A kind of cetirizine hydrochloride solid preparation and preparation method thereof
A technology of cetirizine hydrochloride and solid preparations, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problems of excessive substances, poor compliance, and poor taste-masking effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The influence investigation of embodiment 1PH regulator on preparation stability
[0052] (1) See Table 1 for prescriptions 1-12
[0053] (2) Preparation process
[0054] 1) cetirizine hydrochloride, diluent and pH regulator are mixed homogeneously;
[0055] II) wet the mixture obtained in step I) with 40% (w / w) ethanol-water solution;
[0056] III) stirring the wetting mixture obtained in step II) to bring the pH adjuster in intimate / intimate contact with the active substance and the diluent;
[0057] IV) carry out wet sizing by a swing granulator;
[0058] v) drying the stirred wetting mixture of step IV) until the final moisture content in the suspension-modified particles as measured by loss on drying is <2.0% (w / w);
[0059] VI) Sieve the dry granules obtained in step V), and sieve out particles larger than 20 mesh and fine powders smaller than 80 mesh.
[0060] (3) Detection of related substances
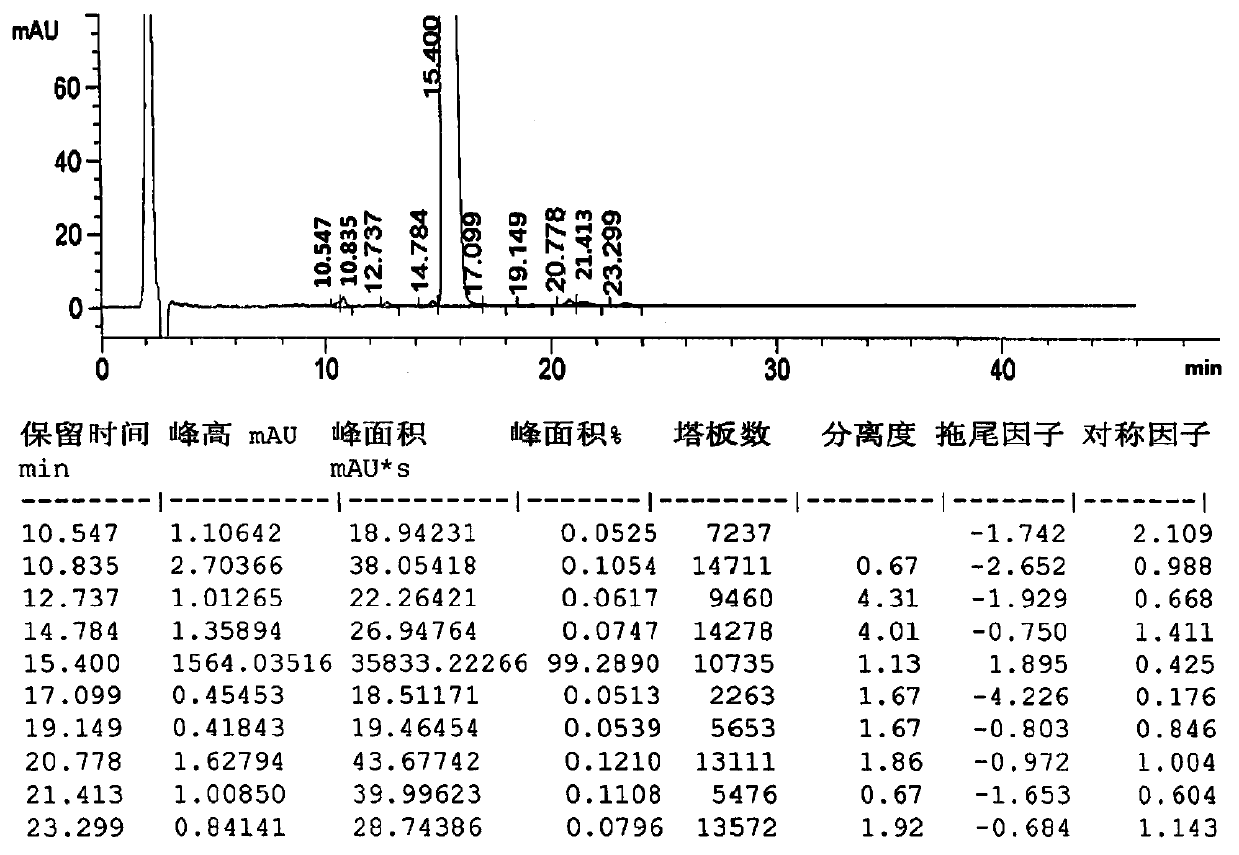
[0061] Take the samples prepared from prescription 1-presc...
Embodiment 2
[0068] The investigation of the type of essence of embodiment 2 to preparation stability
[0069] (1) Prescription 13-16
[0070]
[0071] (2) Preparation process
[0072] 1) cetirizine hydrochloride, sucrose, trisodium citrate dihydrate and specific types of essence are mixed evenly;
[0073] II) wet the mixture obtained in step I) with 40% (w / w) ethanol-water solution;
[0074] III) stirring the wetting mixture obtained in step II) to bring the pH adjuster in intimate / intimate contact with the active substance and the diluent;
[0075] IV) carry out wet sizing by a swing granulator;
[0076] v) drying the stirred wetting mixture of step IV) until the final moisture content in the suspension-modified particles as measured by loss on drying is <2.0% (w / w);
[0077] VI) Sieve the dry granules obtained in step V), and sieve out particles larger than 20 mesh and fine powders smaller than 80 mesh.
[0078] (3) Detection of related substances
[0079] Table 2-2. The resu...
Embodiment 3
[0082] The influence of embodiment 3 solvent on preparation technology
[0083] For prescriptions 17-22, different concentrations of ethanol and water solvents were used as wetting agents, and the influence on the preparation process was investigated. The results are shown in Table 3.
[0084] Table 3. The influence of different solvents on the preparation process
[0085]
[0086] As can be seen from Table 3, it is difficult to use water as a solvent for wet granulation, and the resistance of the granulator is large; as the proportion of ethanol in the solvent increases, the wet granulation gradually becomes smoother, and the resistance of the granulator is small, but when the proportion of ethanol reaches 80%. , wet particles are easy to stick together. Considering the overall process and cost, when the proportion of ethanol is in the range of 20%-60%, the process effect is better. When the proportion of ethanol is 40%, the wet granulation is smooth and the process ef...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com