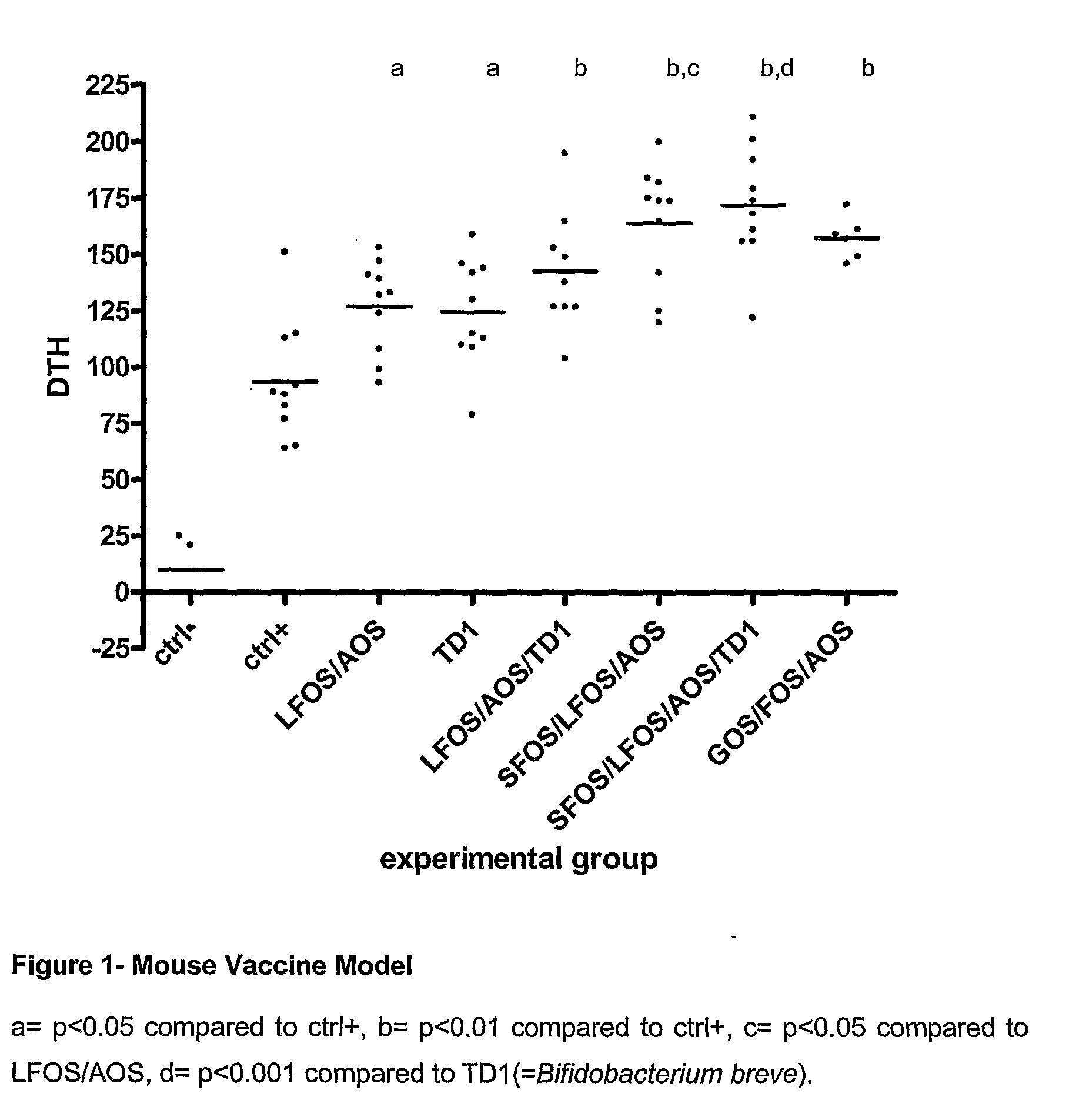
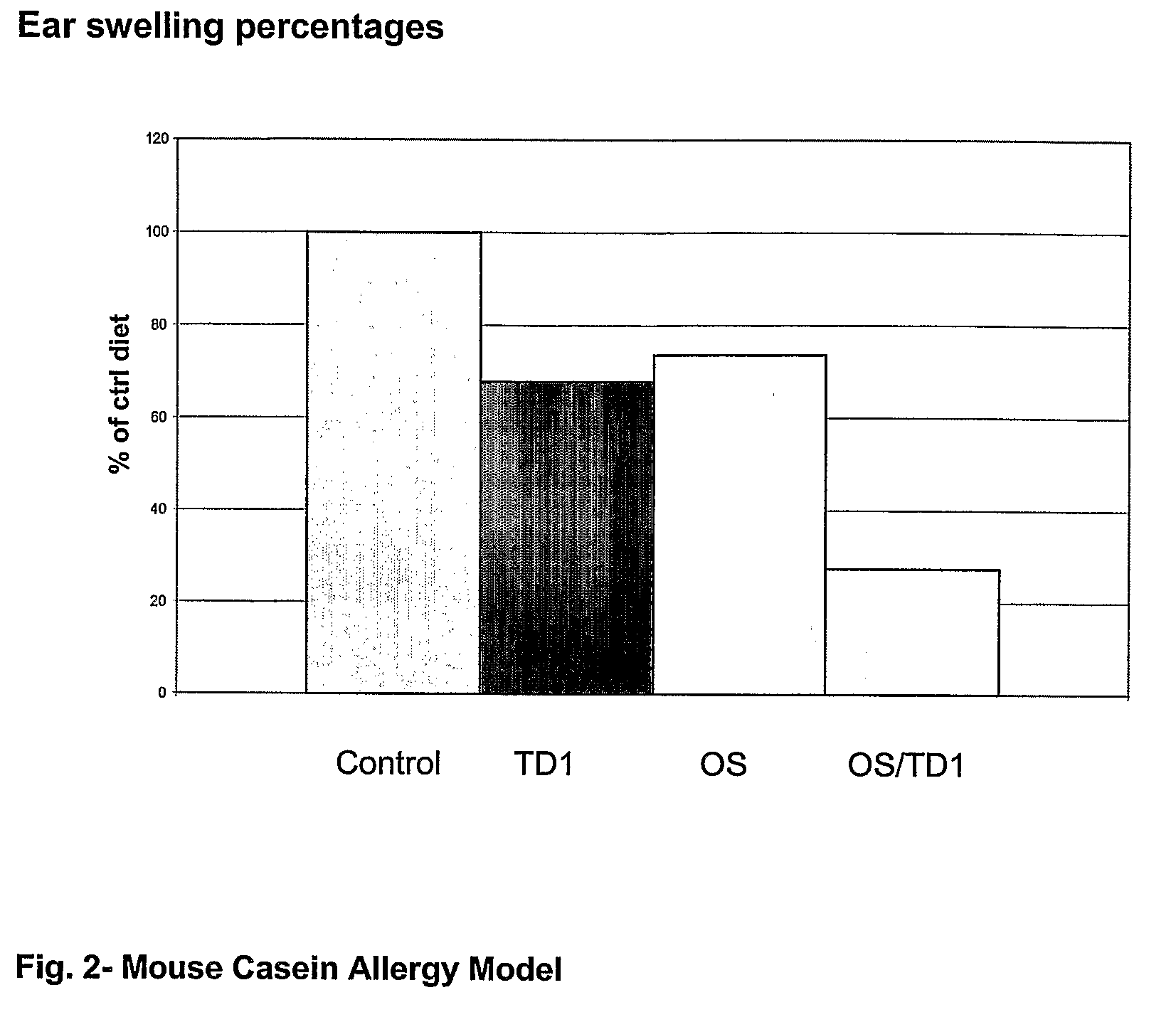
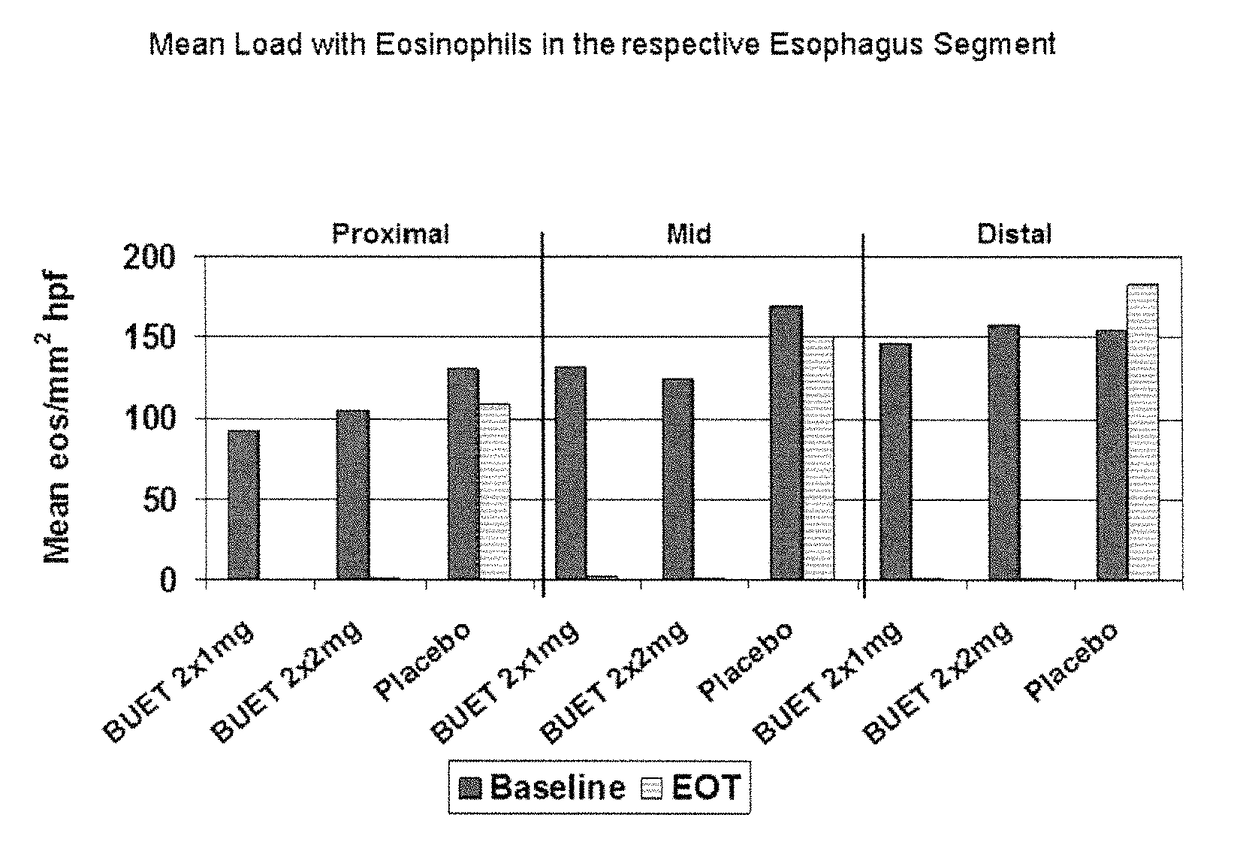
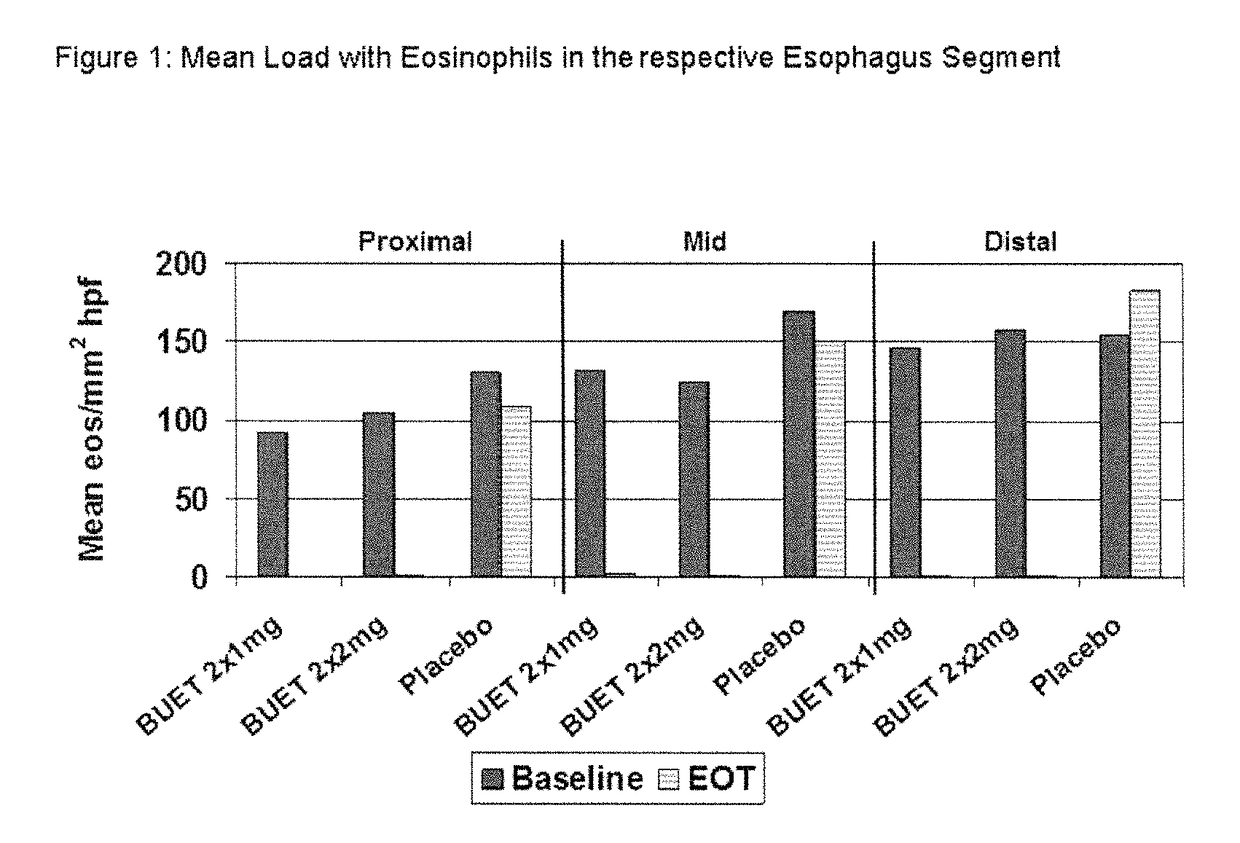
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
44 results about "Eosinophilic esophagitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
An allergic inflammation of esophagus which involves build up of eosinophils in its lining.
