Preparation method of high purity favipiravir impurity
The technology of a compound, hydroxypyrazine, is applied in the field of preparation of impurity 6-chloro-3-hydroxypyrazine-2-carboxamide, which achieves the effect of simple operation, short steps and reduced risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 36.8 g of the compound 6-nitro-3-hydroxypyrazine-2-carboxamide of formula (2) into a 250 ml reaction bottle, add 75 ml of phosphorus oxychloride, add 50 ml of pyridine dropwise, and react at room temperature for 1 hour; 80°C, react for 2h; heat up to reflux, react for 5h, TLC monitors the completion of the raw material reaction, concentrate under reduced pressure until no more liquid flows out, add 150g of ice water and 200ml of toluene to the residual solution, stir at room temperature for 0.5h, and separate the toluene layer , the toluene layer was washed with 150ml of water, 150ml of saturated brine, and evaporated to dryness to obtain 31.5g of yellow powder formula (3) compound 3.6-dichloropyrazine 2-carbonitrile, with a yield of 90% and a purity of 98.5% by HPLC;
Embodiment 2
[0026] Add 31.5g of the compound of formula (3) into a 500ml reaction flask, add 125ml of dichloromethane, stir and dissolve at room temperature, add 5.8g of tetrabutylammonium bromide, add 60ml of 28% sodium hydroxide, stir at room temperature for 3h, and monitor the raw materials by TLC After the reaction was complete, the organic layer was separated and washed with 100 ml of water and 100 ml of saturated brine, and evaporated to dryness to obtain 23.8 g of yellow oil compound 6-chloro-3-hydroxypyrazine-2-carbonitrile of formula (4). Yield 85%, HPLC purity 98.9%;
Embodiment 3
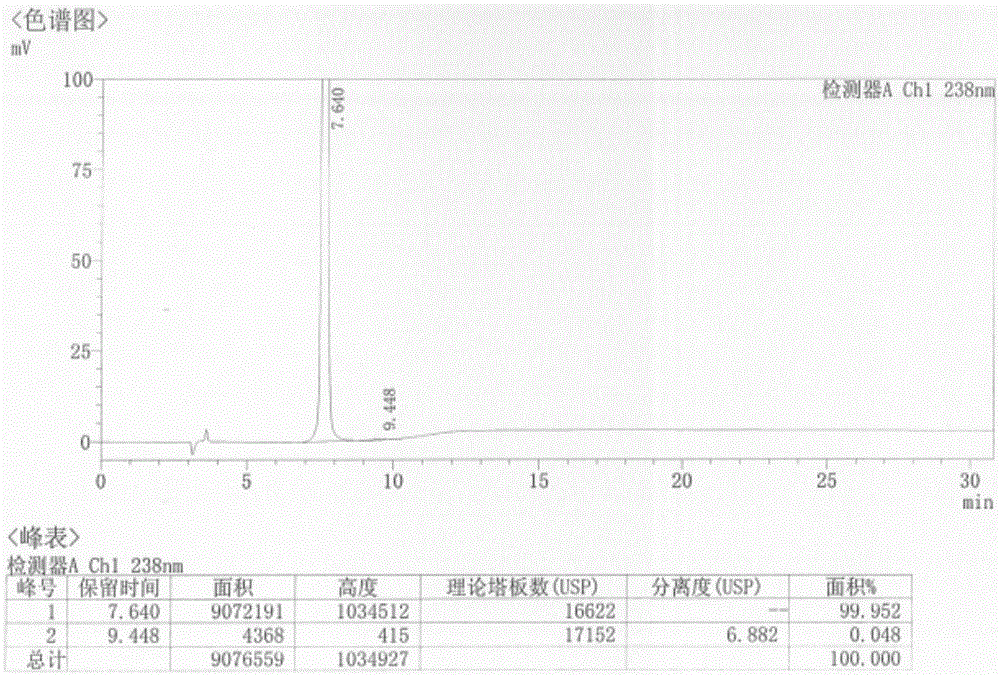
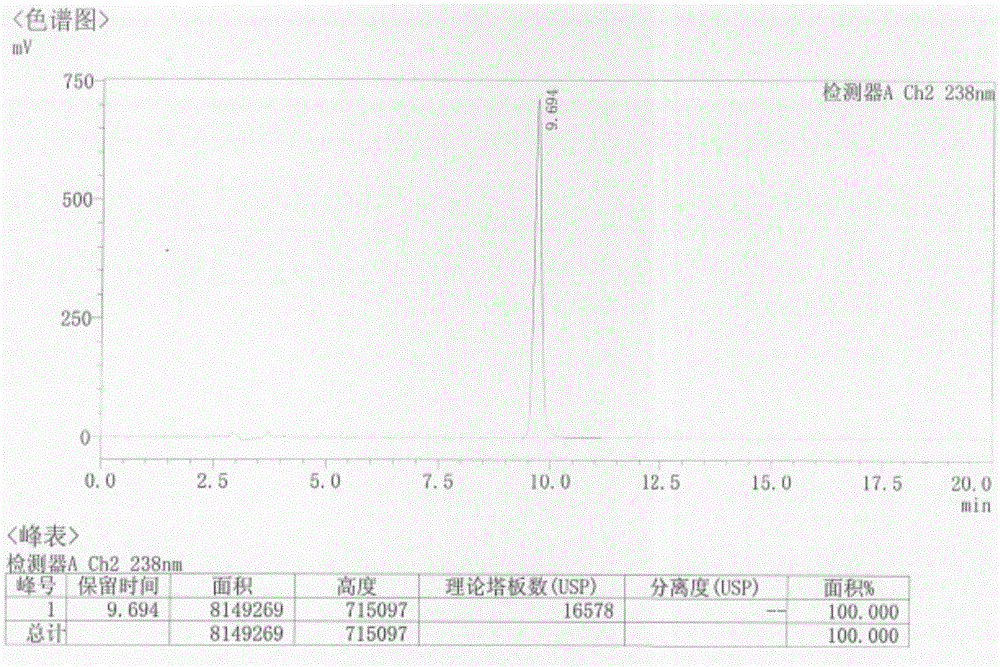
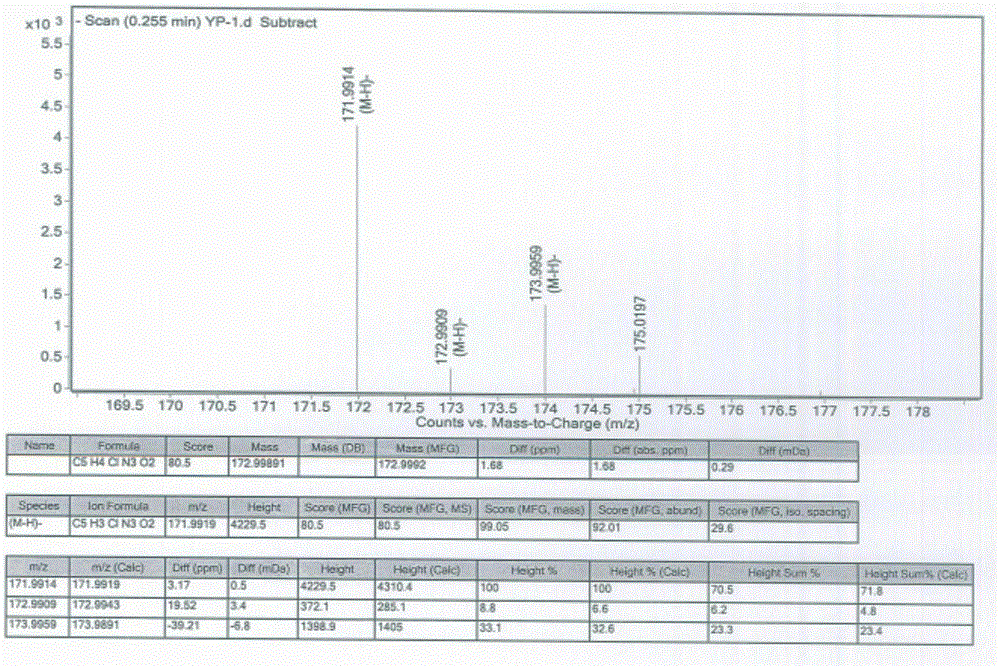
[0028] Take 23.8g of the compound of formula (4) and add it to a 100ml reaction flask, add 40ml of concentrated sulfuric acid, heat up to 50°C, react for 2h, cool down to 5°C, stir for 0.5h, a yellow solid precipitates, filter with suction, and wash the filter cake with 20ml of water , dried to obtain 19.1g of formula (1) 6-chloro 3-hydroxypyrazine-2-carboxamide crude product, yield 70%, HPLC purity 98.5%;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com