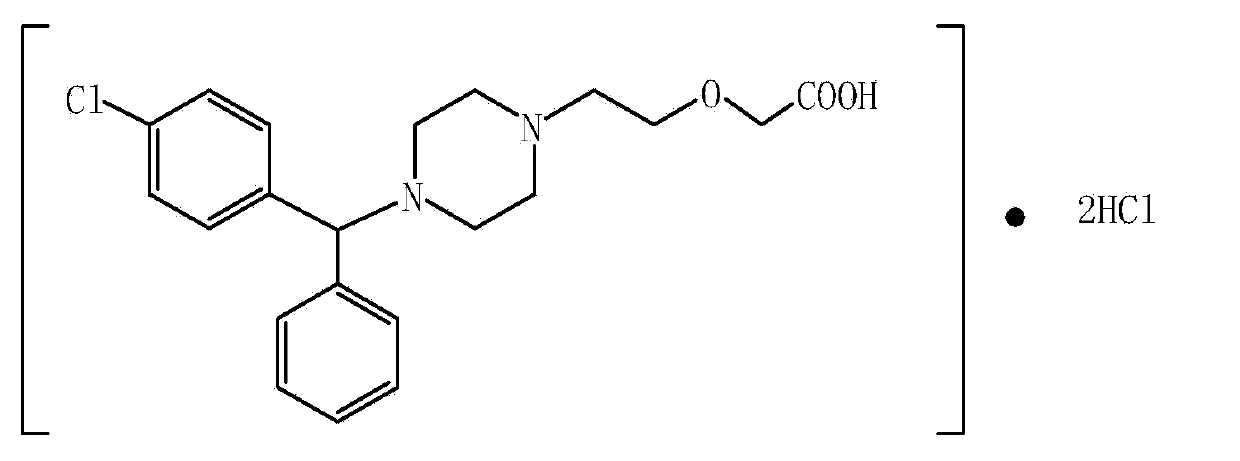
Cetirizine hydrochloride oral solution and preparation method thereof
A technology of cetirizine hydrochloride and oral solution, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of sorbitol usage inspection and other problems, and achieve low production costs , easy to take, significant anti-allergic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1 The screening of auxiliary material kind
[0044] 1. The main drug cetirizine hydrochloride and other auxiliary materials are prepared according to the proportioning ratio in Table 1, wherein, each prescription is prepared with 20 bottles of 1000ml, each bottle is 50ml;
[0045] Table 1 The composition table of raw materials and ingredients of prescription 1-6
[0046] Composition
Prescription 1
Prescription 2
Prescription 3
Prescription 4
Prescription 5
Prescription 6
50mg
50mg
50mg
50mg
50mg
50mg
50mg
50mg
50mg
50mg
50mg
50mg
sodium benzoate
50mg
50mg
50mg
50mg
50mg
50mg
/
5g
/
/
/
/
/
/
5g
/
/
/
/
/
/
5ml
/
/
polyethylene glycol 6000
/
/
/
/
5...
Embodiment 2
[0066] The screening of embodiment 2 auxiliary materials
[0067] 1. The main drug cetirizine hydrochloride and other auxiliary materials are prepared according to the proportioning as shown in Table 3, wherein, each prescription is prepared with 20 bottles of 1000ml, each bottle is 50ml;
[0068] The composition list of raw materials and ingredients of prescription 7-11 of table 3
[0069] Composition
Prescription 7
Prescription 8
Prescription 9
Prescription 10
Prescription 11
50mg
50mg
50mg
50mg
50mg
[0070] aspartame
50mg
50mg
50mg
50mg
50mg
sodium benzoate
50mg
50mg
50mg
50mg
50mg
5g
5g
5g
/
/
5g
/
5g
/
5ml
/
5ml
/
/
1g
1g
1g
Sodium dihydrogen phosphat...
Embodiment 3
[0090] Embodiment 3 Screening of auxiliary material consumption
[0091] According to the type of the cetirizine hydrochloride oral solution auxiliary material screened, the proportioning dosage of the auxiliary material is screened.
[0092] 1. The principal ingredient cetirizine hydrochloride and the auxiliary materials are prepared according to the ratio of tables 5 and 6, wherein, each prescription is prepared with 20 bottles of 1000ml, 50ml per bottle;
[0093] Table 5 Composition table of raw materials and ingredients of prescription 12-17
[0094] composition
Prescription 12
Prescription 13
Prescription 14
Prescription 15
Prescription 16
Prescription 17
50mg
50mg
50mg
50mg
50mg
50mg
50mg
50mg
50mg
50mg
50mg
50mg
sodium benzoate
50mg
50mg
50mg
50mg
50mg
50mg
5g
5g
5g
7.5g
7.5g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com