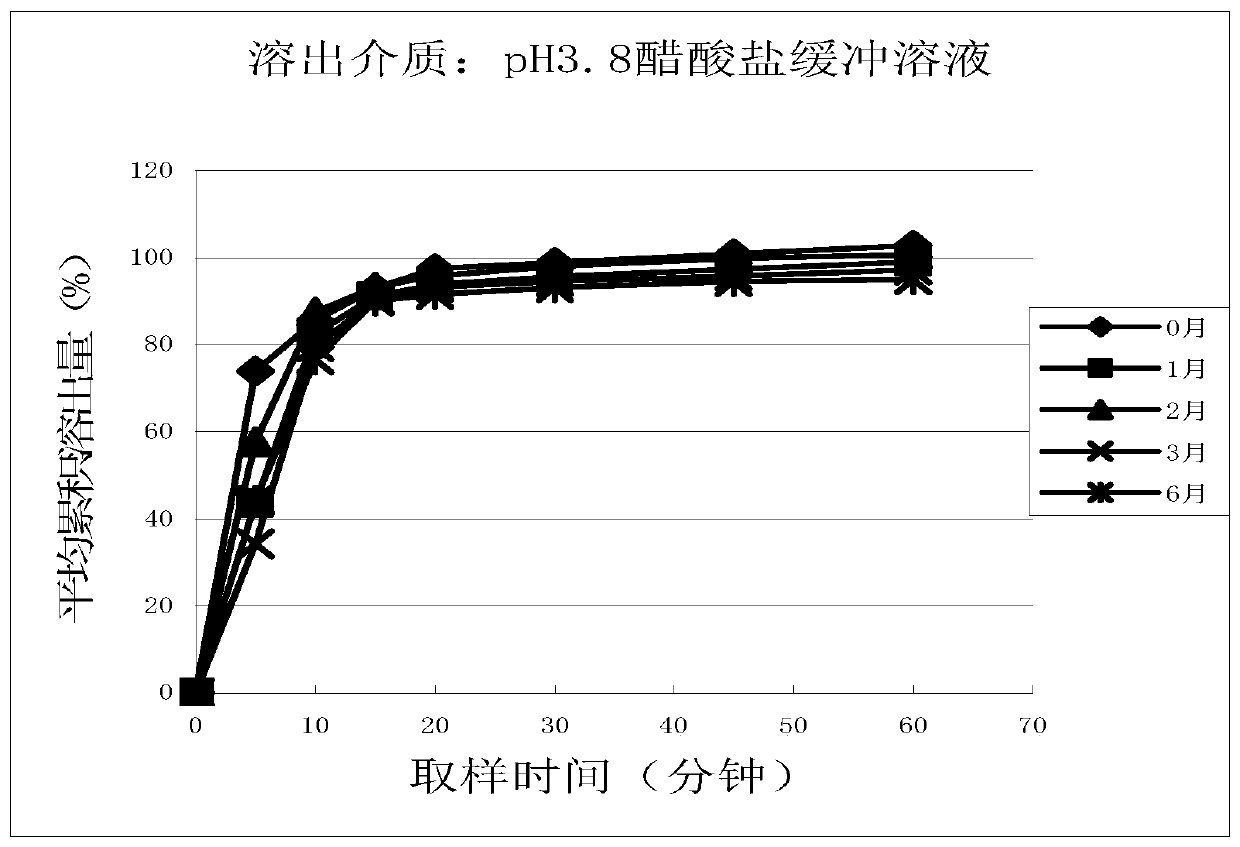
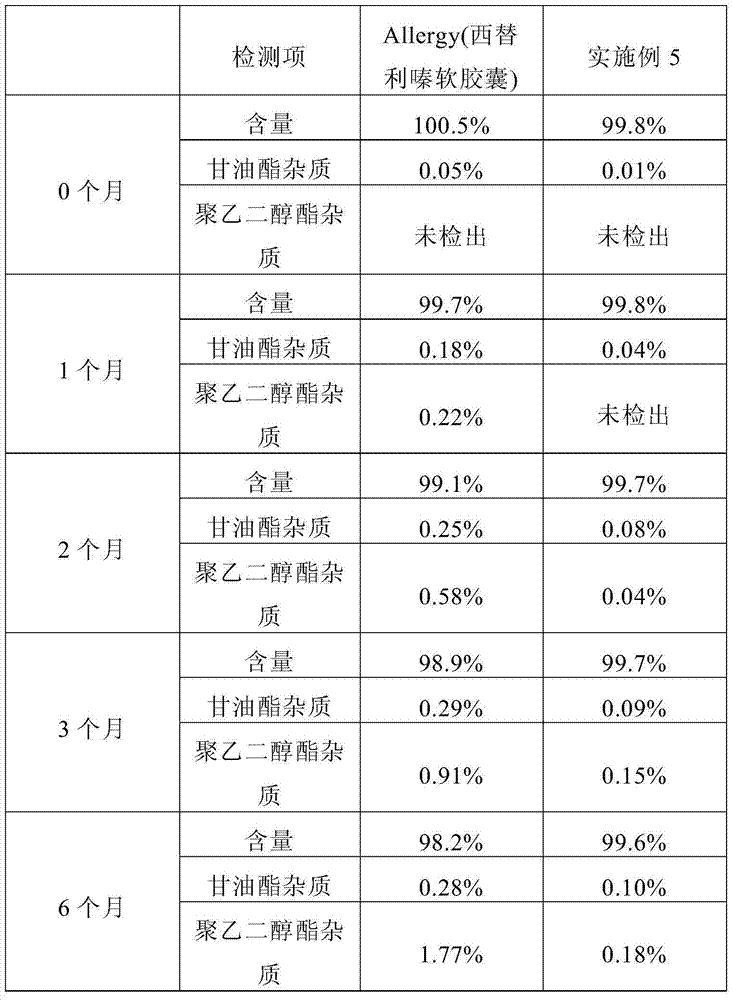
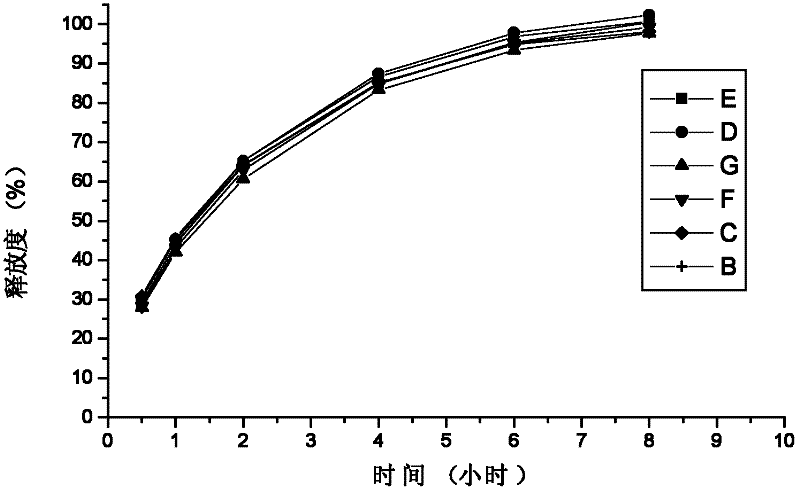
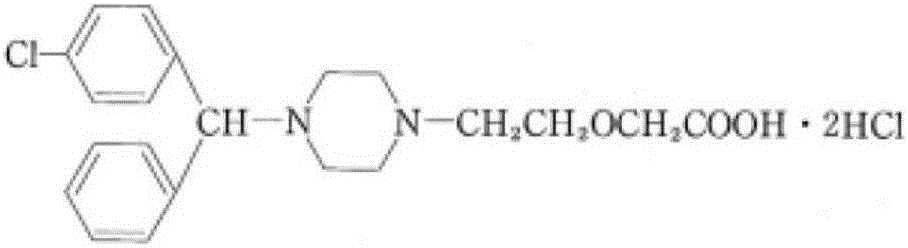
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
82 results about "Cetirizine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
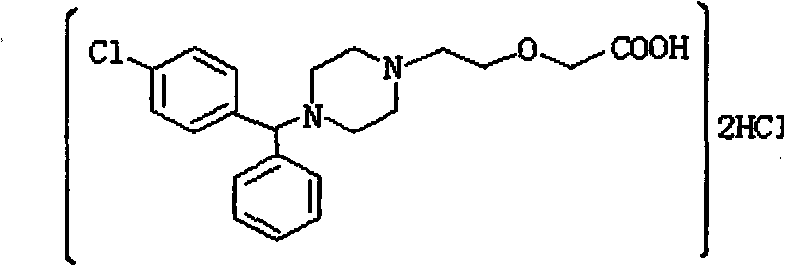
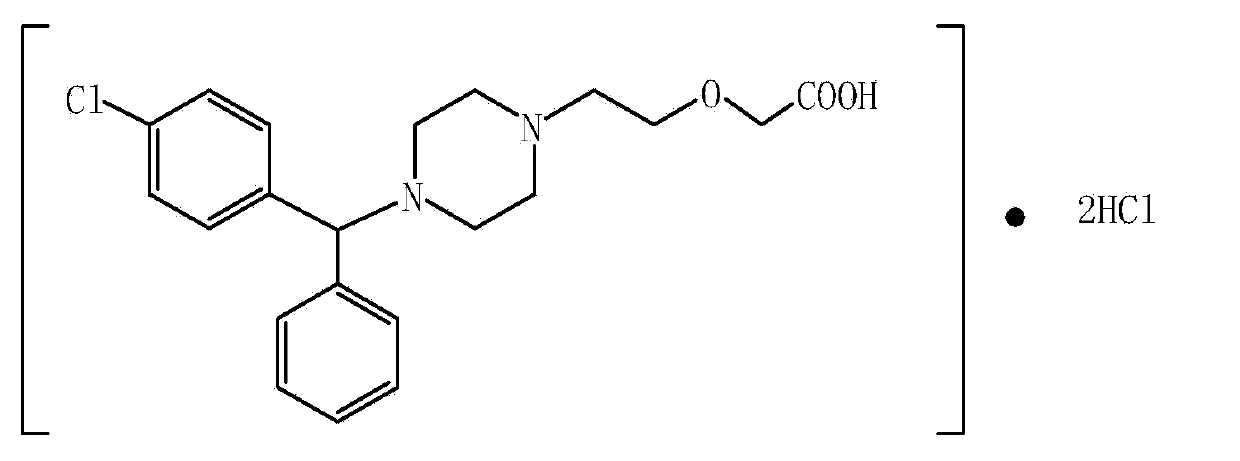
A synthetic phenylmethyl-piperazinyl derivative, antihistaminic Cetirizine is a metabolite of hydroxyzine and a selective peripheral histamine H1-receptor antagonist. It is used for symptomatic treatment of seasonal and perennial allergic rhinitis and for chronic urticaria. (NCI04)
Combination of tablet to swallow of containing Cetirizine Hydrochloride
InactiveCN1660098AImprove complianceEasy to takeOrganic active ingredientsImmunological disordersCetirizine HydrochlorideCyclodextrin
An orally-taken composite medicine containing cetirizine hydrochloride is prepared from cetirizine hydrochloride, cyclodextrin or its derivative, and flavouring. It features its agreeable taste.
Owner:LUNAN PHARMA GROUP CORPORATION
Externally applied formulation of cetirizine hydrochloride
InactiveCN1634063APharmacologically activeIncreased free drug concentrationOrganic active ingredientsAerosol deliverySkin sensitizationWhole body
Pharmacological test shows that the externally-used preparation of Cetirizine has ideal allergy resistant and anti-inflammatory action to the rat passive cutaneous anaphylaxis (Rat PCA) model and dimethylbenzene caused mouse otitis model, and also prevents the adverse effect to the central system caused by whole body administration. It is also found in the test, that the externally used preparation of Cetirizine also has very fine inhibitory action to dinitrofluorobenzene caused mouse porphyria hypersensitivity (PTH), the novel pharmacological action of the Cetirizine shows that the Cetirizine external preparation has good therapeutic action to skin inflammations which mainly include porphyria hypersensitivity.