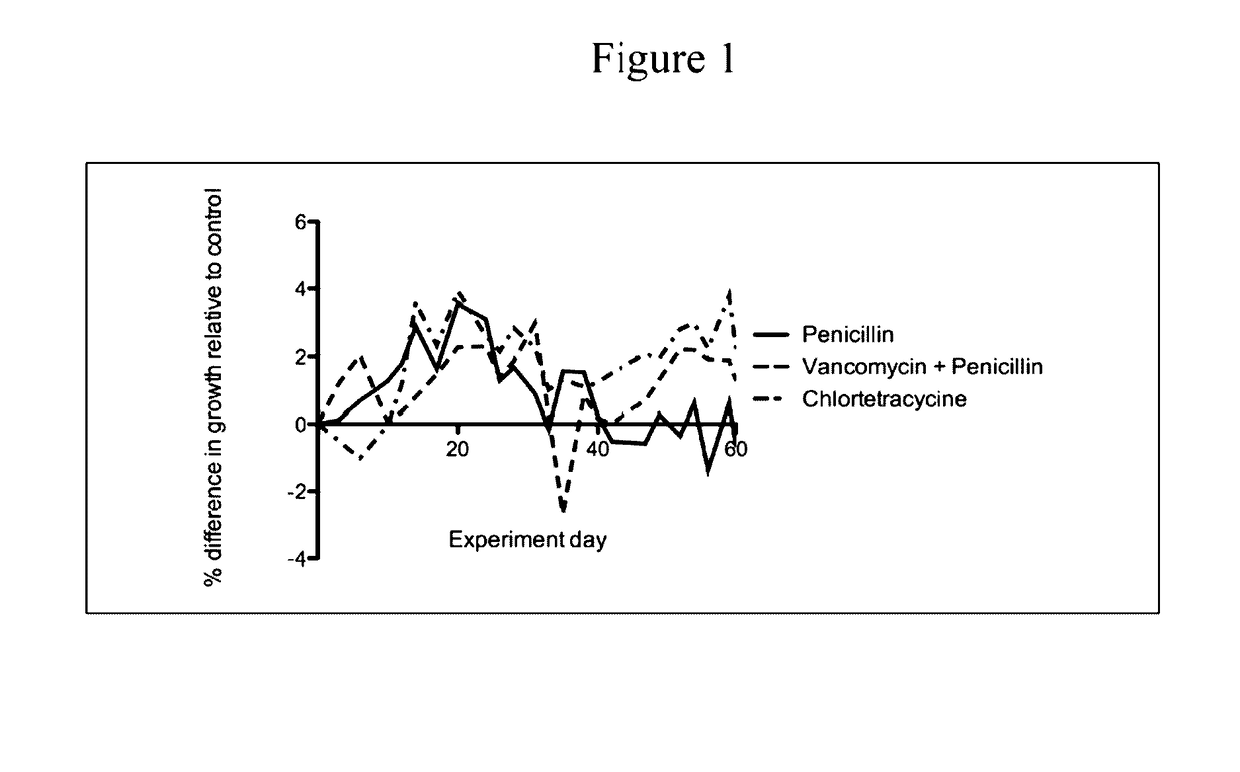
Compositions and methods for characterizing and restoring gastrointestinal, skin, and nasal microbiota
ActiveUS20100074872A1Growth inhibitionFacilitate calorie uptakeBiocideMetabolism disorderBacteroidesDisease
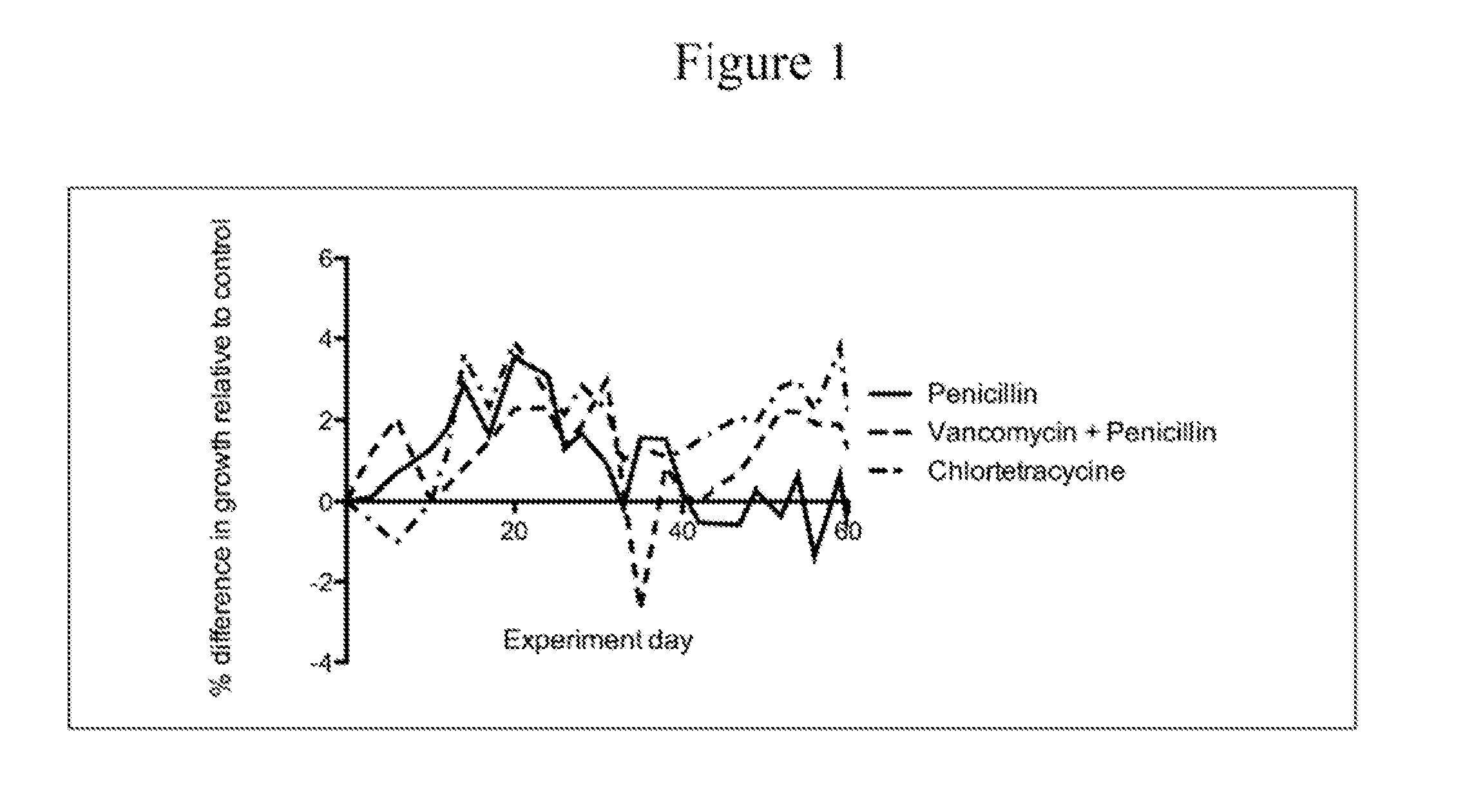
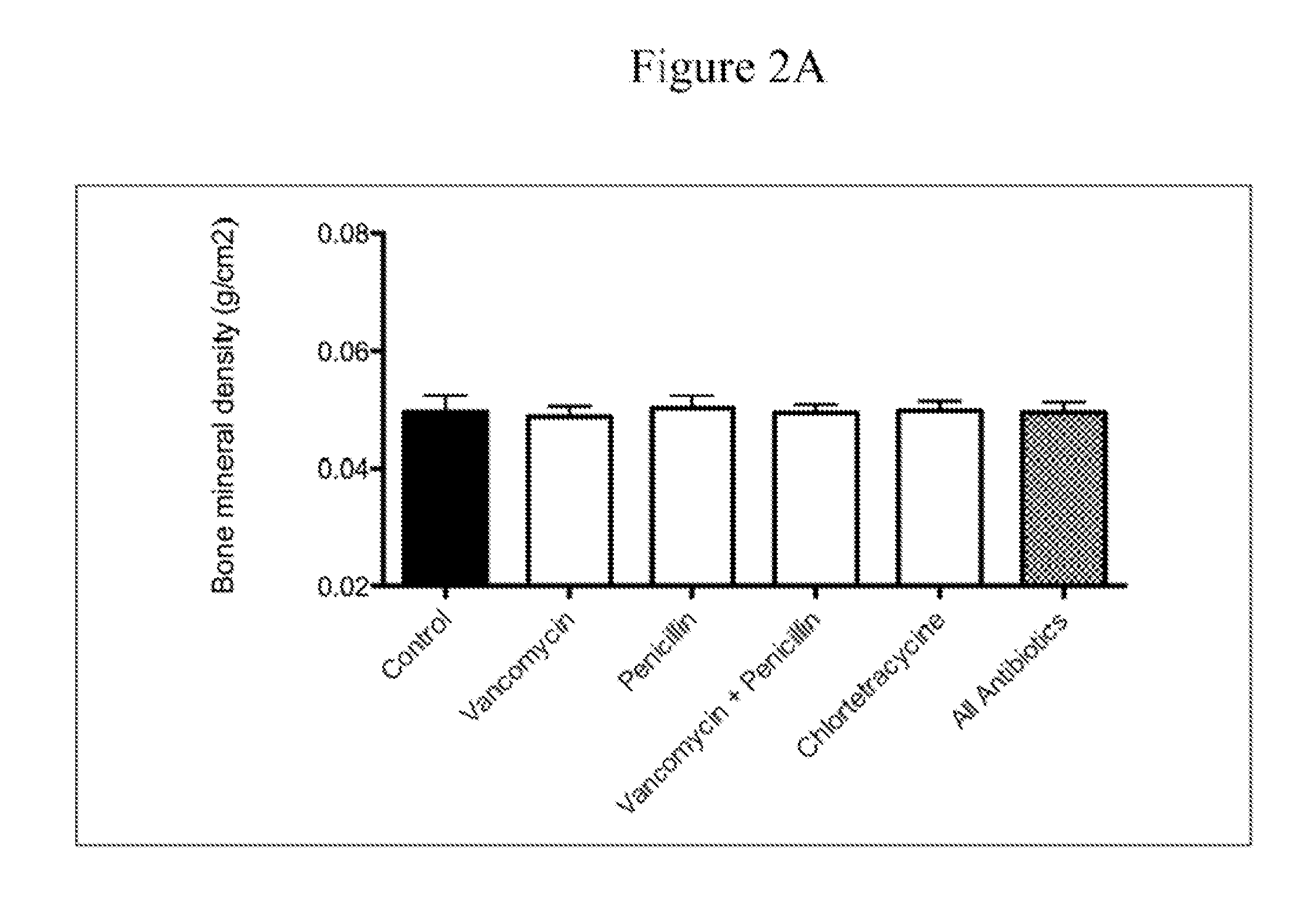
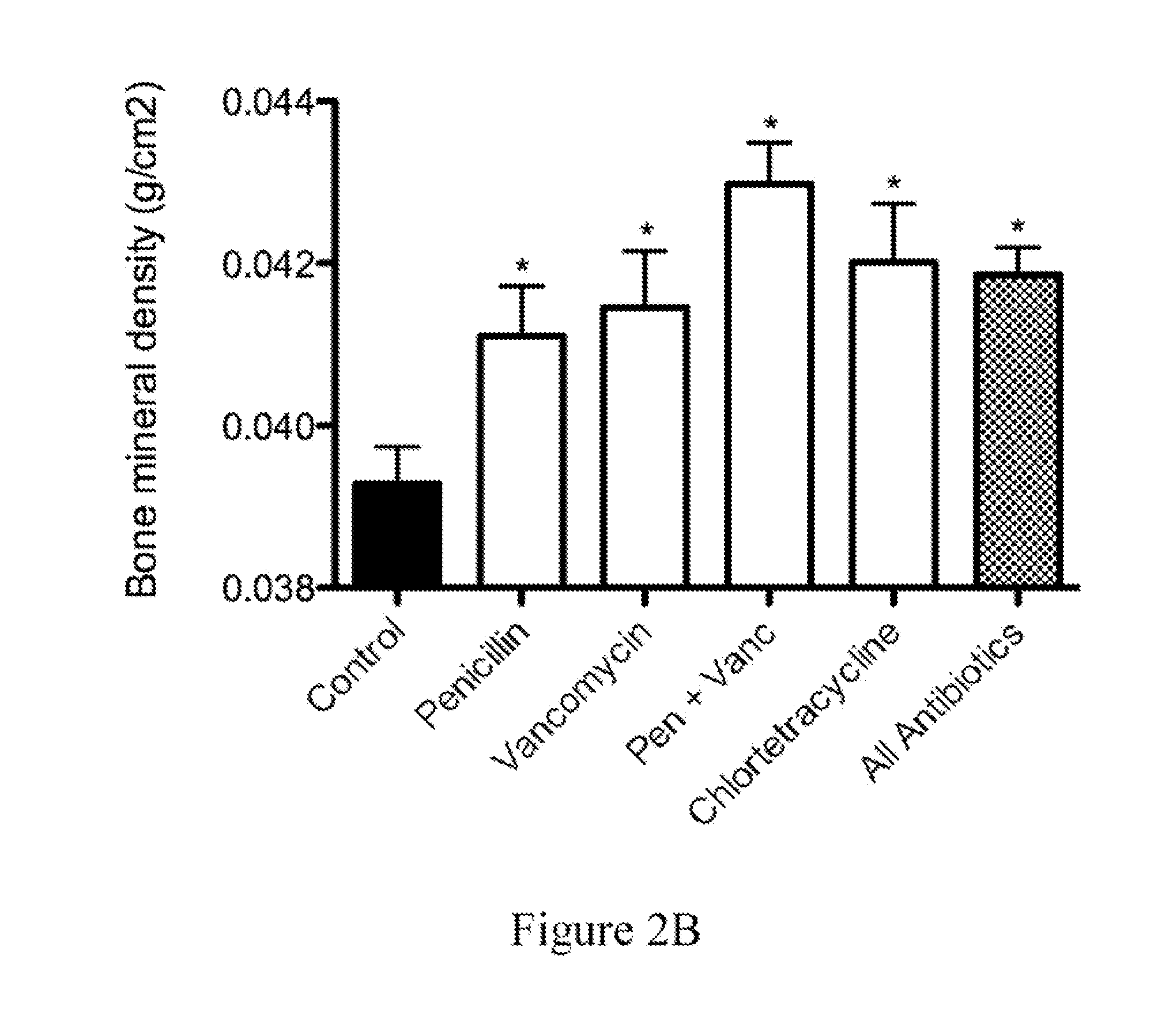
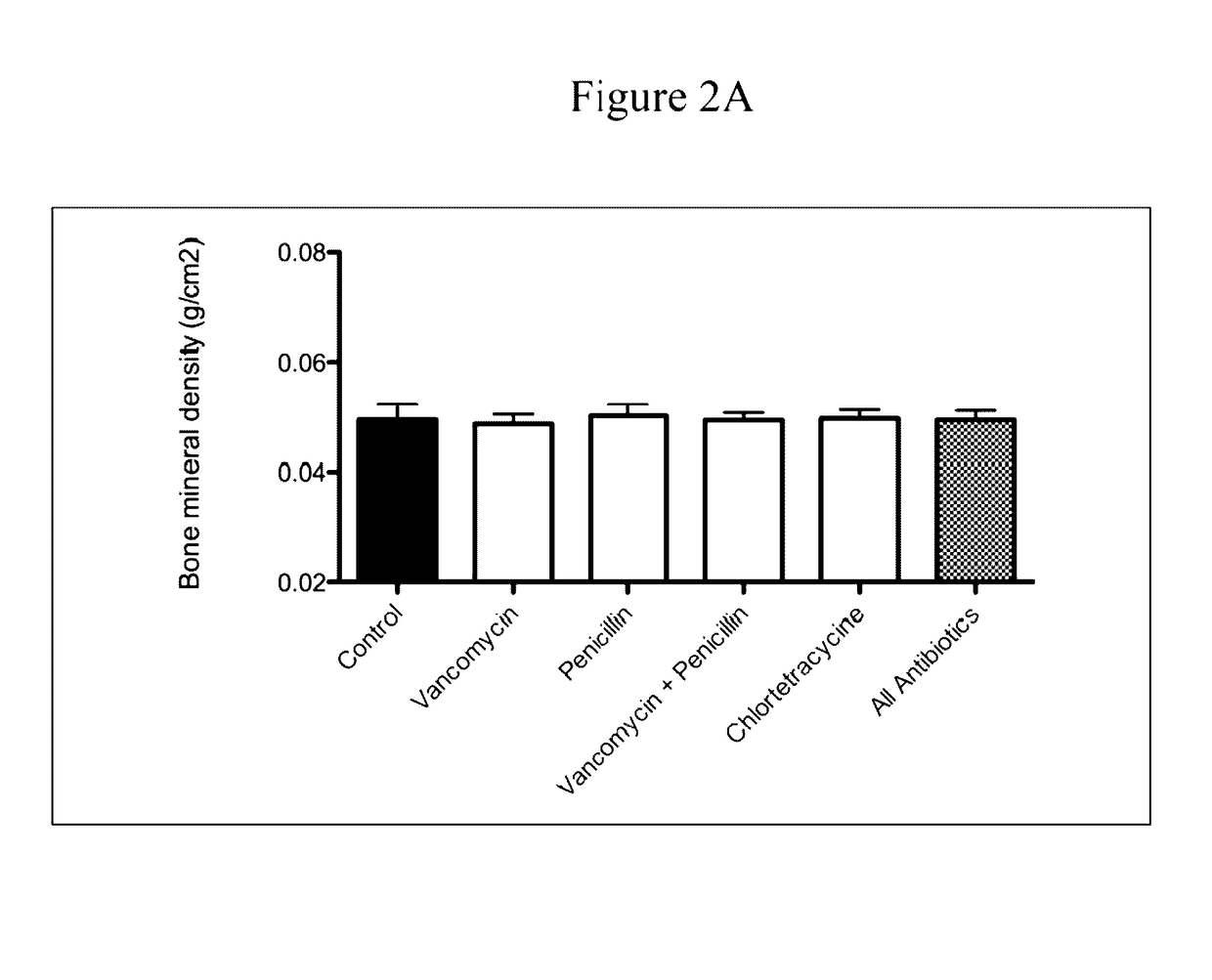
The present invention relates to characterizing changes in mammalian bacterial gastrointestinal, cutaneous and nasal microbiota associated with antibiotic treatment and various disease conditions (such as asthma, allergy, obesity, metabolic syndrome, gastrointestinal reflux disease (GERD), eosinophilic esophagitis, gastro-esophageal junction adenocarcinomas (GEJAC), infections due to bacteria that are resistant to antibiotics, including Methicillin-resistant Staphylococcus aureus (MRSA), Clostridium difficile, vancomycin-resistant enterococci, etc.) and related diagnostic and therapeutic methods. Therapeutic methods of the invention involve the use of live bacterial inoculants that are capable of restoring healthy mammalian bacterial gastrointestinal, skin, and nasal microbiota.
