Orally disintegrating tablets containing aripiprazole and preparation method of orally disintegrating tablets
An orally disintegrating tablet and aripiprazole technology, which is applied to the orally disintegrating tablet of aripiprazole and the field of preparation thereof, can solve the problems that the overall stability of the preparation is not significantly improved, and the patient's medication experience is affected, and the medication experience can be achieved. Improvement, good mouthfeel acceptance, long-term dissolution improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
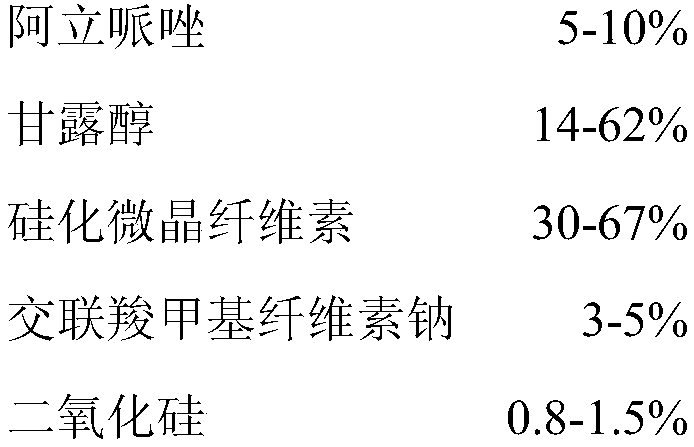
100
[0073] Preparation:
[0074] (1) Take the prescribed amount of aripiprazole, mannitol, silicified microcrystalline cellulose, croscarmellose sodium, silicon dioxide, and sucralose and mix them in a three-dimensional mixer for 20 minutes;
[0075] (2) Add the magnesium stearate of the prescribed amount to the mixed granules prepared in step (1), mix for 5 minutes, and tablet to obtain.
Embodiment 2
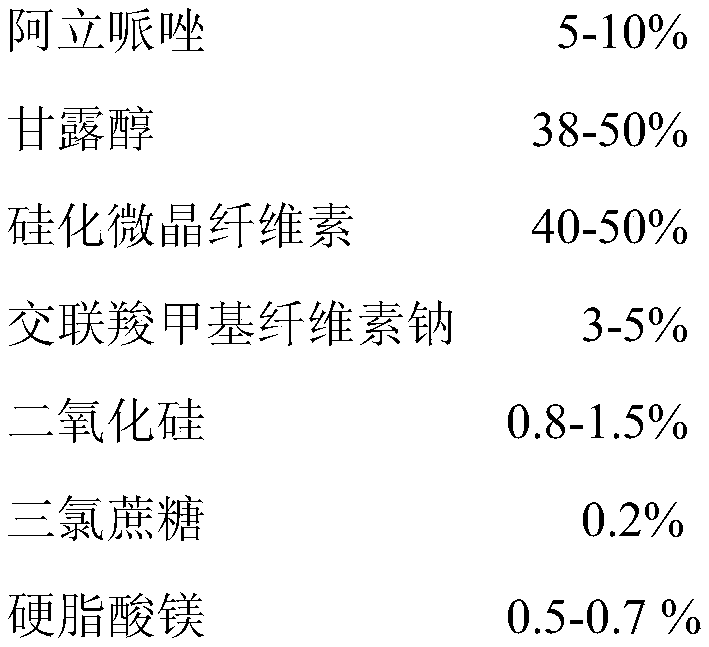
100
[0078] Preparation:
[0079] (1) Take the prescription amount of aripiprazole, lactose, silicified microcrystalline cellulose, croscarmellose sodium, silicon dioxide, and aspartame and mix them in a three-dimensional mixer for 20 minutes;
[0080] (2) Add the magnesium stearate of the prescribed amount to the mixed granules prepared in step (1), mix for 5 minutes, and tablet to obtain.
Embodiment 3
[0082]
[0083]
[0084] Preparation:
[0085] (1) Take the prescribed amount of aripiprazole, starch lactose, silicified microcrystalline cellulose, croscarmellose sodium, silicon dioxide, and acesulfonate potassium and mix them in a three-dimensional mixer for 20 minutes;
[0086] (2) Add the magnesium stearate of the prescribed amount to the mixed granules prepared in step (1), mix for 5 minutes, and tablet to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com