Owner:LUNAN PHARMA GROUP CORPORATION
Cetirizine hydrochloride tablet and preparation method thereof
InactiveCN103340865AImprove solubilityDissolution does not hinderOrganic active ingredientsPharmaceutical non-active ingredientsCetirizine HydrochlorideAlcohol
The invention discloses a cetirizine hydrochloride tablet and a preparation method thereof. The preparation method of the cetirizine hydrochloride tablet comprises the following steps of: dissolving hydroxy propyl cellulose and cetirizine hydrochloride in alcohol, adding blank pellets and slowly adhering the main drug which is coated by the hydroxy propyl cellulose on the surfaces of the pellets in a slow solvent removal process; and uniformly mixing the drug-carrying pellets and pharmaceutically acceptable auxiliary materials and directly tabletting the mixture to form the cetirizine hydrochloride tablet. According to the preparation method of the cetirizine hydrochloride tablet disclosed by the invention, the cetirizine hydrochloride can be prevented from directly contacting with the external environment, so that a preparation device corrosion problem caused by strong drug acidity during a preparation production process can be avoided.
Owner:广东彼迪药业有限公司
Cetirizine hydrochloride gel
InactiveCN1457765AImprove complianceAvoid gastrointestinal irritationOrganic active ingredientsPharmaceutical delivery mechanismCetirizine HydrochlorideOral medication
The present invention relates to the field of medicine technology and is one externally applied form of Cetirizine hydrochloride gel as one histamine resisting medicine and its preparation. The present invention prepares the gel with Cetirizine hydrochloride as main medicine component and different supplementary material, and the gel is administrated via skin or mucous membrane. The present invention can avoid liver reaction and stimulation to gstrointestinal tract of the medicine, reduce medicine concentration in blood, reduce central nerve suppressing effect, raise the medicine concentration in local affected part, decrease the administration times and raise patient's compatibility.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Cetirizine hydrochloride orally disintegrating tablets and preparation method thereof
InactiveCN102038654AOvercome the disadvantages of single-actingGood disintegrationOrganic active ingredientsPill deliverySodium bicarbonateOrally disintegrating tablet
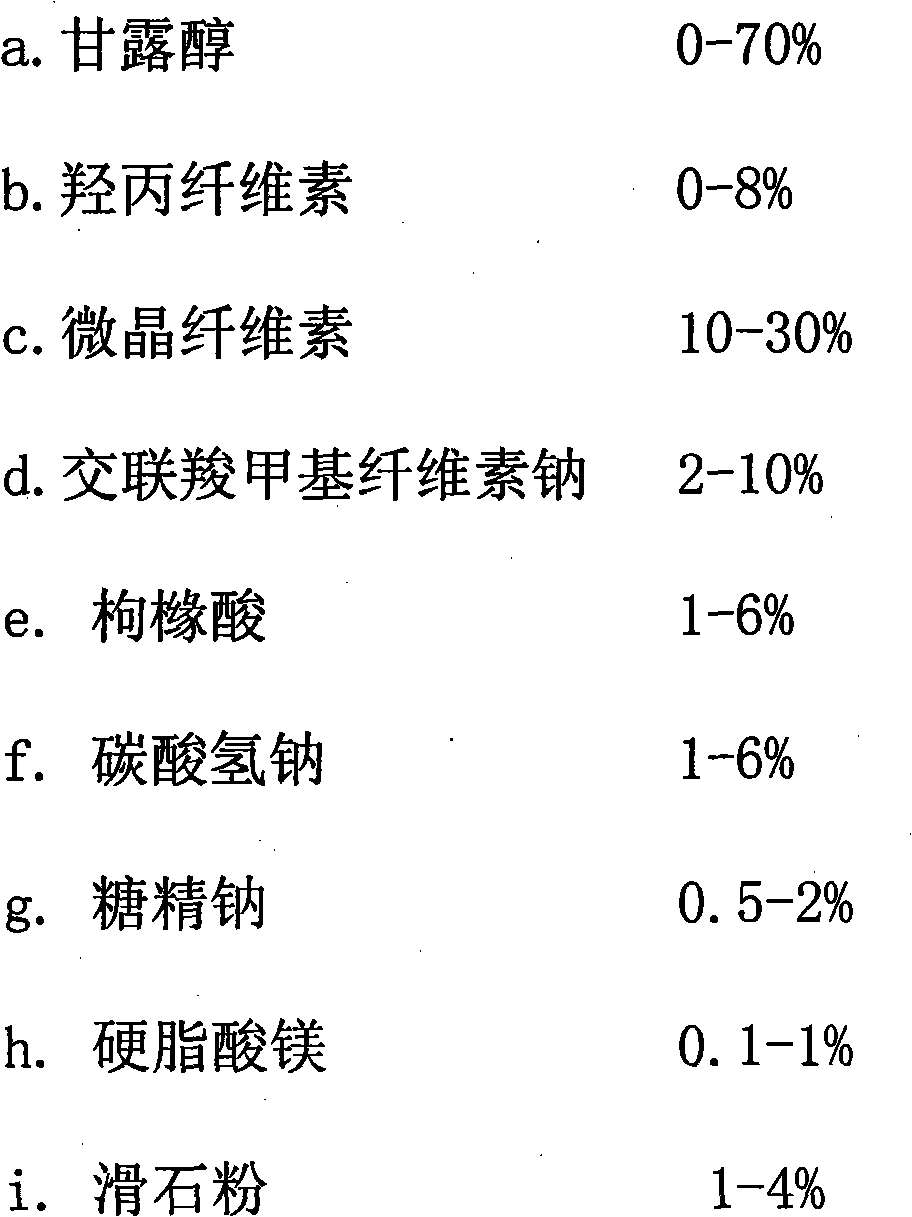
The invention discloses cetirizine hydrochloride orally disintegrating tablets, which comprise the main drug of cetirizine hydrochloride and the auxiliary materials of mannitol, microcrystalline cellulose, hydroxypropyl cellulose, croscarmellose sodium, sodium bicarbonate, citric acid, saccharin sodium, magnesium stearate, talcum powder and essence. The cetirizine hydrochloride orally disintegrating tablets have favorable curative effect on allergic rhinitis, allergen-induced urticaria and pruritus, are convenient to take, can disintegrate rapidly, and provide convenience for the old people, children or patients who suffer from medicine-taking disorders or cannot fetch water conveniently.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Cetirizine hydrochloride oral solution and preparation method thereof
ActiveCN103463089AHigh clarityHas a refreshing tasteOrganic active ingredientsPharmaceutical non-active ingredientsCelluloseCetirizine Hydrochloride
The invention discloses a cetirizine hydrochloride oral solution, which comprises cetirizine hydrochloride, mannitol, hydroxy propyl cellulose and water. The oral solution provided by the invention effectively covers up the strong titter taste of the active component cetirizine, obviously improves the stability of the oral solution, effectively reduces the chemical degradation and the toxic or side effects of the cetirizine, reduces the molecular aggregation phenomenon of the active component, reduces the using risk and improves the medicine safety.