Owner:NEW YORK UNIV
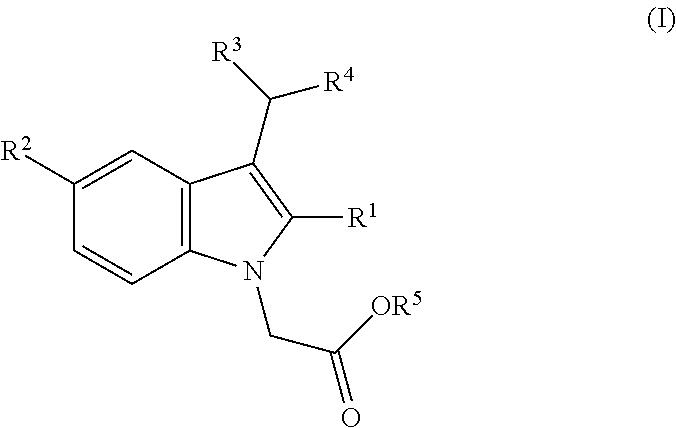
Treating eosinophilic esophagitis
InactiveUS20090264392A1Avoid developmentQuantity minimizationOrganic active ingredientsDigestive systemSteroid CompoundEosinophilic esophagitis
This document provides methods and compositions suitable for treating eosinophilic esophogitis. For example, this document provides methods that involve administering a steroid and mucoadherent to a mammal (e.g., a human). Kits comprising compositions containing a steroid in combination with a mucoadherent also are provided.
Owner:MERITAGE PHARMA INC
Method of treating eosinophilic esophagitis
ActiveUS20120207815A1Reduce penetrationDecrease of the other histological patterns of EEDigestive systemAllergen ingredientsDermatologySkin exposure
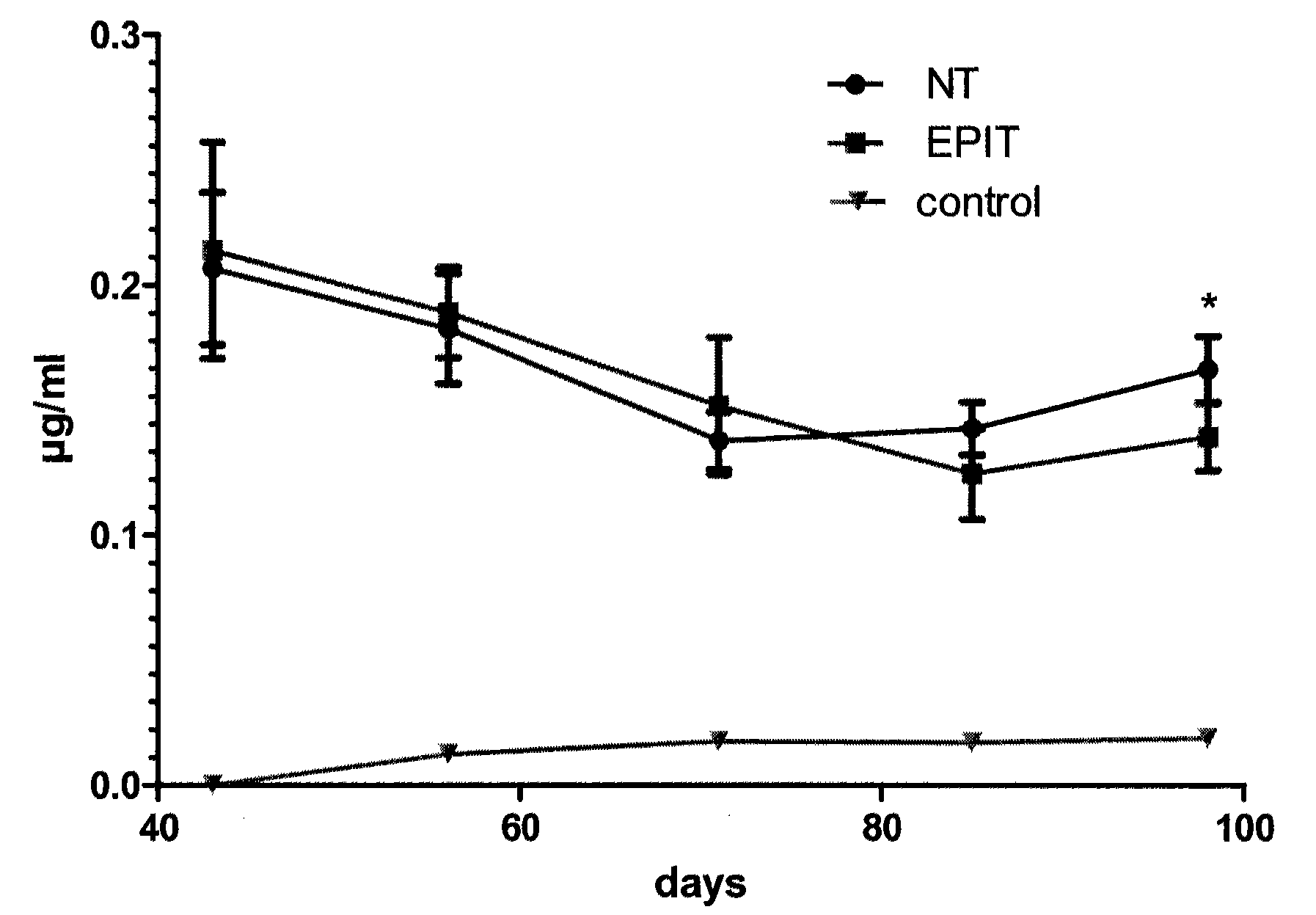
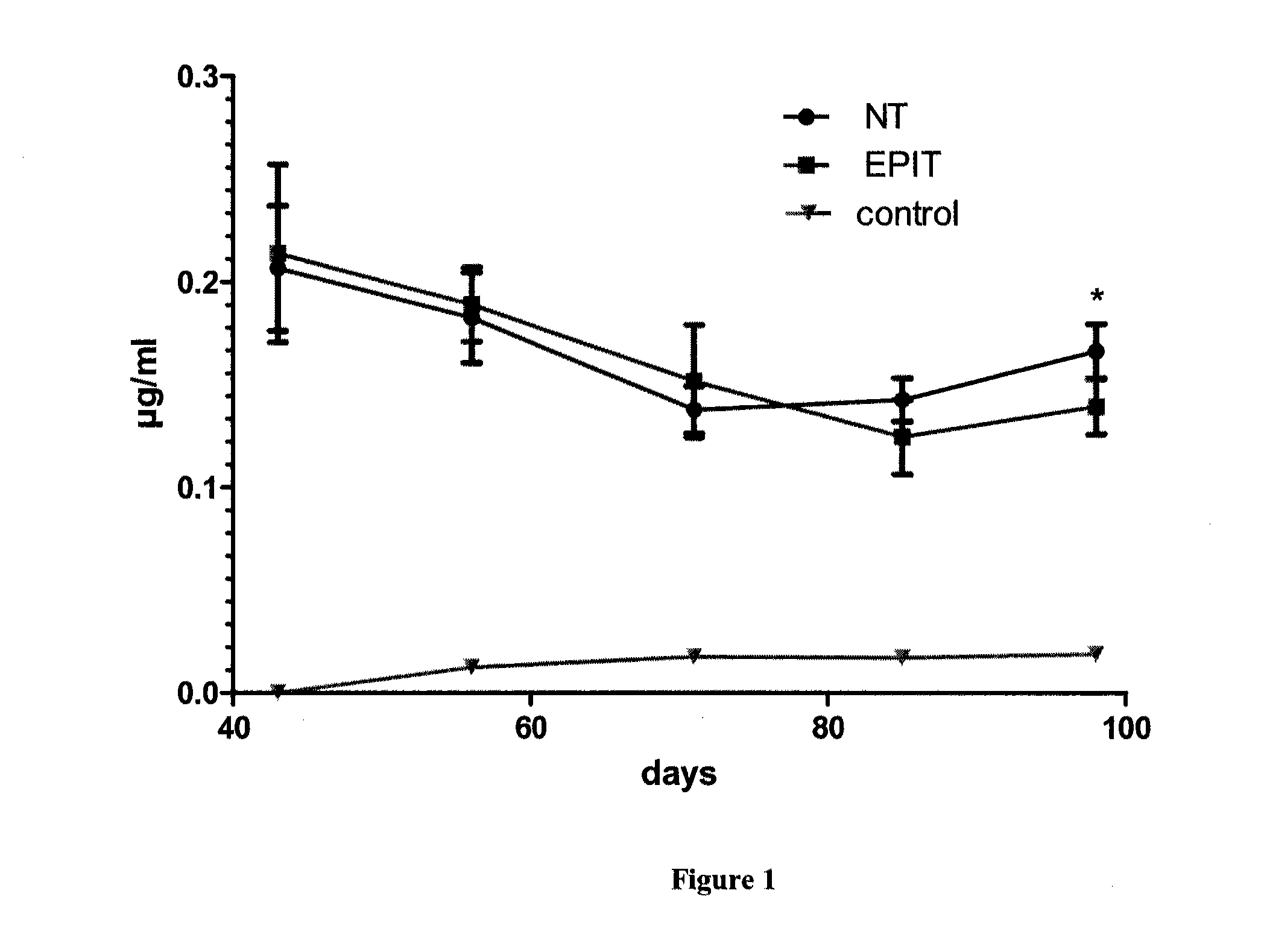
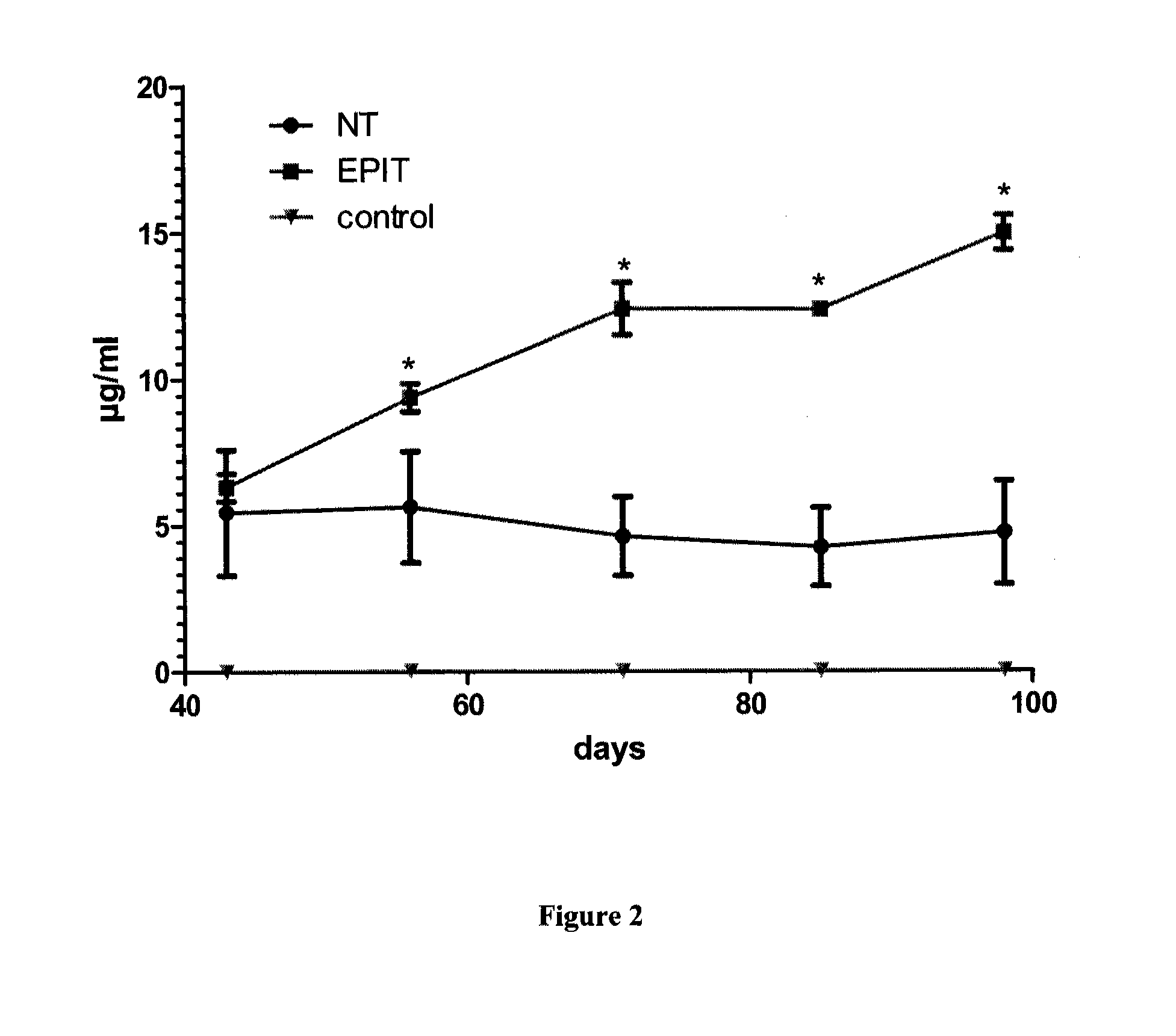
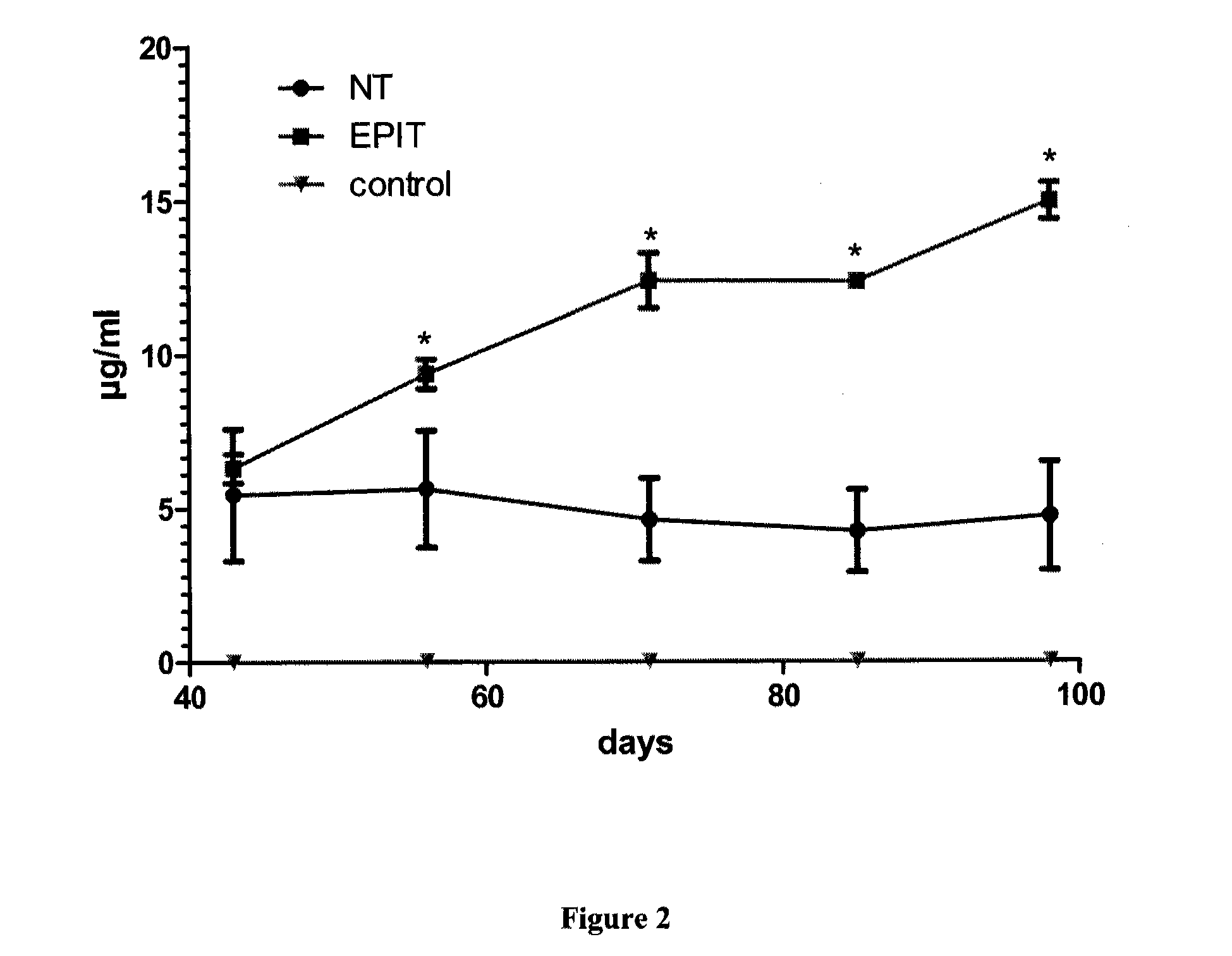
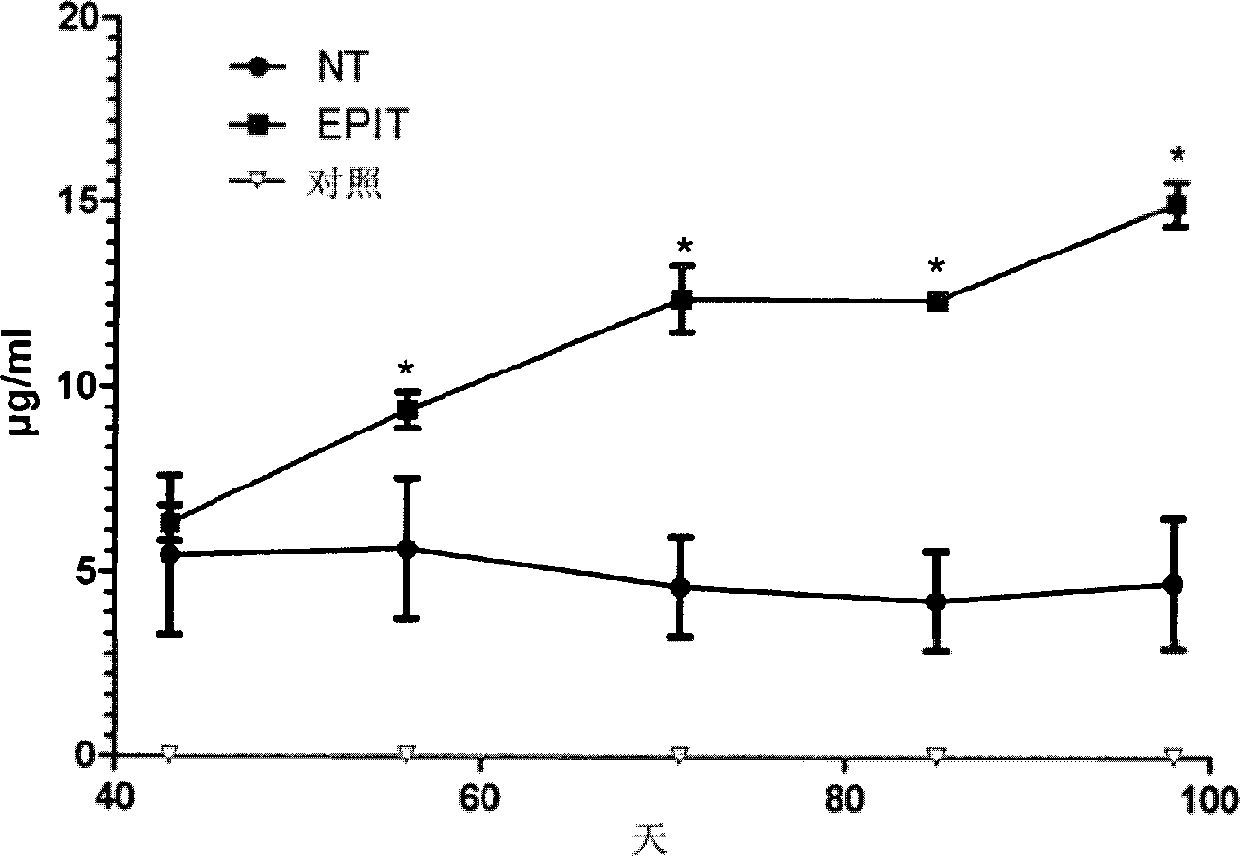
The present invention relates to the treatment of EE. More specifically, the invention relates to a new method of treating EE through the epicutaneous route. In particular, the method of the invention comprises applying to the skin of the subject a skin patch device, comprising a composition, under conditions allowing a contact between said composition and the skin. The present invention also relates to the skin patch device and to a use of the skin patch device in the manufacture of a composition for treating eosinophilic esophagitis in a subject.
