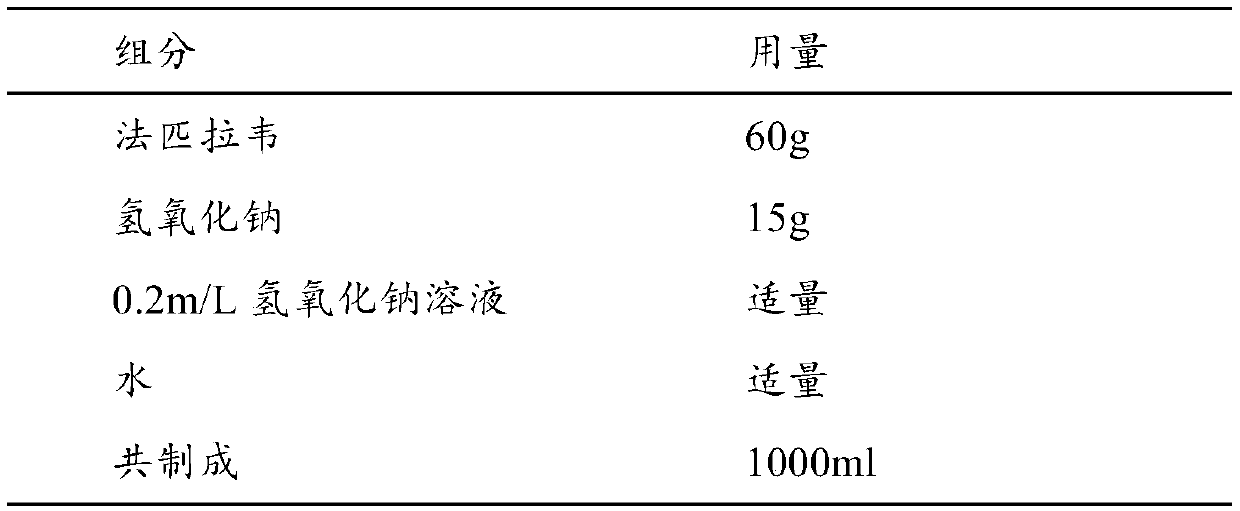
[0076] 100 prescriptions
[0077]
[0078] Preparation
[0079] (1) Preparation of Favipiravir freeze-dried preparation intermediate solution
[0080] Add the prescribed amount of Favipiravir to 90% water for injection, control the temperature between 15-20°C, and form a suspension state, add tert-butanol therein, shear and stir until a clear solution is obtained. Then add sodium hydroxide, adjust the pH to 6.0-9.0, add water to the full amount, filter with a 0.2 μm polyethersulfone filter membrane, fill in 20ml vials, each bottle has a volume of 10ml; the vials are half-stoppered and placed in a freeze dryer. Freeze drying is carried out.
[0081] (2) Pre-freezing of Favipiravir freeze-dried preparation
[0082] Rapidly lower the temperature of the intermediate solution of the above-mentioned favipiravir freeze-dried preparation to -25~-50°C, and maintain it for 0.5~2h.
[0083] (3) Sublimation and analytical drying
[0084] Turn on the vacuum pump to evacuate. After ...
Embodiment 2
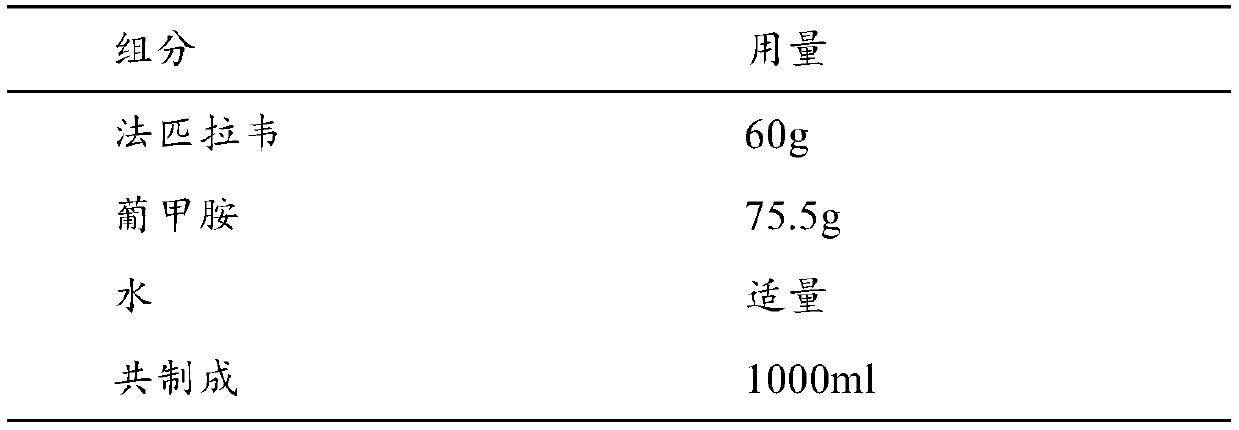
[0087] 100 prescriptions
[0088]
[0089] Preparation
[0090] (1) Preparation of Favipiravir freeze-dried preparation intermediate solution
[0091] Add the prescribed amount of Favipiravir to 90% water for injection, control the temperature between 25 and 35°C to form a suspension, add tert-butanol to it, and shear and stir until a clear solution is obtained. Then add sodium hydroxide, adjust the pH to 6.0-9.0, add water to the full amount, filter with a 0.2 μm polyethersulfone filter membrane, fill in 20ml vials, each bottle has a volume of 10ml; the vials are half-stoppered and placed in a freeze dryer. Freeze drying is carried out.
[0092] (2) Pre-freezing of Favipiravir freeze-dried preparation
[0093] Rapidly lower the temperature of the intermediate solution of the above-mentioned favipiravir lyophilized preparation to -35~-60°C, and maintain it for 2~4h.
[0094] (3) Sublimation and analytical drying
[0095] Turn on the vacuum pump to evacuate. After the v...
Embodiment 3
[0098] 100 prescriptions
[0099]
[0100]
[0101] Preparation
[0102](1) Preparation of Favipiravir freeze-dried preparation intermediate solution
[0103] Add the prescribed amount of Favipiravir to 90% water for injection, control the temperature between 20 and 25°C to form a suspension, add tert-butanol to it, and shear and stir until a clear solution is obtained. Then add sodium hydroxide, adjust the pH to 7.0-8.0, replenish the water to the full amount, filter with a 0.2 μm polyethersulfone filter membrane, fill in 20ml vials, and the volume of each bottle is 10ml; the vials are half-stoppered and placed in a freeze dryer. Freeze drying is carried out.
[0104] (2) Pre-freezing of Favipiravir freeze-dried preparation
[0105] Rapidly lower the temperature of the intermediate solution of the above-mentioned favipiravir lyophilized preparation to -35~-50°C, and maintain it for 2~5h.
[0106] (3) Sublimation and analytical drying
[0107] Turn on the vacuum pum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com