Owner:北京柏雅联合药物研究所有限公司
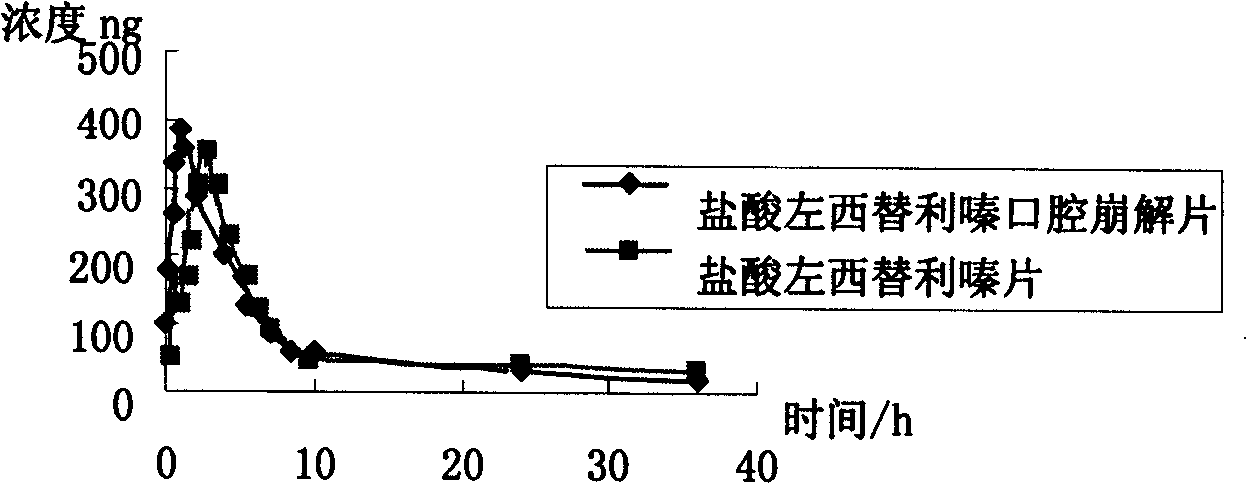
Levo-cetirizine hydrochloride orally disintegrating tablets and preparation method thereof
InactiveCN101310711AFast disintegrationFast absorptionOrganic active ingredientsPill deliveryCetirizine HydrochlorideTreatment effect
The invention relates to a hydrochloric acid levocetirizine orally disintegrating tablet and a preparation method thereof. The hydrochloric acid levocetirizine orally disintegrating tablet is obtained by directly pressing principal medicine and accessories. The tablet of the invention can be dissolved fast in a mouth cavity and has no grit feeling and high biological availability, which takes effect fast and can have curative effect faster.
Owner:海南高升医药科技开发股份有限公司
Method for preparing high-purity cetirizine hydrochloride
ActiveCN101492430AHigh puritySimple processOrganic chemistryImmunological disordersSodium chloroacetatePhenyl group
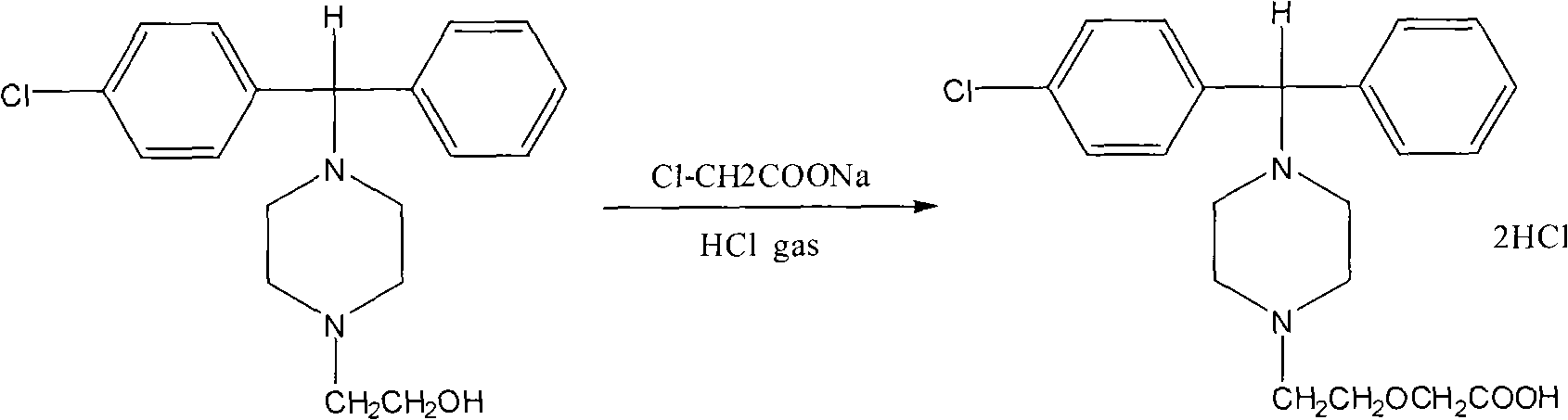
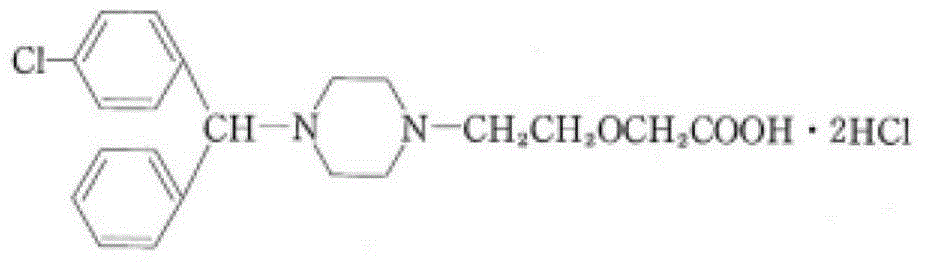
The invention provides a preparation method of cetirizine hydrochloride with high purity. The method comprises: 4-[(4-chlorophenyl) phenyl]-1-hydroxyethyl piperazine and sodium chloroacetate are used as reaction substrates and alkali metal hydroxide is used as catalyst for the condensation reaction in organic solvent at 5-40 DEG C; when the condensation reaction is finished, cetirizine mother nucleus compounds obtained after the reaction are separated and the salt-forming reaction is carried out between the cetirizine mother nucleus compounds and hydrogen chloride gas. The cetirizine hydrochloride is obtained. The method of the invention has the advantages of simple technical process, easily obtained raw materials, safe operation, little produced three wastes and high purity of obtained cetirizine hydrochloride products and satisfies standards of British pharmacopeia and European pharmacopoeia.