Owner:DBV TECH INC
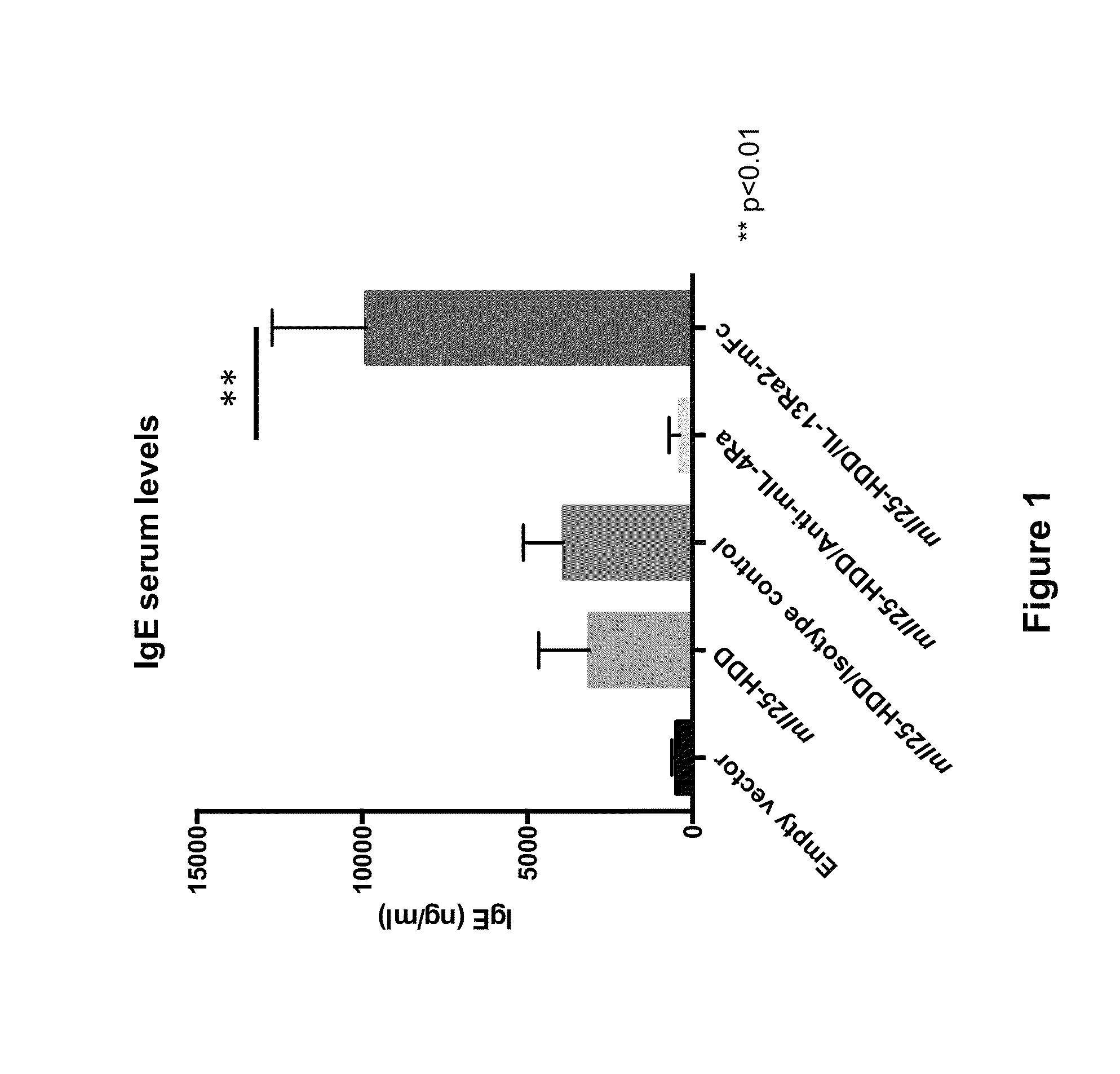
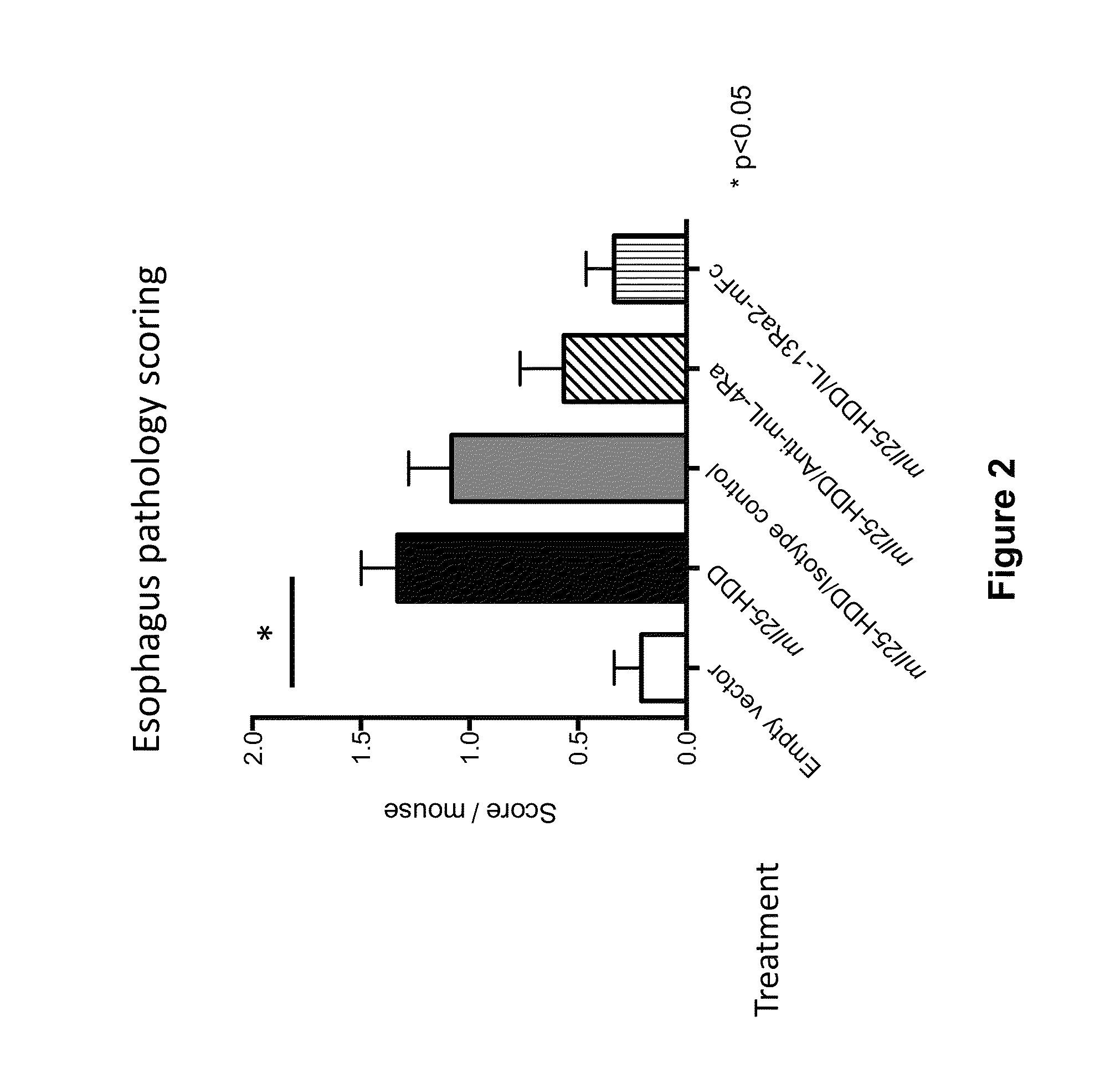
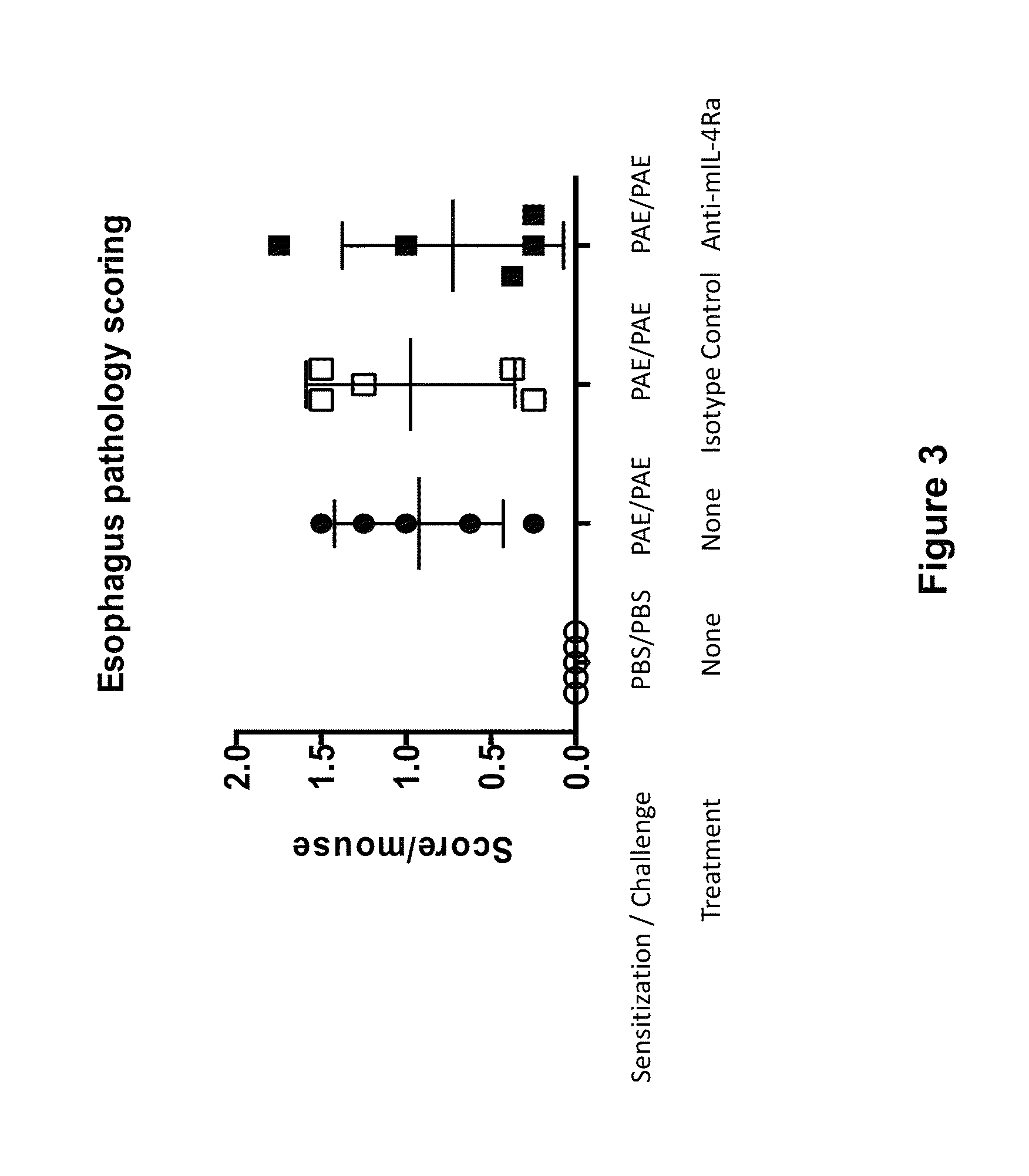
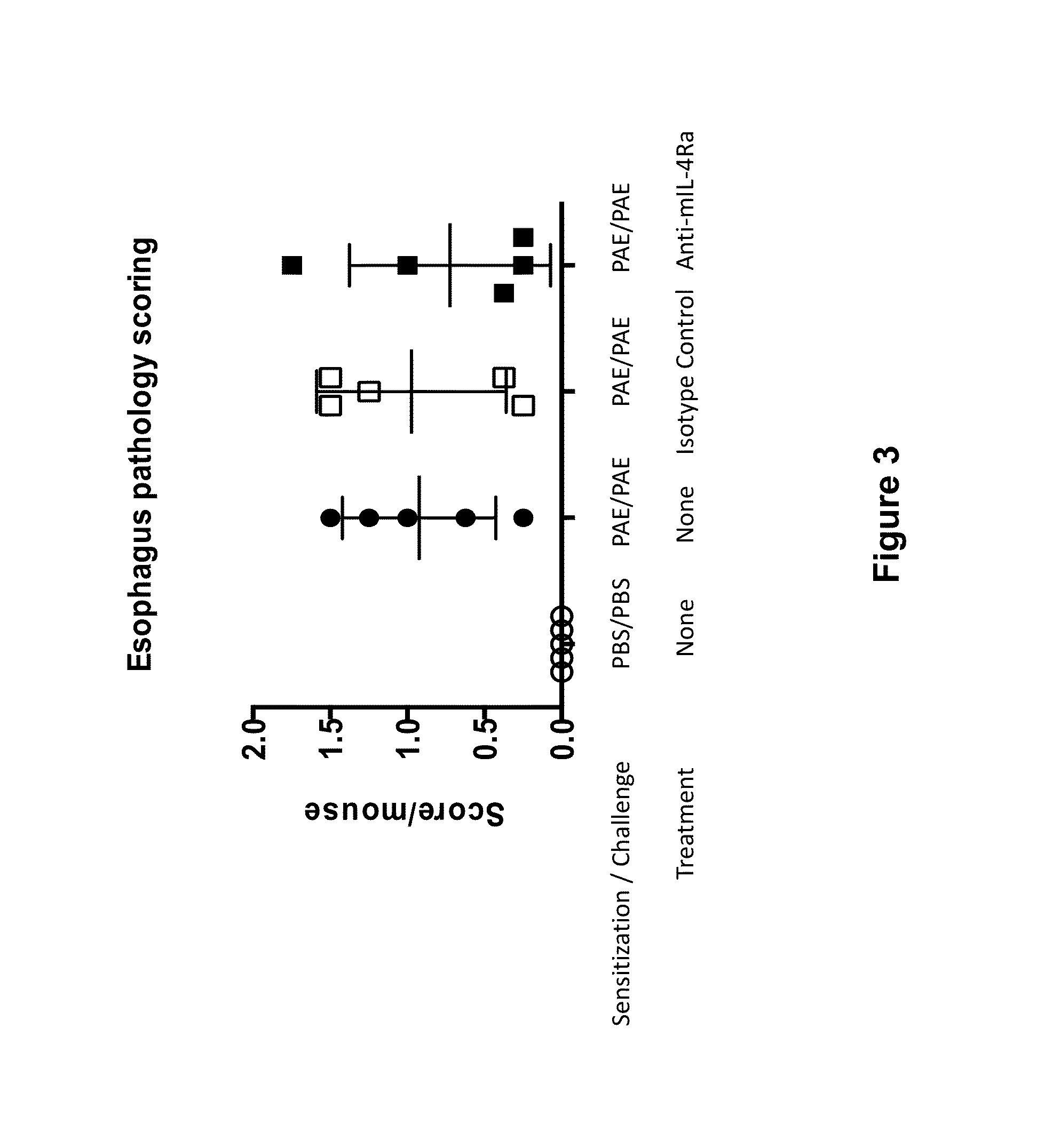
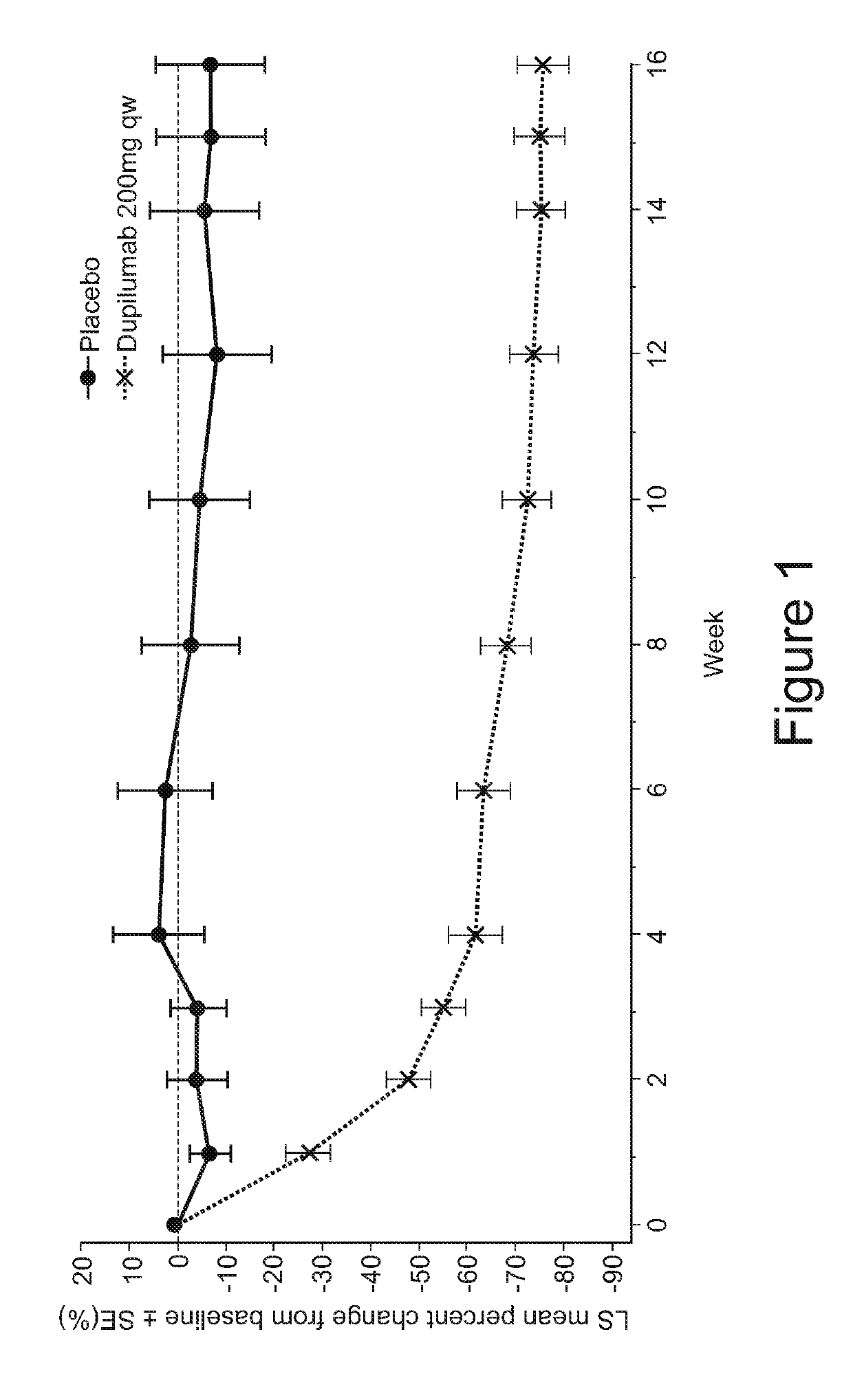
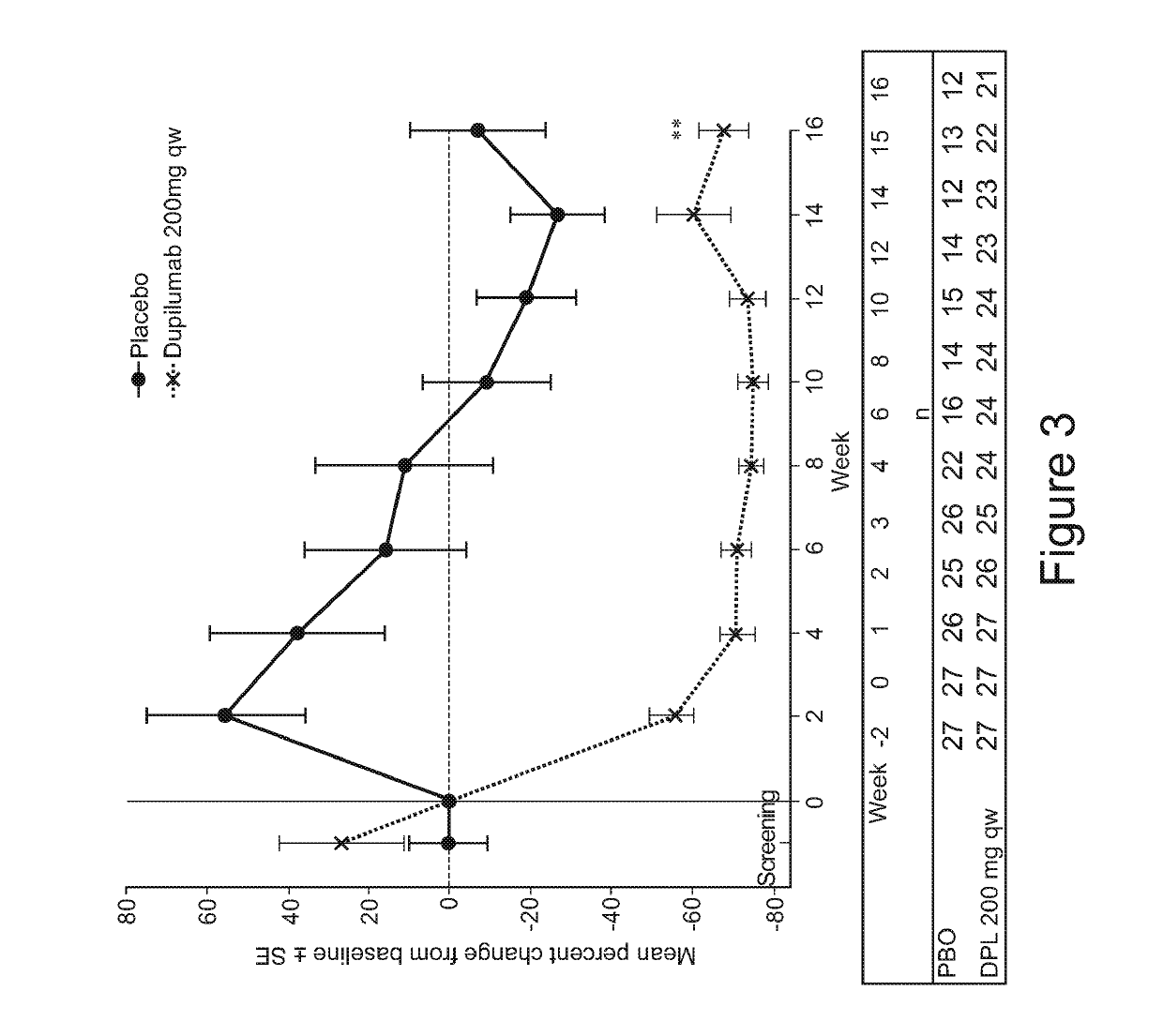
Methods for treating eosinophilic esophagitis by administering an IL-4R inhibitor
The present invention provides methods for treating, preventing or reducing the severity of eosinophilic esophagitis. The methods of the present invention comprise administering to a subject in need thereof a therapeutic composition comprising an interleukin-4 receptor (IL-4Rα) inhibitor such as an anti-IL-4Rα antibody.
Owner:REGENERON PHARM INC
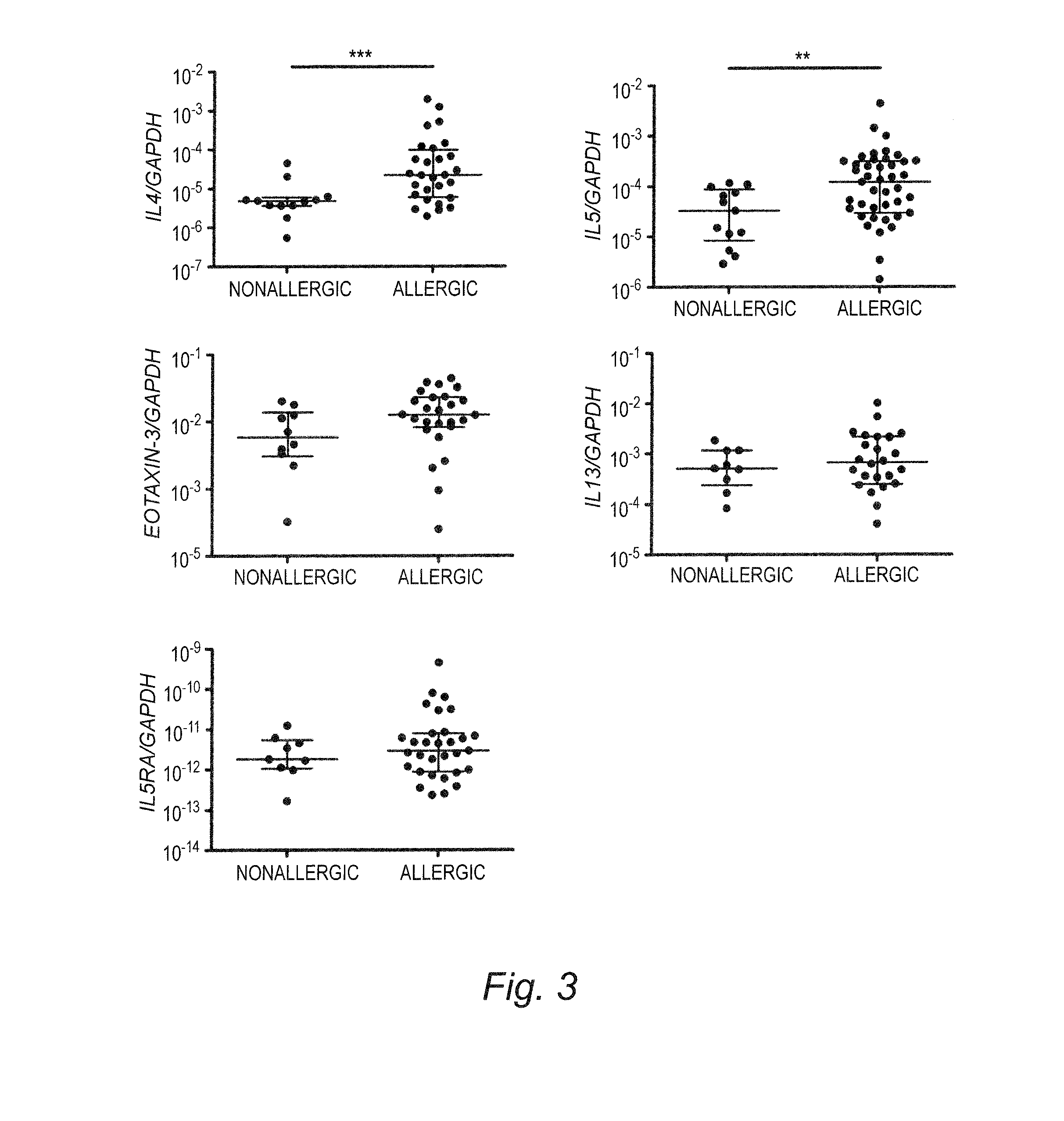
Esophageal cytokine expression profiles in eosinophilic esophagitis
InactiveUS20130324435A1Easy diagnosisNucleotide librariesMicrobiological testing/measurementFactor iiCytokine Expression Profile
Methods and compositions disclosed herein generally relate to methods of providing or enhancing a diagnosis of eosinophilic esophagitis (EE). In particular, the invention relates to obtaining a sample from a patient having at least one indication of EE, then quantifying from the sample an amount of one or more cytokines associated with EE or an mRNA corresponding to the cytokine or its receptor, wherein an altered level of the cytokine or mRNA correlates with a positive diagnosis of EE. An EE diagnosis can then be provided or enhanced, based upon the quantifying step. The invention further relates to diagnostic kits, tests, and / or arrays that can be used to quantify the one or more cytokines associated with EE or an mRNA corresponding to the cytokine or its receptor.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Methods for treating eosinophilic esophagitis by administering an IL-4R inhibitor
The present invention provides methods for treating, preventing or reducing the severity of eosinophilic esophagitis. The methods of the present invention comprise administering to a subject in need thereof a therapeutic composition comprising an interleukin-4 receptor (IL-4Rα) inhibitor such as an anti-IL-4Rα antibody.
