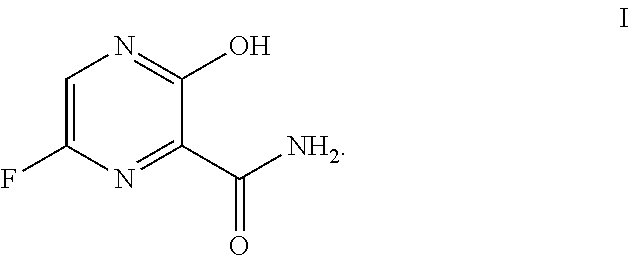
Use of favipiravir in treatment of coronavirus infection
a coronavirus and favipiravir technology, applied in the field of coronavirus infection treatment with favipiravir, can solve the problems of high death probability and dysfunction of the death organ
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
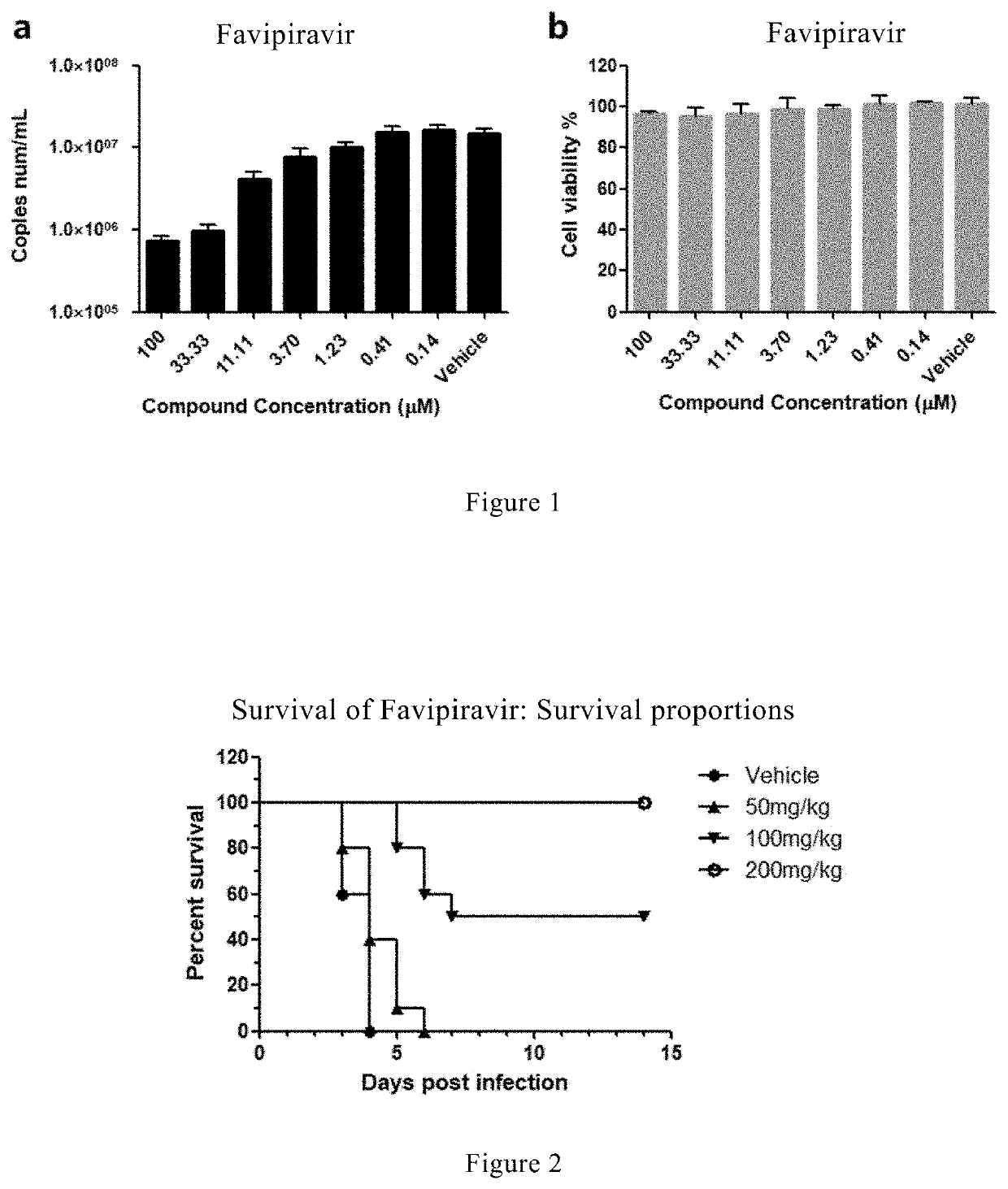
Experiment of Favipiravir in Protecting SARS Coronavirus-Infected Mice from Death
[0097](1) Grouping and Marking of Mice
[0098]A129 mice aged 3-4 weeks and weighed 9-13 g (provided by Institut Pasteur of Shanghai, Chinese Academy of Sciences) were randomly divided into 4 groups, namely the virus control group, the high-dose administration group, the middle-dose administration group and the low-dose administration group, 10 mice per group, marked with ear studs.
[0099](2) Preparation of Drug
[0100]0.5% CMC-Na was used as solvent to dissolve Favipiravir. First, the drug Favipiravir was accurately weighed, added with an appropriate amount of 0.5% CMC-Na solution, alternately treated under vortex and ultrasonic condition for 15 min until the sample was a uniform particle suspension, then diluted with 0.5% CMC-Na by 2-fold, and the dilution was carried out according to administration dose of 200 mg / kg, 100 mg / kg and 50 mg / kg, respectively. The resultant drug solutions were stored at 4° C. fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com