Owner:HANGZHOU HEZE PHARMA TECH
Cetirizine hydrochloride syrup
ActiveCN104306331AImprove stabilityGreat tasteOrganic active ingredientsSolution deliveryCetirizine HydrochlorideSodium Glutamate
The invention provides cetirizine hydrochloride syrup. The syrup comprises cetirizine hydrochloride, sodium glutamate, xanthan gum, saccharose, a pH regulator, a flavoring agent and water, wherein the weight ratio of cetirizine hydrochloride to xanthan gum is 1:(1-10), preferably 1:5; the using amount of sodium glutamate accounts for 0.5-1.0 percent of the total weight of the syrup; the pH regulator is a disodium hydrogen phosphate-citric acid buffer solution; and the flavoring agent is aspartame, steviosin or sodium saccharin. The cetirizine hydrochloride syrup has excellent taste, stable property and simple preparation process.
Owner:LUNAN BETTER PHARMA
Preparation containing Cetirizine Hydrochloride and hydrochloric pseudoephedrine and its prepn. method
InactiveCN1498617AOrganic active ingredientsPharmaceutical delivery mechanismCetirizine HydrochlorideUse medication
A medicine in the form of tablet is composed of the core tablet prepared from pseudoephedrine hydrochloride and pharmacologically receptable carrier and the coated layer prepared from cetirizine hydrochloride and pharmacologically receptable carrier. Its advantages are quick release of cetirizine hydrochloride, slow release of pseudoephedrine hydrochloride.
Owner:SHANGHAI SINE PHARMA LAB
Cetirizine hydrochloride pseudo ephedrine sustained-release pellet and preparation method thereof
InactiveCN104490880AIncreased surface distribution areaReduce absorption rateOrganic active ingredientsRespiratory disorderCetirizine HydrochlorideTherapeutic effect
The invention relates to a cetirizine hydrochloride pseudo ephedrine sustained-release pellet. The cetirizine hydrochloride pseudo ephedrine sustained-release pellet comprises a medicated pellet body and a coating layer; the coating layer wraps the medicated pellet body; the medicated pellet body comprises 5 mg of cetirizine hydrochloride, 125 mg of pseudoephedrine hydrochloride, 80 mg of core pellets, 100-200 mg of fillers, 25-125 mg of lubricating agents and 5-50 mg of binding agents; the coating layer comprises 45-225 mg of Eudragit NE30D and 14-68 mg of talcum powder; the preparation method includes the steps that (1) material preparing; (2) mixing; (3) binding agent preparing; (4) pelleting; (5) coating material preparing, (6) coating; (7) filling; and (8) conducting aluminum plastic and obtaining end products. The cetirizine hydrochloride pseudo ephedrine sustained-release pellet is used for remitting nasal symptoms or non-nasal symptoms caused by perennial or seasonal anaphylactic rhinitis; a novel sustained release preparation and a novel pellet preparation are adopted, the treatment effect is stable, and the bioavailability is higher.
Owner:HARBIN SHENGJI PHARMA
Cetirizine and pseudoephedrine sustained-release capsule and preparation method thereof
InactiveCN101474166AWell mixedQuick effectOrganic active ingredientsPharmaceutical delivery mechanismCetirizine HydrochlorideSustained Release Capsule
The invention discloses a cetirizine pseudoephedrine slow release capsule. Cetirizine hydrochloride is prepared to quick release pellets and pseudoephedrine hydrochloride is prepared to slow release pellets, in accordance with the weight proportion that each 1000 preparations contain 5g of cetirizine hydrochloride and 120g of pseudoephedrine hydrochloride, the two pellets are uniformly mixed and encapsulated; or the two pellets are encapsulated respectively in proportion. The quick release part of the cetirizine hydrochloride and the slow release part of the pseudoephedrine hydrochloride are synthesized together subsequent to being prepared to the pellets respectively, so as to prepare the capsule with two different medicine-releasing speeds, the medicine-releasing speed of each pellet is uniform and the medicine effect is steady. The capsule has the advantages of even and extensive distribution of the medicine in vivo subsequent to the administering, sufficient absorption of the medicine, small stimulation to gastrointestinal tracts and good reproducibility of the medicine-releasing rule, and improves, while maintaining the properties of rapid effecting and long half-life of the cetirizine, the pharmacokinetic properties of the pseudoephedrine hydrochloride to administer twice per day instead of having to administer four times per day, so that both reach the optimal cooperative curative effect.
Owner:QINGDAO HUANGHAI PHARM CO LTD
Cetirizing hydrochloride cataplasm preparation
InactiveCN1457781AIncrease concentrationImprove complianceOrganic active ingredientsPharmaceutical non-active ingredientsCetirizine HydrochlorideOral medication
The present invention relates to medicine technology and is cataplasm as one new form of Cetirizine hydrochloride. There are available oral forms of Cetirizine hydrochloride, such as tablet, capsule, solution preparation, etc. The present invention prepares the cataplasm with Cetirizine hydrochloride as main medicine component and different supplementary material, and the cataplasm is administrated via skin. The present invention can avoid stimulation to gastrointestinal tract of the medicine, reduce medicine concentration in blood, reduce central nerve suppressing effect, raise the medicine concentration in local affected part, decrease the administration times and raise patient's compatibility.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Cetirizine hydrochloride liniment
InactiveCN101623253AReduce stimulationFast transdermal absorptionOrganic active ingredientsInorganic non-active ingredientsCetirizine HydrochlorideIrritation
The invention belongs to the field of medicines, in particular to an external preparation of cetirizine hydrochloride, i.e., cetirizine hydrochloride liniment. Aiming at the defects of poor tissue infiltration capacity, limited dosage reaching target positions, and the like existed in the prior external preparation of cetirizine hydrochloride, the invention provides the cetirizine hydrochloride liniment with more remarkable antianaphylaxis healing effect by optimizing a prescription and screening more suitable accessories in an experiment process according to the physicochemical property of the cetirizine hydrochloride. The cetirizine hydrochloride liniment provided by the invention has simple preparation process and lower cost, little skin irritation and favorable treatment effect on partial skin redness and pruritus caused by mosquito bites.