Owner:REGENERON PHARM INC
Compositions and methods for restoring gastrointestinal microbiota following antibiotic treatment
ActiveUS9603876B2Increase the number ofGrowth inhibitionBiocideMetabolism disorderDiseaseIntestinal microorganisms
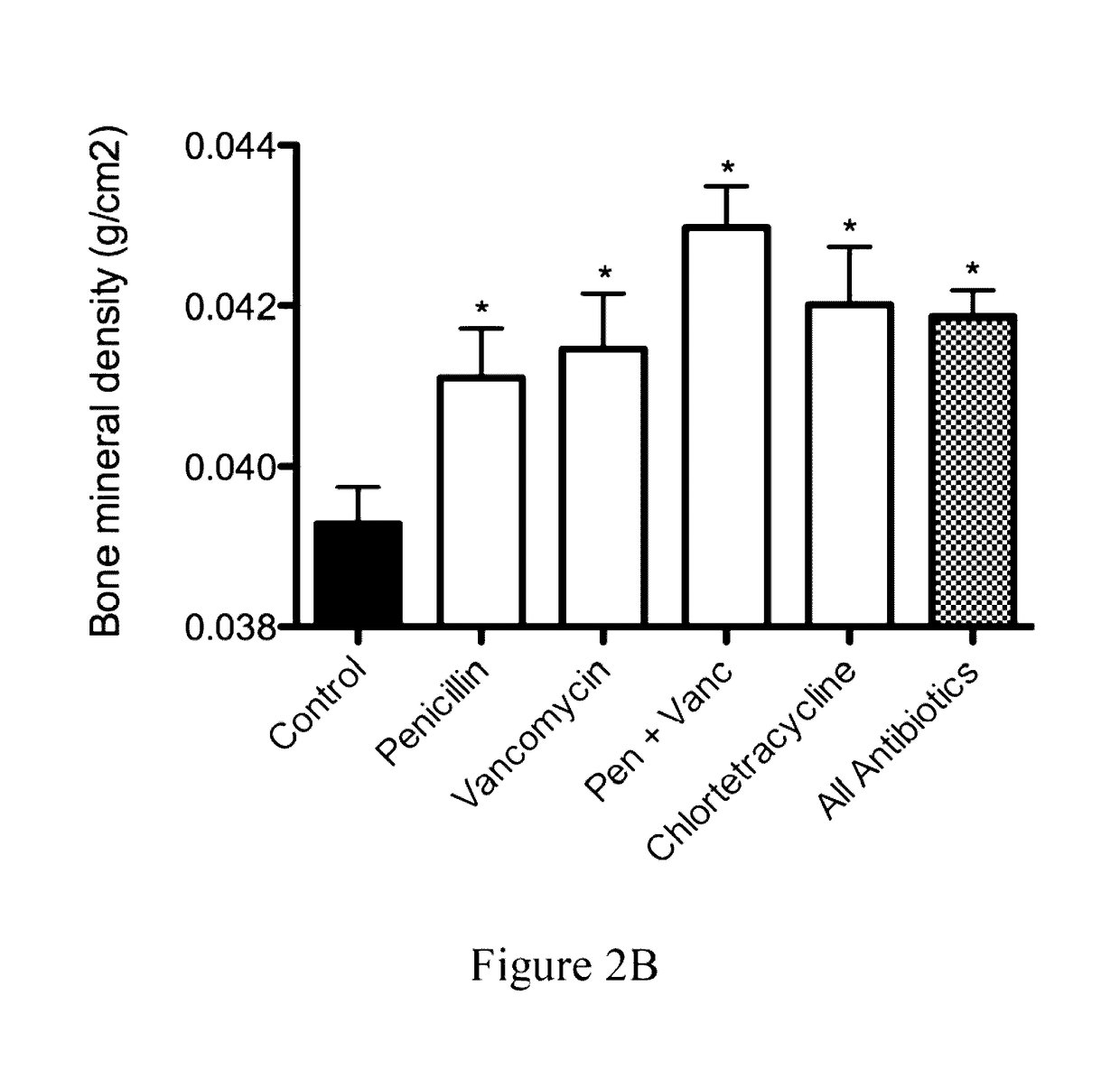
The present invention relates to characterizing changes in mammalian bacterial gastrointestinal, cutaneous and nasal microbiota associated with antibiotic treatment and various disease conditions (such as asthma, allergy, obesity, metabolic syndrome, gastrointestinal reflux disease (GERD), eosinophilic esophagitis, gastro-esophageal junction adenocarcinomas (GEJAC), infections due to bacteria that are resistant to antibiotics, including Methicillin-resistant Staphylococcus aureus (MRSA), Clostridium difficile, vancomycin-resistant enterococci, etc.) and related diagnostic and therapeutic methods. Therapeutic methods of the invention involve the use of live bacterial inoculants that are capable of restoring healthy mammalian bacterial gastrointestinal, skin, and nasal microbiota.
Owner:NEW YORK UNIV
Methods of treating eosinophilic esophagitis
ActiveUS20180133145A1Raise countNo worseningOrganic active ingredientsAntipyreticGastrointestinal inflammationUpper gastrointestinal
The present disclosure provides methods of treating inflammation of the upper gastrointestinal tract, especially the esophagus, by administering an oral corticosteroid. In some cases, the methods include treating eosinophilic esophagitis (EoE) by administering an oral corticosteroid in an induction phase and a maintenance phase to improve peak eosinophilic counts and symptoms. In embodiments, the methods include treating EoE by administering the oral corticosteroid at nighttime and / or while the patient is lying down.
Owner:ELLODI PHARM LP
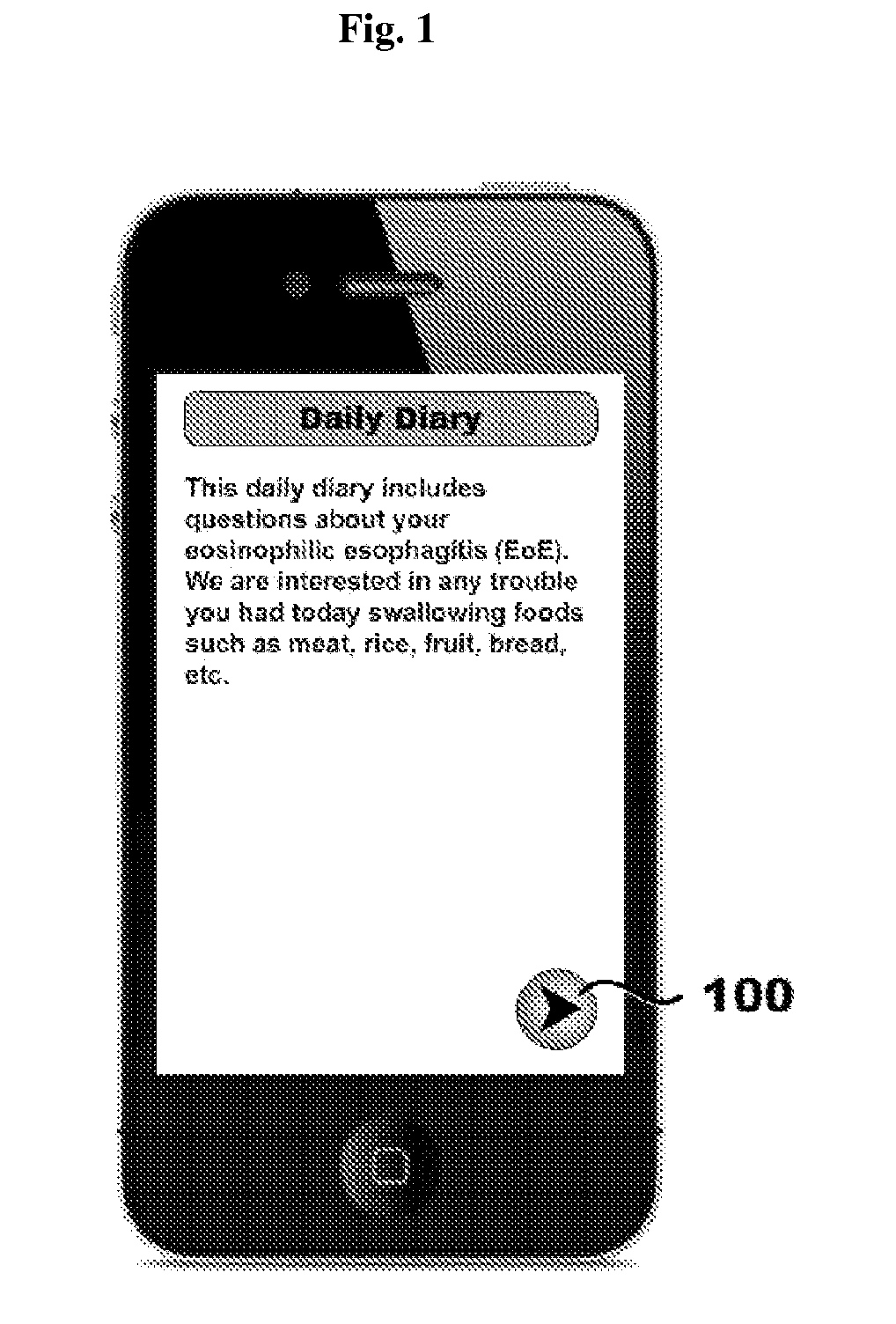
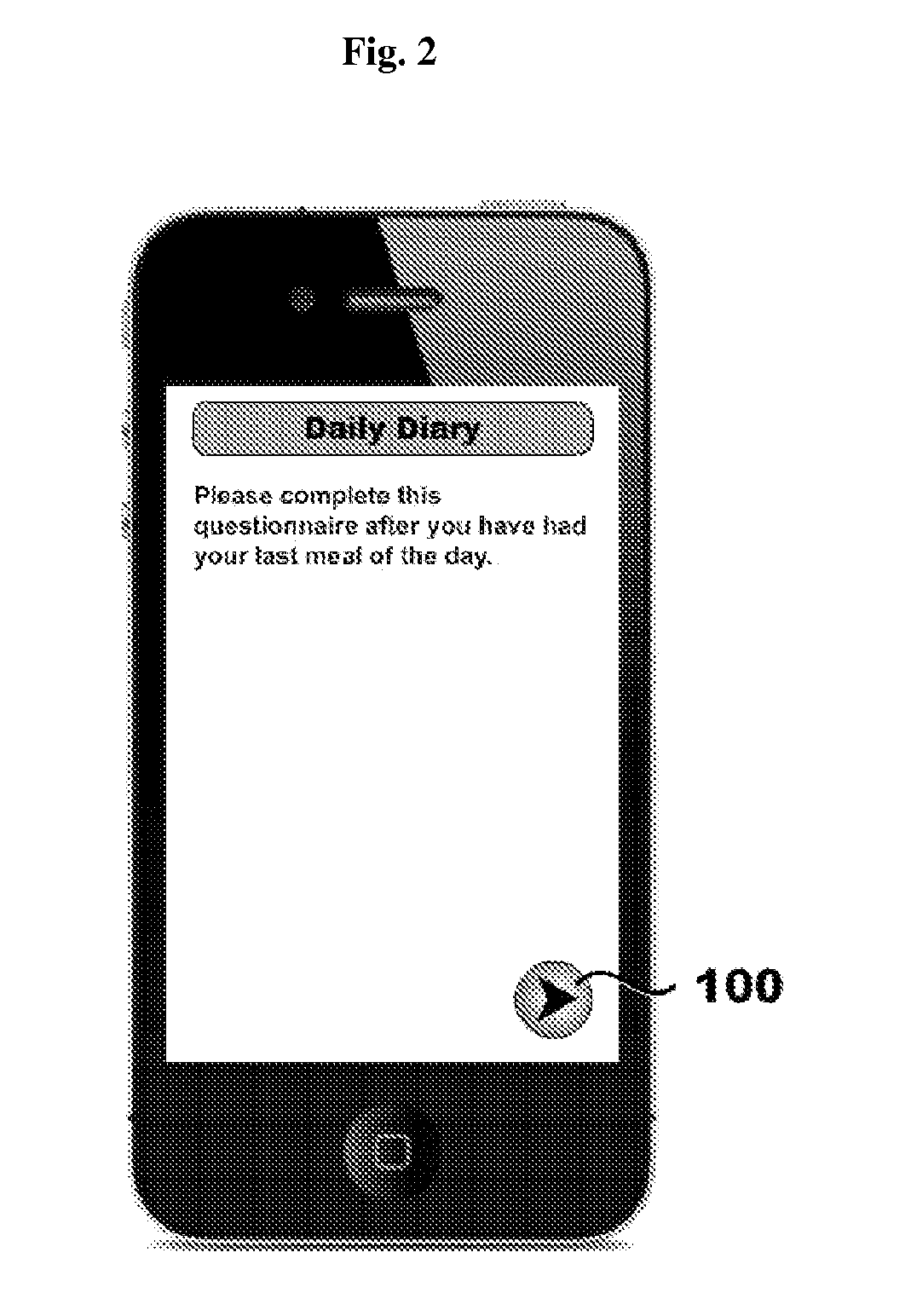
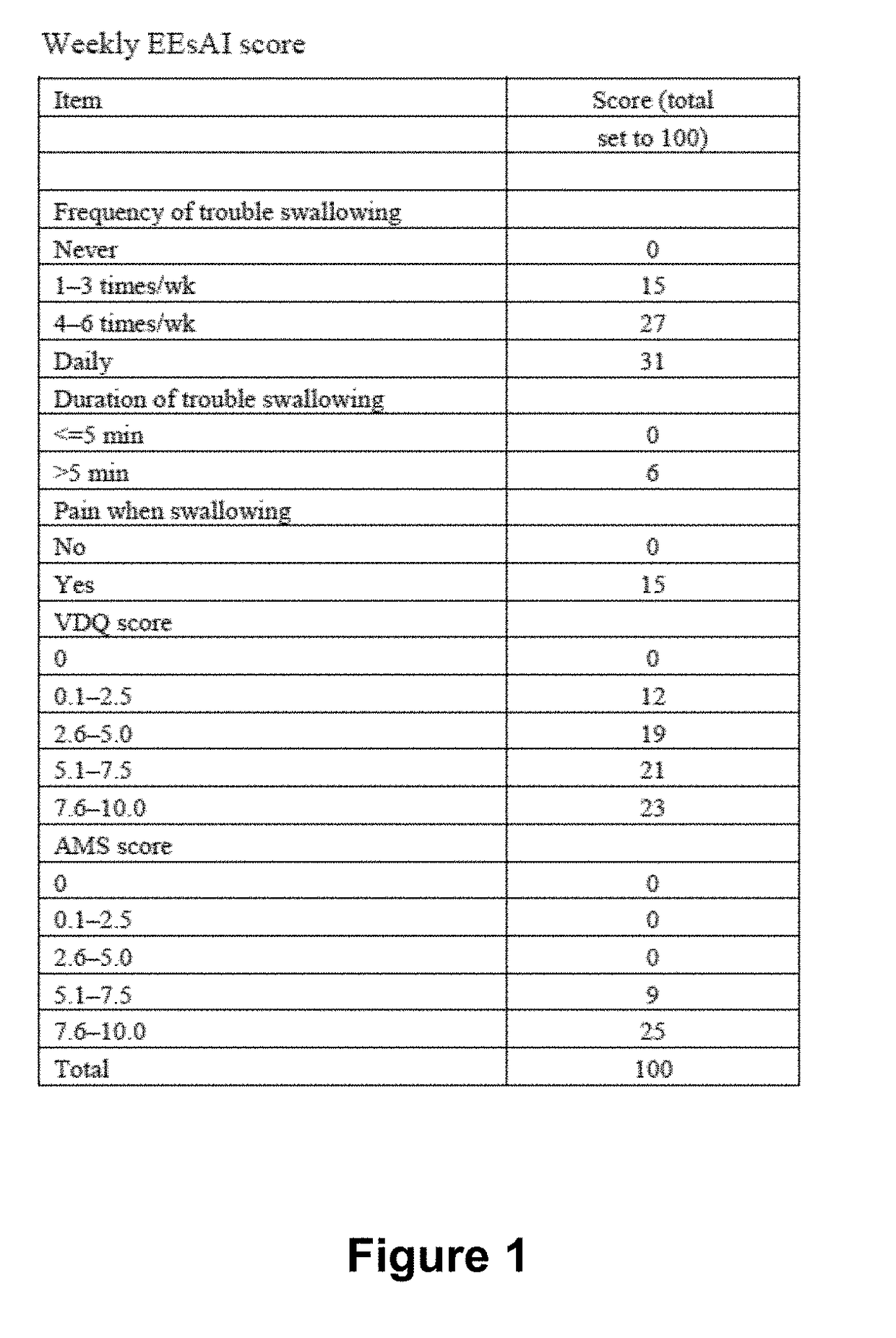
Systems, methods, and software for providing a patient-reported outcome measure of dysphagia patients with eosinophilic esophagitis
ActiveUS20160078186A1Reduction in esophageal inflammationData processing applicationsComputer-assisted medical data acquisitionPatient questionnaireSwallow food
Provided herein are computer-based systems, software, and methods of using the same including a daily patient questionnaire, the questionnaire comprising: a question for determining whether the patient avoided solid food; a question for determining whether the patient had difficulty swallowing solid food; a question for determining what action the patient took to correct or relieve difficulty swallowing food; a question for determining the amount of pain the patient experienced while swallowing food; and a software module configured to apply an algorithm to answers to one or more of said questions to determine a score, wherein said score illustrates one or more selected from the group consisting of: (1) severity, intensity, or frequency of patient dysphagia; (2) suitability of a patient for a particular diagnostic tool, diagnostic method, or therapy for dysphagia; and (3) efficacy of a particular therapy for dysphagia.
Owner:VIROPHARMA HLDG
Corticosteroid containing orally disintegrating tablet compositions for eosinophilic esophagitis
ActiveUS20160206627A1High blend uniformity/homogeneityOrganic active ingredientsAntipyreticGastrointestinal inflammationOral medication
The present invention is directed to orally administered compositions of topically acting corticosteroids for the treatment of inflammation of the gastrointestinal tracts such as eosinophilic esophagitis. The present invention also provides a method for treating conditions associated with inflammation of the gastrointestinal tract in an individual. The method comprises administering to an individual in need thereof a phermaceutical compossition of the present invention as orally disintegrating tablets comprising a topically active corticosteroid adsorbed onto a pharmaceutically acceptable carrier such as silicified microcrystalline cellulose.
