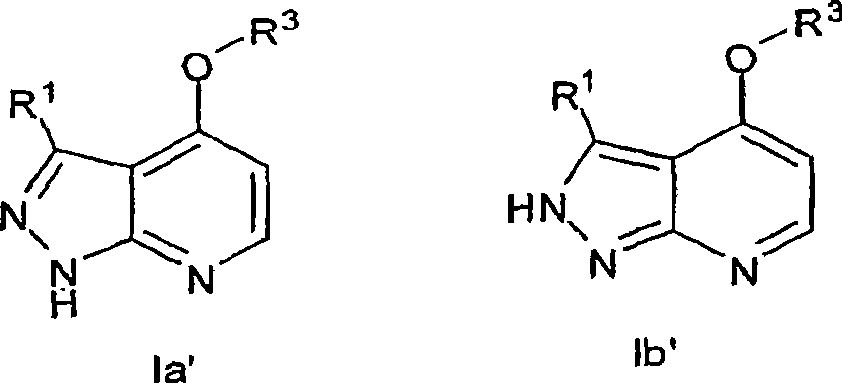
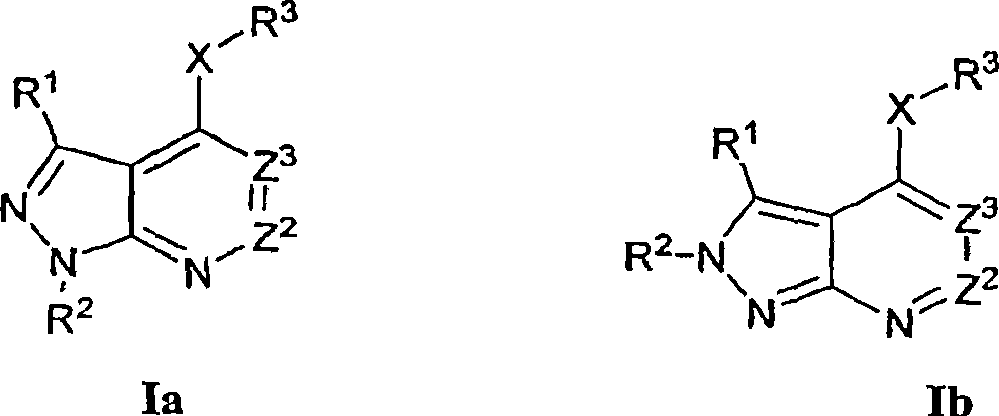Heterobicyclic pyrazole compounds and methods of use
A compound, heterocyclic group technology, applied in the field of heterobicyclic pyrazole compounds, can solve the problem of tumor growth deceleration and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0551] N-(4-(1H-pyrazolo[3,4-b]pyridin-4-yloxy)-3-fluorophenylcarbamoyl)-2-phenyl Preparation of acetamide
[0552]
[0553] As described, and / or by routine modification of reaction conditions. Additionally, other reactions disclosed herein or known in the art should be recognized as applicable to the preparation of other compounds of the invention.
[0554] In the examples described below, all temperatures are expressed in degrees Celsius unless otherwise indicated. Reagents were purchased from commercial suppliers such as Aldrich Chemical Company, Lancaster, TCI or Maybridge and were used without further purification unless otherwise stated.
[0555] Reactions described below are generally performed in anhydrous solvents under positive pressure of nitrogen or argon, or using drying tubes (unless otherwise stated), and reaction flasks are generally fitted with rubber septa for introduction of substrates and reagents via syringes.
[0556] Glassware is oven-dried and / ...
Embodiment 2
[0576] N-(4-(1H-pyrazolo[3,4-b]pyridin-4-yloxy)-3-fluorophenyl)-N-(4-fluorophenyl)cyclopropane - Preparation of 1,1-dicarboxamide
[0577]
[0578] Step A: N-(4-(1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridin-4-yloxy)-3-fluorophenyl)-N- Preparation of (4-fluorophenyl)cyclopropane-1,1-dicarboxamide:
[0579] To stirred 4-(1-(4-methoxybenzyl)-1H-pyrazolo[3,4-b]pyridin-4-yloxy)-3-fluoroaniline (73 mg, 0.20 mmol; obtained from In step D) of Example 1 and ((4-fluorophenyl)carbamoyl)cyclopropanecarboxylic acid (49mg, 0.220mmol; from cyclopropane-1,1-dicarboxylic acid and 4-fluoroaniline using WO 2005 / 030140 and Shih and Rankin, Synth.Comm.1996, 26 (4), prepared by the method of 833-836) in DMA (2mL) mixture, add N1-((ethylimino) methylene)-N3, N3 - Dimethylpropane-1,3-diamine hydrochloride (EDCI) (77 mg, 0.400 mmol). The reaction was stirred at room temperature for 1 hour. The reaction was diluted with EtOAc (10ml) and water (10ml). The phases were separated and the orga...
Embodiment 3
[0585] N-(3-fluoro-4-(1-methyl-1H-pyrazolo[3,4-b]pyridin-4-yloxy)phenyl)-N-(4-fluorophenyl)ring Preparation of propane-1,1-dicarboxamide
[0586]
[0587] Step A: Preparation of 4-(2-fluoro-4-nitrophenoxy)-1H-pyrazolo[3,4-b]pyridine:
[0588] in N 2 Next, stirred 1-(4-methoxybenzyl)-4-(2-fluoro-4-nitrophenoxy)-1H-pyrazolo[3,4-b]pyridine (27.6g, 70.0 mmol; obtained from Example 1, step C) and TFA (53.9 mL, 700 mmol) was heated at reflux for 18 hours. The reaction was cooled to room temperature, then concentrated in vacuo and azeotroped with toluene (4 x 100 mL) to remove residual TFA. The resulting residue was diluted with EtOAc (200 mL) and washed with saturated NaHCO 3 Aqueous solution (100 mL) was carefully neutralized (pH=8-9). The biphasic suspension was stirred at room temperature for 30 minutes. The suspension is filtered. The resulting solid was azeotropically dried with toluene (2 x 200 mL) to afford the product (18.7 g, 97%).
[0589] 1 H NMR (DMSO-d6, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com