Owner:LUNAN PHARMA GROUP CORPORATION
Method for detecting relevant substances of cetirizine hydrochloride granules
ActiveCN104458932AGood compatibilityExtended retention timeComponent separationCetirizine HydrochlorideUltraviolet detectors
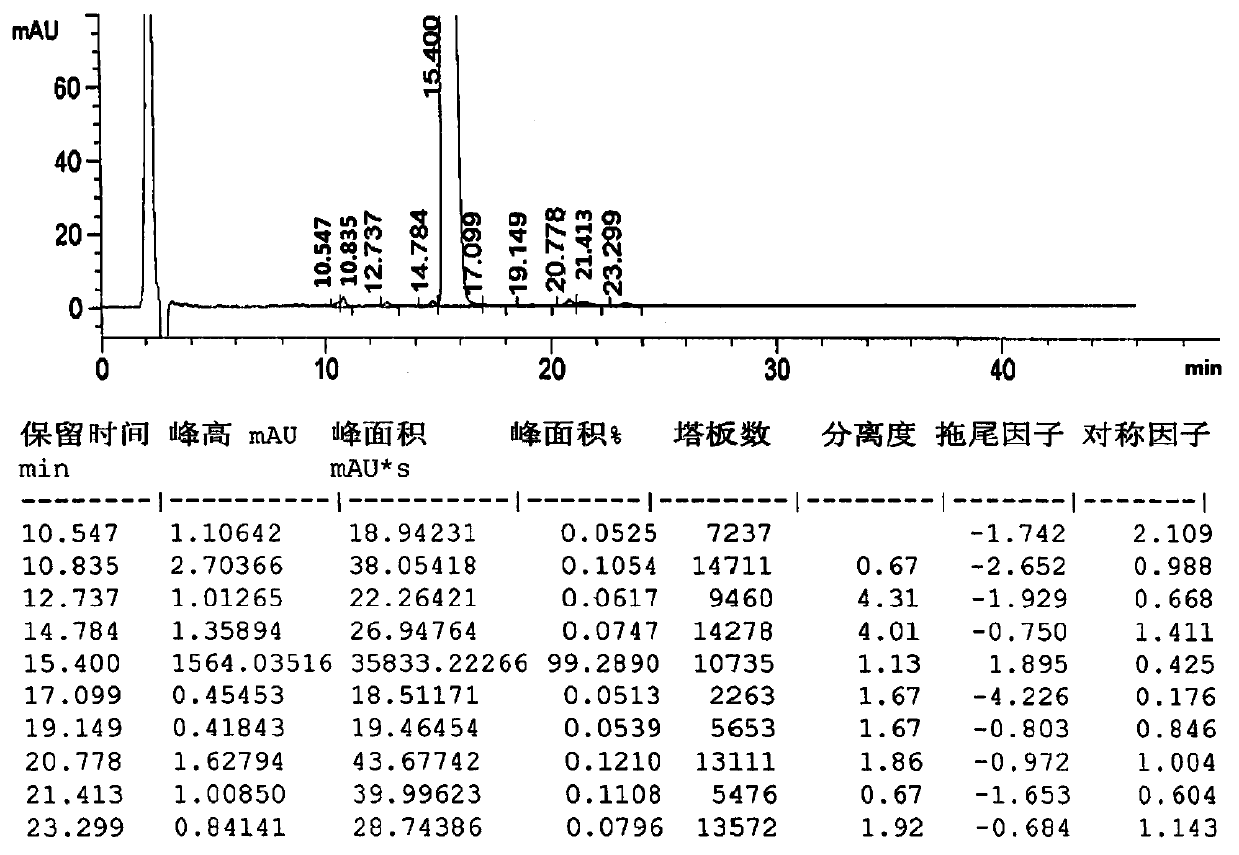
The invention provides a method for detecting relavant substances of cetirizine hydrochloride granules. The detection method is characterized in that a phenyl column is adopted, the phenyl column is an Agilent Eclipse XDB phenyl 5-micrometer chromatographic column in the area of 4.6mm*250mm; a mixed solution of acetonitrile and ammonium acetate buffer solution in a volume ratio of (2: 8) to (4: 6) is adopted as a flow phase, isocratic elution is carried out on a high-efficiency liquid phase chromatographic instrument, and the relevant substances of the cetirizine hydrochloride granules are detected by utilizing an ultraviolet detector. The detection method is good in sensitivity, specificity, precision and accuracy, simple to operate and suitable for detecting relevant substances of the cetirizine hydrochloride granules.
Owner:SUNSHINE LAKE PHARM CO LTD
Cetirizine pseudoephedrine sustained-release capsules
InactiveCN101181244AAvoid molding qualityHigh yieldOrganic active ingredientsCapsule deliveryCetirizine HydrochlorideSustained Release Tablet
The invention relates to a cetirizine pseudoephedrine sustained-release capsule preparation. The interior of the capsule is provided with two tablets with different drug releasing speeds, namely, the cetirizine hydrochloride immediate-release tablet and the pseudoephedrine hydrochloride sustained-release tablet. The preparation has simple preparation process, and the ordinary tablet press machine can complete the process by only one time of tablet pressing; when taken, the cetirizine hydrochloride and the pseudoephedrine hydrochloride can be released simultaneously, so as to better achieve the purpose of combined treatment.
Owner:BEIJING SUNHO PHARMA
A kind of cetirizine hydrochloride tablet and preparation technology thereof
ActiveCN104887635BReduce penetrationAntioxidantOrganic active ingredientsPill deliveryCetirizine HydrochlorideMedicine
The invention relates to a cetirizine hydrochloride tablet. The cetirizine hydrochloride tablet comprises cetirizine hydrochloride lipidosome, a filler and a disintegrating agent. The cetirizine hydrochloride lipidosome comprises cetirizine hydrochloride, soya bean lecithin, cholesterol, vitamin E and Tween. The cetirizine hydrochloride tablet is advantaged by being rapid in dissolution, high in bioavailability, good in stability, simple in preparing process, suitable for industrial production, and the like.