Owner:ADARE PHARMA US LP
Methods for preventing or treating allergy by administering an il-4r antagonist
ActiveUS20190183973A1Treat and reduce and prevent allergy and allergen sensitizationPeptide/protein ingredientsPharmaceutical delivery mechanismDiseaseAtopic dermatitis
The present invention provides methods for preventing or treating allergy. Also provided are methods for reducing the susceptibility to an allergen in a subject in need thereof. In certain embodiments, the subject has a disease or disorder selected from the group consisting of atopic dermatitis, asthma, allergic rhinitis, and eosinophilic esophagitis. The methods of the present invention comprise administering to a subject in need thereof a pharmaceutical composition comprising an interleukin-4 receptor (IL-4R) antagonist such as an anti-IL-4R antibody.
Owner:REGENERON PHARM INC +1
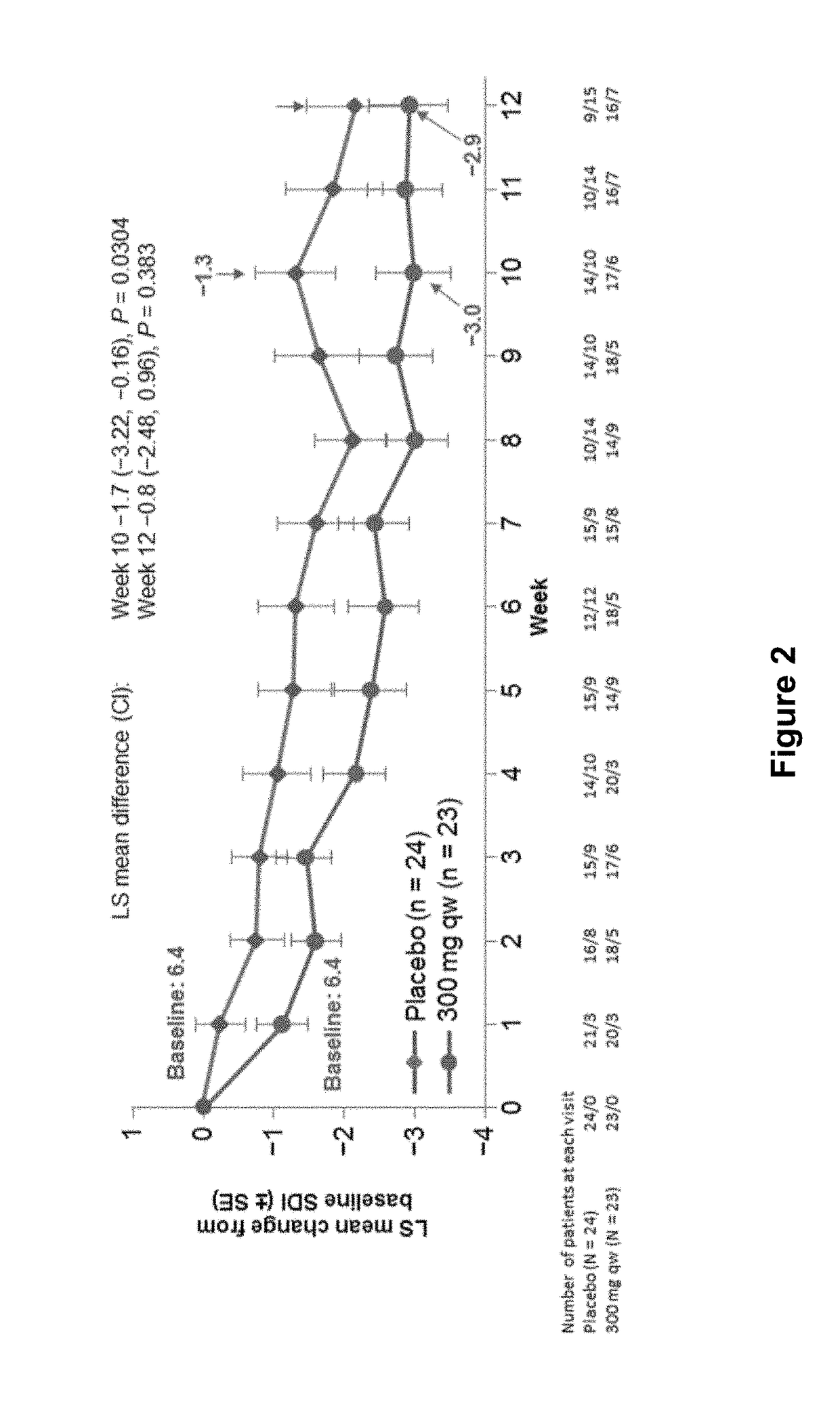
Methods for treating active eosinophilic esophagitis
ActiveUS20190040126A1Improve acceleration performanceImprovement of biomarker levelHybrid immunoglobulinsDigestive systemWhite blood cellInterleukin 13
The present invention provides methods for treating, preventing or reducing the severity of active eosinophilic esophagitis. In certain embodiments, the present invention provides methods of increasing esophageal distensibility. The methods of the present invention comprise administering to a subject in need thereof a therapeutic composition comprising an interleukin-4 / interleukin-13 (IL-4 / IL-13) pathway inhibitor such as an anti-IL-4R antibody.
Owner:REGENERON PHARM INC +1
Protein free formula
ActiveUS20100172876A1Avoid developmentReduce severityBiocidePeptide/protein ingredientsIntact proteinFeces
The invention relates to the use of composition comprising free amino acids as a sole source of protein, a fatty acid source comprising long chain polyunsaturated fatty acids, a carbohydrate source comprising digestible and indigestible carbohydrates, and milk protein free Bifidobacteria for treating a person suffering from (a) colic, congestion, runny nose, wheezing, vomiting, diarrhoea, bloody stools, mucus in stools, rash, eczema, gastroesophageal reflux, eosinophilic esophagitis or asthma; (b) cow's milk allergy and / or food protein intolerance; and / or (c) infections, wherein the indigestible carbohydrate is selected from a milk protein free source and the total composition is essentially free of intact proteins.
Owner:SHS INTERNATIONAL
Optimized pharmaceutical formulation for the treatment of inflammatory conditions of the esophagus
ActiveUS9867780B2Long retention timeWidely distributedOrganic active ingredientsAntipyreticEffervescent tabletHigh rate
Disclosed is an optimized pharmaceutical formulation for the treatment of inflammatory conditions of the esophagus. A pharmaceutical formulation in the form of an orodispersible effervescent tablet is stable, easy to produce, and can be used without dissolving same in a liquid. It is not necessary to drink anything with the tablet as this would reduce the time that the budesonide solution remains in the affected regions of the esophagus. The effervescent tablet of the invention surprisingly resulted in an unexpectedly high rate of histological remission in patients with active eosinophilic esophagitis.
Owner:DR FALK PHARMA GMBH
Protein free formula
ActiveUS8691213B2Avoid developmentReduce severityBiocidePeptide/protein ingredientsSerum rashIntact protein
The invention relates to the use of composition comprising free amino acids as a sole source of protein, a fatty acid source comprising long chain polyunsaturated fatty acids, a carbohydrate source comprising digestible and indigestible carbohydrates, and milk protein free Bifidobacteria for treating a person suffering from (a) colic, congestion, runny nose, wheezing, vomiting, diarrhea, bloody stools, mucus in stools, rash, eczema, gastroesophageal reflux, eosinophilic esophagitis or asthma; (b) cow's milk allergy and / or food protein intolerance; and / or (c) infections, wherein the indigestible carbohydrate is selected from a milk protein free source and the total composition is essentially free of intact proteins.
Owner:SHS INTERNATIONAL
Methods of treating eosinophilic esophagitis
ActiveUS10105315B2Raise countNo worseningOrganic active ingredientsAntipyreticGastrointestinal inflammationUpper gastrointestinal
The present disclosure provides methods of treating inflammation of the upper gastrointestinal tract, especially the esophagus, by administering an oral corticosteroid. In some cases, the methods include treating eosinophilic esophagitis (EoE) by administering an oral corticosteroid in an induction phase and a maintenance phase to improve peak eosinophilic counts and symptoms. In embodiments, the methods include treating EoE by administering the oral corticosteroid at nighttime and / or while the patient is lying down.
Owner:ELLODI PHARM LP
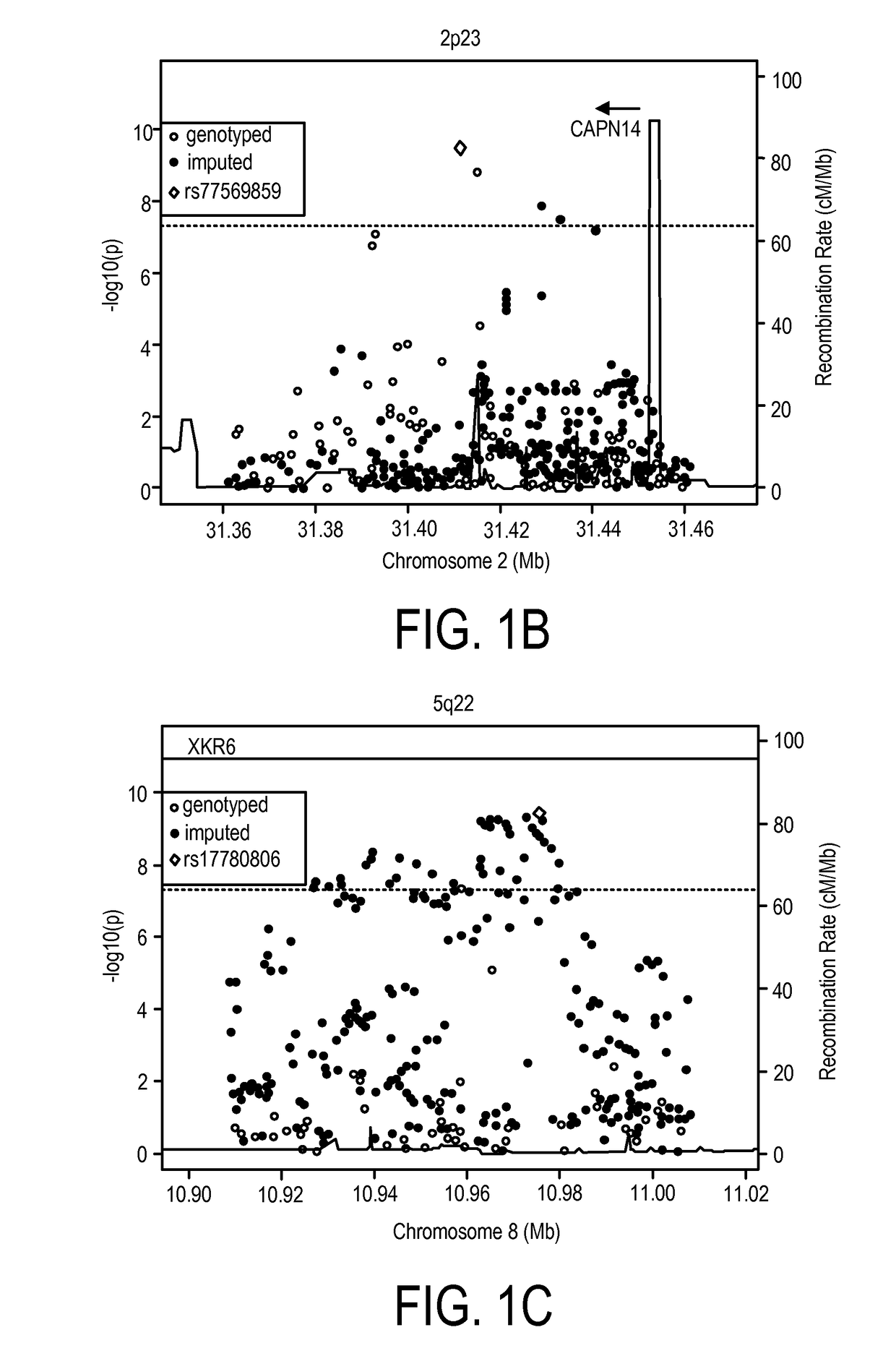
Genetic Test for Determining Susceptibility for Eosinophilic Esophagitis
Methods and compositions disclosed herein generally relate to determination of susceptibility to eosinophilic esophagitis, asthma, and / or allergic diseases, disorders, and / or pulmonary and / or upper gastrointestinal conditions arising therefrom and / or related thereto and the diagnosis, treatment, and / or management of eosinophilic esophagitis, asthma, and / or allergic diseases, disorders, and / or pulmonary and / or upper gastrointestinal conditions arising therefrom and / or related thereto. Embodiments of the invention relate to the association between genes and specific polymorphisms of genes with eosinophilic esophagitis. Embodiments of the invention can be used to determine and manage patient risk factors for development of eosinophilic esophagitis; this determination can then be used to diagnose eosinophilic esophagitis and to treat a patient diagnosed with eosinophilic esophagitis.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
Molecular diagnostic panel of eosinophilic gastrointestinal disorders
ActiveUS20140286896A1Organic active ingredientsAntibody mimetics/scaffoldsIntestinal tract diseasesDisease
Embodiments of the invention are directed to methods of diagnosing eosinophilic gastritis (EG), or remission therefrom in a subject, wherein the methods include applying a sample from the subject to a diagnostic panel that contains selected markers for EG, analyzing the results thereof, and making a determination as to the EG status of the subject. Embodiments of the invention are also directed to methods of monitoring the pathological development or medical prognosis of EG in a subject. Embodiments of the invention are also directed to use of CDH26 as a marker for EG, eosinophilic esophagitis, or allergic inflammatory conditions. Embodiments of the invention also relate to the use of anti-CDH26-based therapeutics to treat allergic inflammatory conditions.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
Systems, methods, and software for providing a patient-reported outcome measure of dysphagia patients with eosinophilic esophagitis
ActiveUS10176301B2Medical automated diagnosisSpecial data processing applicationsPatient questionnaireSwallow food
Provided herein are computer-based systems, software, and methods of using the same including a daily patient questionnaire, the questionnaire comprising: a question for determining whether the patient avoided solid food; a question for determining whether the patient had difficulty swallowing solid food; a question for determining what action the patient took to correct or relieve difficulty swallowing food; a question for determining the amount of pain the patient experienced while swallowing food; and a software module configured to apply an algorithm to answers to one or more of said questions to determine a score, wherein said score illustrates one or more selected from the group consisting of: (1) severity, intensity, or frequency of patient dysphagia; (2) suitability of a patient for a particular diagnostic tool, diagnostic method, or therapy for dysphagia; and (3) efficacy of a particular therapy for dysphagia.