Owner:SUZHOU DAWNRAYS PHARM CO LTD
A kind of cetirizine hydrochloride tablet and preparation method thereof
InactiveCN104800178BImprove liquiditySimple production processOrganic active ingredientsPharmaceutical non-active ingredientsCetirizine HydrochlorideCoated tablets
The invention discloses a cetirizine hydrochloride tablet and a preparation method thereof, belonging to the field of chemical pharmaceutical preparations. The preparation is made of cetirizine hydrochloride into pellets, which are coated and prepared into tablets. The invention avoids The sticky punch is achieved, and the drug release is stable.
Owner:张祥坤
Cetirizine hydrochloride tablet and preparation method thereof
InactiveCN104800178AImprove liquiditySimple production processOrganic active ingredientsPharmaceutical non-active ingredientsDrug releaseCetirizine Hydrochloride
The invention discloses a cetirizine hydrochloride tablet and a preparation method thereof, and belongs to the field of chemical medicine preparations. The cetirizine hydrochloride tablet is characterized in that cetirizine hydrochloride is prepared into pellets, and the pellets are coated, so as to prepare the tablet. The cetirizine hydrochloride tablet has the advantages that the sticking is avoided, and the release of the medicine is stable.
Owner:张祥坤
Compound cetirizine hydrochloride gel
InactiveCN1762360AImprove physical stabilityShort validity periodOrganic active ingredientsPharmaceutical delivery mechanismCetirizine HydrochlorideCurative effect
Disclosed is a cetirizine hydrochloride gelling agent, wherein each 1000g of the gelling agent comprises 100.0g of cetirizine hydrochloride, 0.5-2g of mupirocin, 5.0-10.0g of base, 60-100ml of glycerin propylene glycol, 5.0-10.0g of triethanolamine, right amount of PBS, and balancing distilled water.
Owner:HARBIN MEDICAL UNIVERSITY
Cetirizine hydrochloride tablet and preparation method thereof
ActiveCN108309948BImprove stabilityImprove bioavailabilityOrganic active ingredientsPharmaceutical non-active ingredientsPolyethylene glycolMagnesium stearate
The invention belongs to the technical field of medicinal solid oral-taking preparation, relates to a cetirizine hydrochloride tablet and a preparation method thereof. The cetirizine hydrochloride tablet is prepared from the following raw materials and auxiliary materials of cetirizine hydrochloride, a filling agent, a flow aid, a bonding agent, a plasticizer, an opaquer, wherein the filling agentis prepared from microcrystalline cellulose and lactose; the flow aid is prepared from one or two of silicon dioxide, talcum powder and colloidal silicon dioxide; the lubricating agent is magnesium stearate; the bonding agent is prepared from one or two of hydroxypropyl methylcellulose, starch, dextrin or polyvinyl alcohol; the plasticizer is polyethylene glycol-400; and the opaquer is titanium dioxide. The product has high stability, can keep stable at high temperature and in a high-temperature environment; the product has high stability, can be stored for a longer time under the normal storage conditions, the shelf life of the product is prolonged, the product can be dissolved rapidly, bioavailability is high, as provided by external quality research, the product conforms to the regulation and standard, clinical bioequivalence can meet the requirement of the regulation. The invention further provides a preparation method, and the production prescription process is easy.
Owner:XINHUA PHARMA GAOMI CO LTD
Cetirizine hydrochloride soft capsule and preparation method thereof
InactiveCN104116720AStable pHReduce generationOrganic active ingredientsInorganic non-active ingredientsCetirizine HydrochloridePolyethylene glycol
The invention provides a cetirizine hydrochloride soft capsule and a preparation method thereof. The cetirizine hydrochloride soft capsule comprises a capsule shell and content, wherein the capsule shell is prepared from gelatin, glycerinum, sorbitol and water; the content is prepared from cetirizine hydrochloride, buffer salt, polyethylene glycol and water. The cetirizine hydrochloride soft capsule has the advantages of good stability, little impurity production, relatively long acting period and few potential adverse responses, and is convenient to transport and store.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Preparation for treating allergic rhinitis and preparation method thereof
InactiveCN102302497AFlat surfacePromote absorptionOrganic active ingredientsRespiratory disorderSide effectClinical efficacy
The invention relates to a preparation for treating allergic rhinitis. The preparation is prepared by pressing a quick release layer and a sustained-release layer into bilayer tablets and coating, wherein the quick release layer consists of the following components in percentage by mass: 1 to 10 percent of levocetirizine hydrochloride, 1 to 15 percent of disintegrating agent, 0.5 to 3 percent of lubricating agent, 0 to 8 percent of adhesive and the balance of filler; the sustained-release layer consists of the following components in percentage by mass: 20 to 30 percent of pseudoephedrine hydrochloride, 50 to 60 percent of sustained-release framework material, 0.5 to 3 percent of lubricating agent, 0 to 8 percent of adhesive and the balance of filler; and the mass ratio of the levocetirizine hydrochloride to the pseudoephedrine hydrochloride is 1:48. The sustained-release tablets for treating the allergic rhinitis have bright and clean surfaces, and the release behavior of the main medicine pseudoephedrine hydrochloride of the sustained-release tablets has high consistency with that of American marketed medicine cetirizine hydrochloride and pseudoephedrine hydrochloride sustained-release tablets through detection; and the dissolution rate of the main medicine levocetirizine hydrochloride of the sustained-release tablets in 30 minutes is over 90 percent, so that the sustained-release preparation for treating the allergic rhinitis has high bioavailability, a good absorption effect, comprehensive and effective clinical curative effects, small side effects and ideal safety.
Owner:TIANSHENG PHARMA GROUP
Ophthalmic cetirizine hydrochloride liposome, in-situ gel and preparation method of ophthalmic cetirizine hydrochloride liposome
ActiveCN114392235AReduce releaseImprove permeabilityOrganic active ingredientsSenses disorderCholesterolPhospholipid
The invention discloses cetirizine hydrochloride ophthalmic liposome, in-situ gel and a preparation method of cetirizine hydrochloride ophthalmic liposome, and belongs to the technical field of pharmaceutical preparations. In order to overcome the defects that existing cetirizine hydrochloride eye drops are short in residence time, poor in compliance of semi-solid dosage forms, low in bioavailability, not easy to accept by patients and the like, an ethanol injection method is combined with an ammonium sulfate gradient method to obtain the liposome, and the liposome comprises 0.28-0.30% of cetirizine hydrochloride, 1.4-6.0% of phospholipid, 0.14-1.8% of cholesterol, 0.1-0.15% of an osmotic pressure regulator, 0.05-0.1% of a bacteriostatic agent and the balance of water. And a solvent. The invention further provides the cetirizine hydrochloride liposome-in-situ gel and a preparation method thereof. By optimizing the prescription and the process, the obtained liposome and in-situ gel can delay the drug release, increase the retention time of eyes, improve the cornea permeability and improve the bioavailability.
Owner:CHENGDU UNIV
A kind of cetirizine hydrochloride syrup
ActiveCN104306331BImprove stabilityGreat tasteOrganic active ingredientsSolution deliveryCetirizine HydrochlorideSodium Glutamate
The invention provides cetirizine hydrochloride syrup. The syrup comprises cetirizine hydrochloride, sodium glutamate, xanthan gum, saccharose, a pH regulator, a flavoring agent and water, wherein the weight ratio of cetirizine hydrochloride to xanthan gum is 1:(1-10), preferably 1:5; the using amount of sodium glutamate accounts for 0.5-1.0 percent of the total weight of the syrup; the pH regulator is a disodium hydrogen phosphate-citric acid buffer solution; and the flavoring agent is aspartame, steviosin or sodium saccharin. The cetirizine hydrochloride syrup has excellent taste, stable property and simple preparation process.
Owner:LUNAN BETTER PHARMA
Cetirizine hydrochloride tablet and preparation method thereof
ActiveCN109481409ANot growing fasterSimple and fast operationOrganic active ingredientsSenses disorderCetirizine HydrochlorideAqueous solution
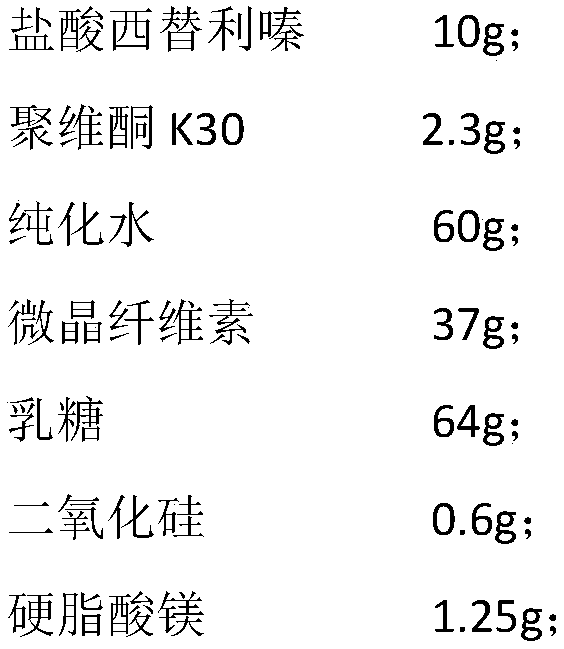
The invention relates to the technical field of pharmaceutical solid oral preparations and specifically relates to a cetirizine hydrochloride tablet and a preparation method thereof. The cetirizine hydrochloride tablet is prepared by using the following method: dissolving cetirizine hydrochloride into a povidone aqueous solution; then, carrying out wet granulation on cetirizine hydrochloride and microcrystalline cellulose to obtain a cetirizine hydrochloride pretreated particle; and finally, uniformly mixing and tabletting the cetirizine hydrochloride pretreated particle and pharmaceutically available auxiliary materials, wherein the weight ratio of cetirizine hydrochloride to the microcrystalline cellulose is 1:1 to 1:15.
Owner:微研优仿医药科技(江苏)有限公司
A kind of cetirizine hydrochloride tablet
ActiveCN104546772BPromote dissolutionNo corrosionOrganic active ingredientsPill deliverySolubilityCetirizine Hydrochloride
Owner:LUNAN BETTER PHARMA
A kind of cetirizine hydrochloride solid preparation and preparation method thereof
ActiveCN106177973BOrganic active ingredientsPharmaceutical non-active ingredientsCetirizine HydrochloridePharmaceutical formulation
The invention relates to a cetirizine hydrochloride solid preparation and a preparation method thereof, and belongs to the technical field of medicinal preparations. The solid preparation comprises cetirizine and a pH regulator, the pH regulator and the corresponding use amount of the pH regulator make the pH value of a solution obtained by dissolving the prepared solid preparation in water with the weight 10 times the weight of the solid preparation be 5.0-7.2, and the solid preparation meeting the above condition has good stability. The preparation method of the solid preparation has the advantages of simplicity in operation, low cost, and suitableness for industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Cetirizine hydrochloride tablet
ActiveCN104546772APromote dissolutionNo corrosionOrganic active ingredientsPill deliverySolubilityCetirizine Hydrochloride
The invention discloses a cetirizine hydrochloride tablet and a preparation method thereof. The method comprises the following steps: dissolving cetirizine hydrochloride into water, and adding an alkaline matter to form cetirizine alkali so that the solubility is reduced and the cetirizine alkali is separated out; filtering, drying and crushing the cetirizine alkali, so as to obtain cetirizine powder; dissolving the cetirizine alkali and deoxycholic acid into ethyl alcohol, and carrying out rotary evaporation to remove ethyl alcohol, so as to obtain cetirizine deoxycholic acid dispersion powder; and evenly mixing the cetirizine deoxycholic acid dispersion powder with pharmaceutically available auxiliary materials and tabletting, so as to obtain the rapidly dissolved cetirizine tablet.
Owner:LUNAN BETTER PHARMA
Purification method of cetirizine hydrochloride
ActiveCN104045607AHigh purityHigh yieldOrganic chemistryCetirizine HydrochloridePurification methods
The invention relates to a purification method of cetirizine hydrochloride. The method comprises the following steps: (1) dissolving cetirizine hydrochloride and a small amount of potassium iodide in a mixed solution of water and ethanol, and stirring evenly; (2) adding a proper amount of potassium bicarbonate solution with concentration of 40% to the mixed reaction solution, adjusting the pH value of the reaction solution to 7.5-8.5, and standing for 15-30 min; (3) continuing adding a proper amount of a mixed solution of ethanol and dichloromethane, adding hydrochloric acid to regulate the pH value of the reaction solution to 6-7, and crystallizing for 1-1.5 h; and (4) filtering, washing the products with methylene chloride more than 2 times, and drying to obtain the products.
Owner:LIVZON PHARM GRP INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com