Owner:VIROPHARMA HLDG
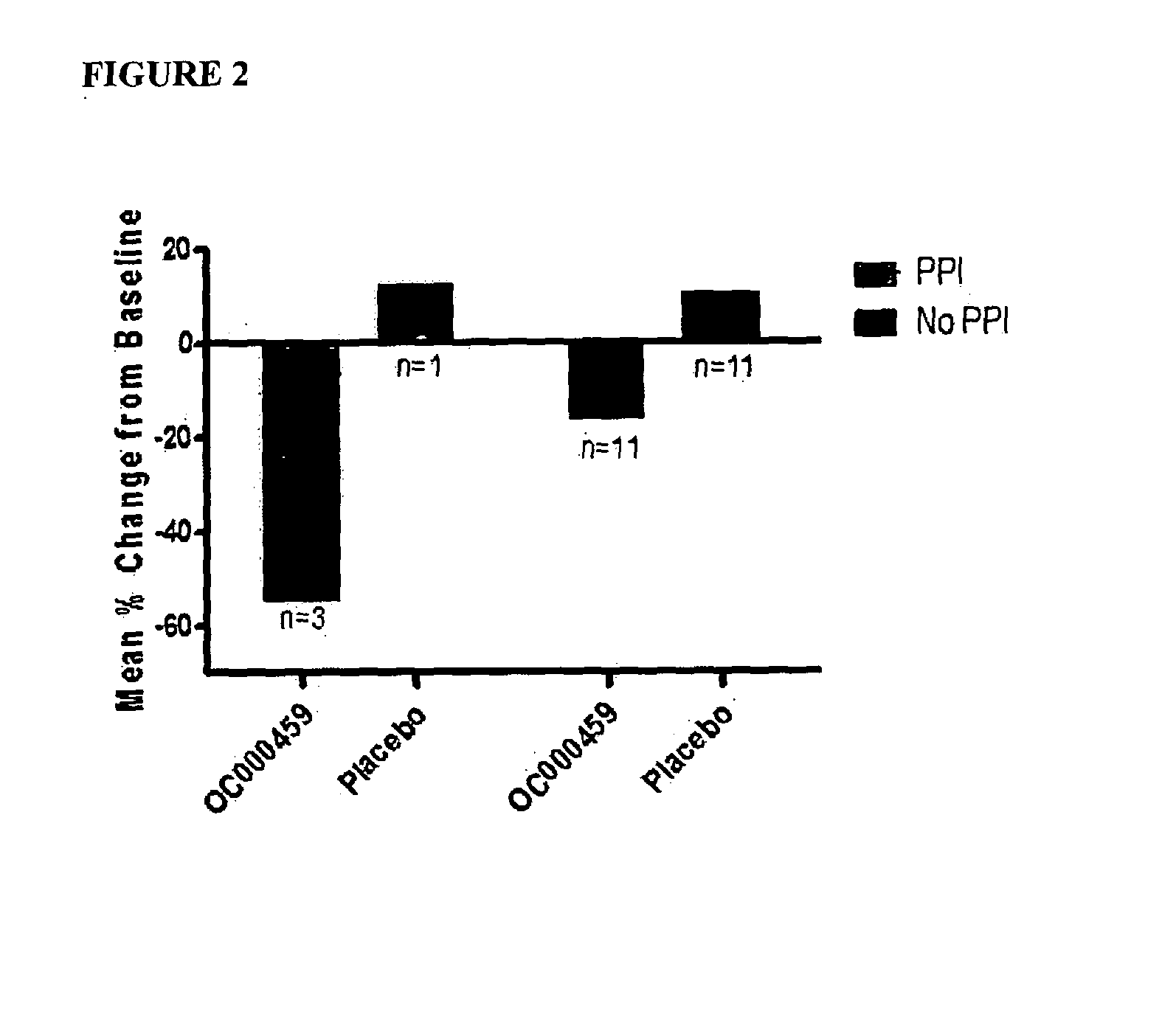
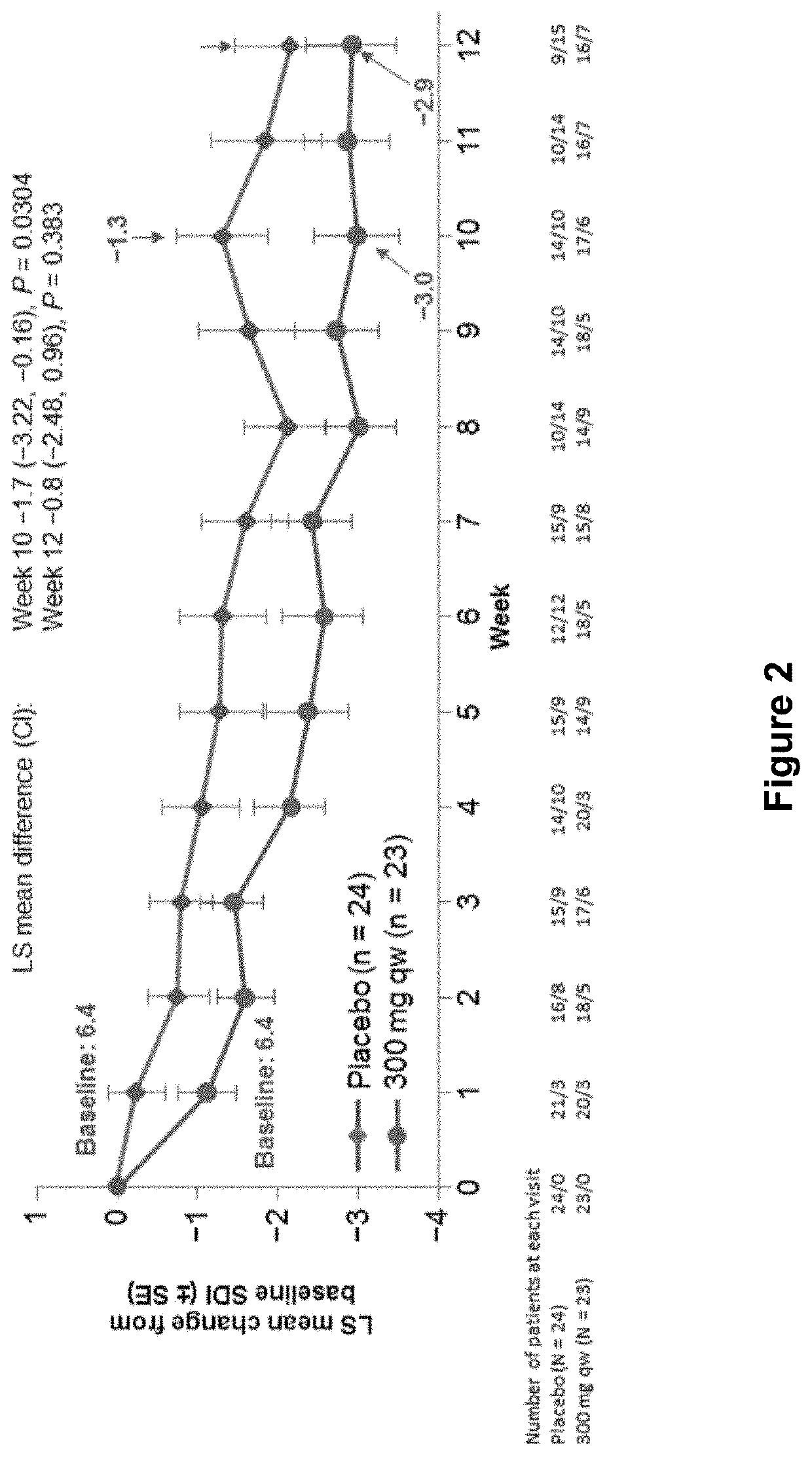
Combination of CRTH2 Antagonist and a Proton Pump Inhibitor for the Treatment of Eosinophilic Esophagitis
Disclosed are methods and compositions for preventing, treating, or ameliorating eosinophilic esophagitis (EoE) in an individual, comprising administering to the individual a therapeutically effective amount of at least one CRTH2 antagonist or a pharmaceutically acceptable salt thereof and at least one proton pump inhibitor (PPI) or a pharmaceutically acceptable salt thereof. Also disclosed are compositions comprising at least one CRTH2 antagonist or a pharmaceutically acceptable salt thereof and at least one proton pump inhibitor or a pharmaceutically acceptable salt thereof.
Owner:ATOPIX THERAPEUTICS
Method of treating eosinophilic esophagitis
ActiveUS8932596B2Reduce penetrationDecrease of the other histological patterns of EEAnthropod material medical ingredientsPeptide/protein ingredientsDermatologySkin exposure
The present invention relates to the treatment of EE. More specifically, the invention relates to a new method of treating EE through the epicutaneous route. In particular, the method of the invention comprises applying to the skin of the subject a skin patch device, comprising a composition, under conditions allowing a contact between said composition and the skin. The present invention also relates to the skin patch device and to a use of the skin patch device in the manufacture of a composition for treating eosinophilic esophagitis in a subject.
Owner:DBV TECH INC
Method of treating eosinophilic esophagitis
The present invention relates to the treatment of EE. More specifically, the invention relates to a new method of treating EE through the epicutaneous route. In particular, the method of the invention comprises applying to the skin of the subject a skin patch device, comprising a composition, under conditions allowing a contact between said composition and the skin. The present invention also relates to the skin patch device and to a use of the skin patch device in the manufacture of a composition for treating eosinophilic esophagitis in a subject.
Owner:DBV TECH INC
Corticosteroid containing orally disintegrating tablet compositions for eosinophilic esophagitis
ActiveUS10471071B2High blend uniformity/homogeneityOrganic active ingredientsAntipyreticGastrointestinal inflammationOrally disintegrating tablet
The present invention is directed to orally administered compositions of topically acting corticosteroids for the treatment of inflammation of the gastrointestinal tracts such as eosinophilic esophagitis. The present invention also provides a method for treating conditions associated with inflammation of the gastrointestinal tract in an individual. The method comprises administering to an individual in need thereof a pharmaceutical composition of the present invention as orally disintegrating tablets comprising a topically active corticosteroid adsorbed onto a pharmaceutically acceptable carrier such as silicified microcrystalline cellulose.
Owner:ADARE PHARMA US LP
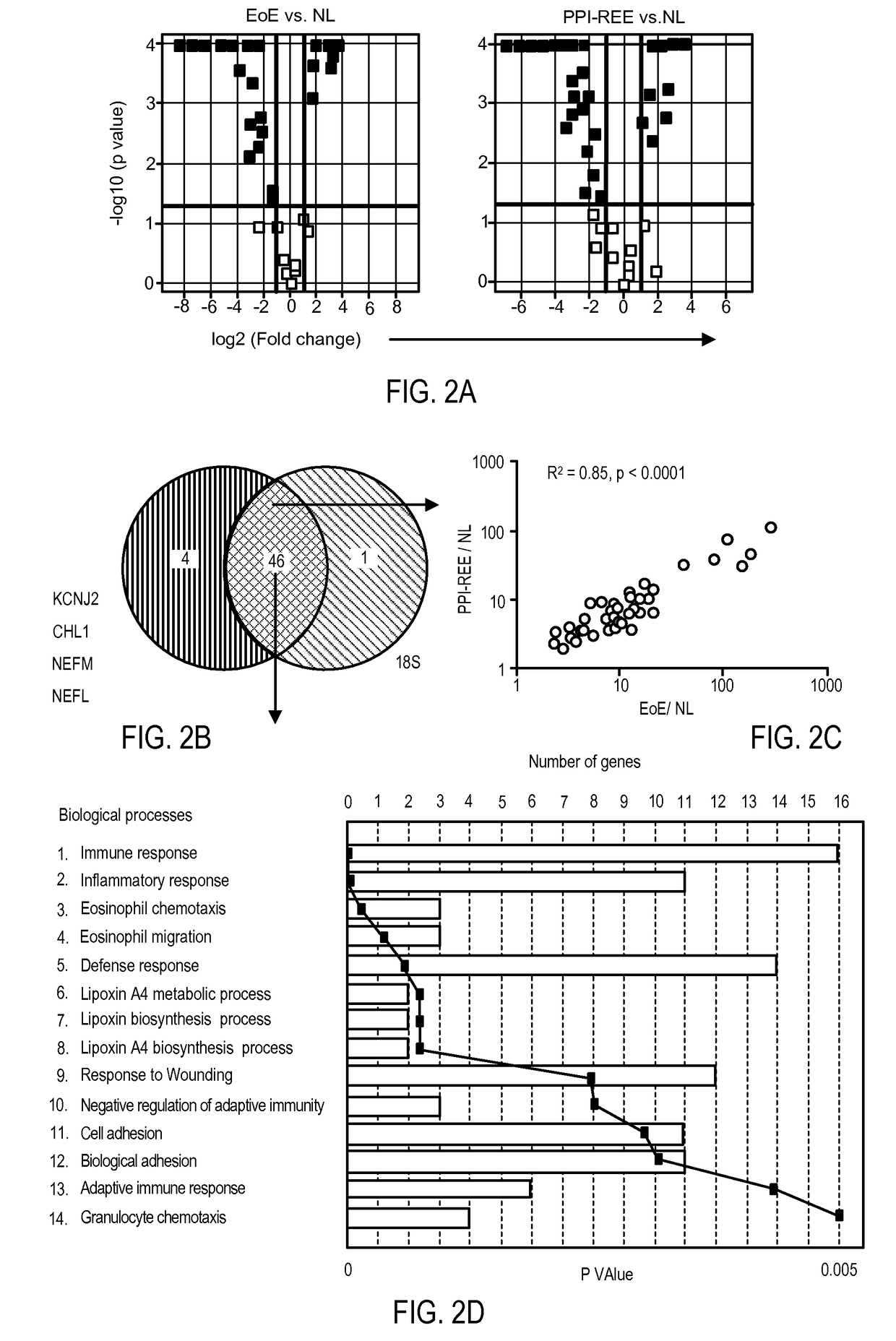
Differential Expression of Novel Protein Markers for the Diagnosis and Treatment of Eosinophilic Esophagitis
ActiveUS20150355180A1Reduce severityRelieve symptomsBiocideOrganic active ingredientsMetaboliteALOX15
A method is described for diagnosing eosinophilic esophagitis by studying the levels of expression of novel markers, including ALOX15 or metabolites thereof, TNFAIP6, FLG, SLURP1, or CRISP3. Also described are methods for treating eosinophilic esophagitis.
Owner:RHODE ISLAND HOSPITAL
Diagnostic method for distinguishing forms of esophageal eosinophilia
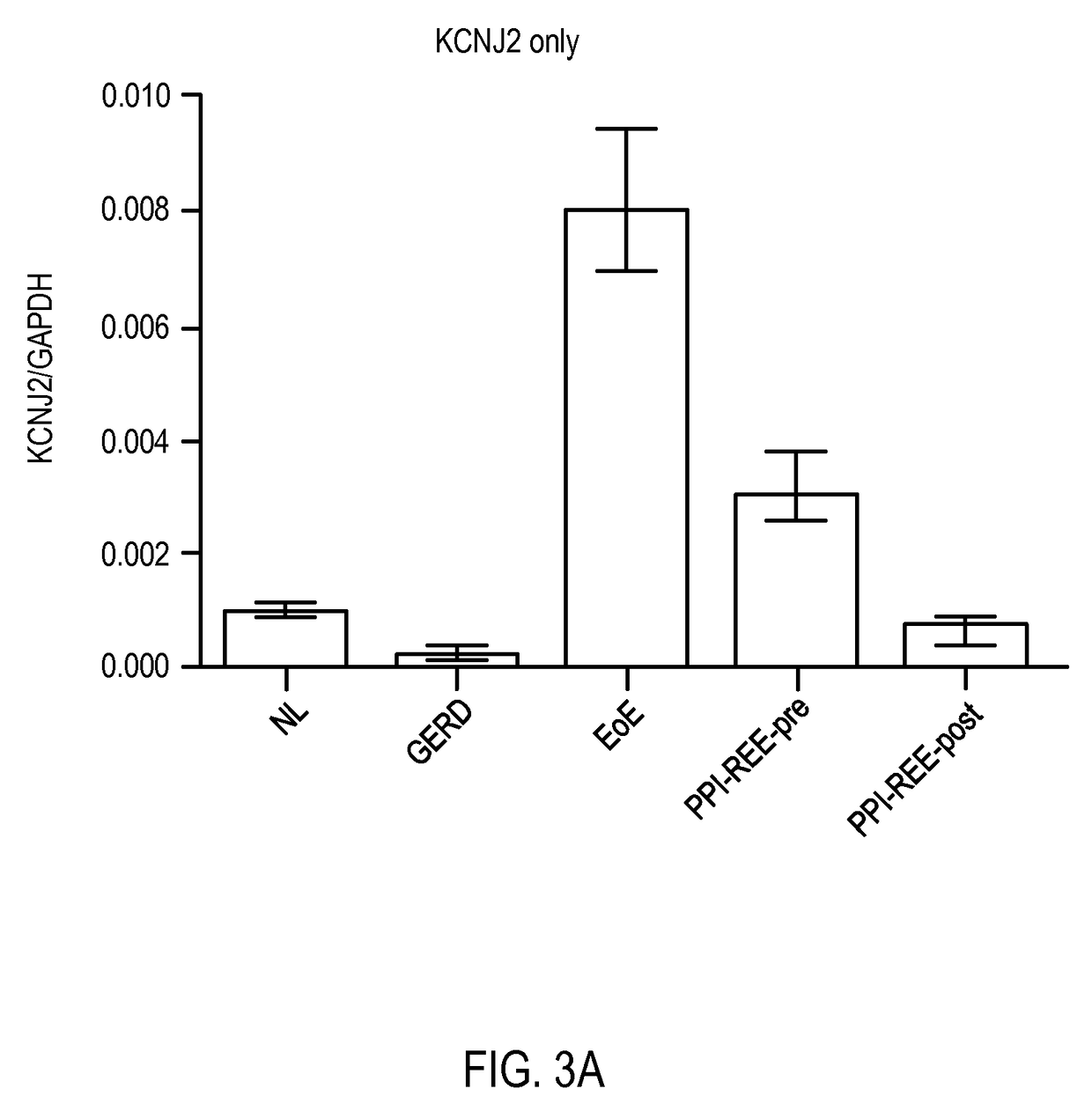
ActiveUS20170233813A1Improves care for EoE patientsOrganic active ingredientsMicrobiological testing/measurementCytosisOncology
The invention provides methods for diagnosing eosinophilic esophagitis in a patient using a biomarker based assay directed to KCNJ2 / Kir2.1 and related compositions, kits, and computer program products.
Owner:RGT UNIV OF CALIFORNIA +1
Methods for treating active eosinophilic esophagitis
The present invention provides methods for treating, preventing or reducing the severity of active eosinophilic esophagitis. In certain embodiments, the present invention provides methods of increasing esophageal distensibility. The methods of the present invention comprise administering to a subject in need thereof a therapeutic composition comprising an interleukin-4 / interleukin-13 (IL-4 / IL-13) pathway inhibitor such as an anti-IL-4R antibody.
Owner:REGENERON PHARM INC +1
A sterically stabilized carrier for subcutaneous, sublingual, and oral therapeutics, compositions and methods for treating a mammal
Disclosed herein are pharmaceutical compositions and methods for oral, sublingual or subcutaneous administration comprising a sterically stabilized liposome carrier comprising: i) poly (ethylene glycol) distearoylphosphatidylethanolamine (PEG-DSPE); and ii) at least one of phosphatidylglycerol and phosphatidylcholine. Compositions and methods for treatment of food allergy and eosinophilic esophagitis are also described.
Owner:VGSK TECH
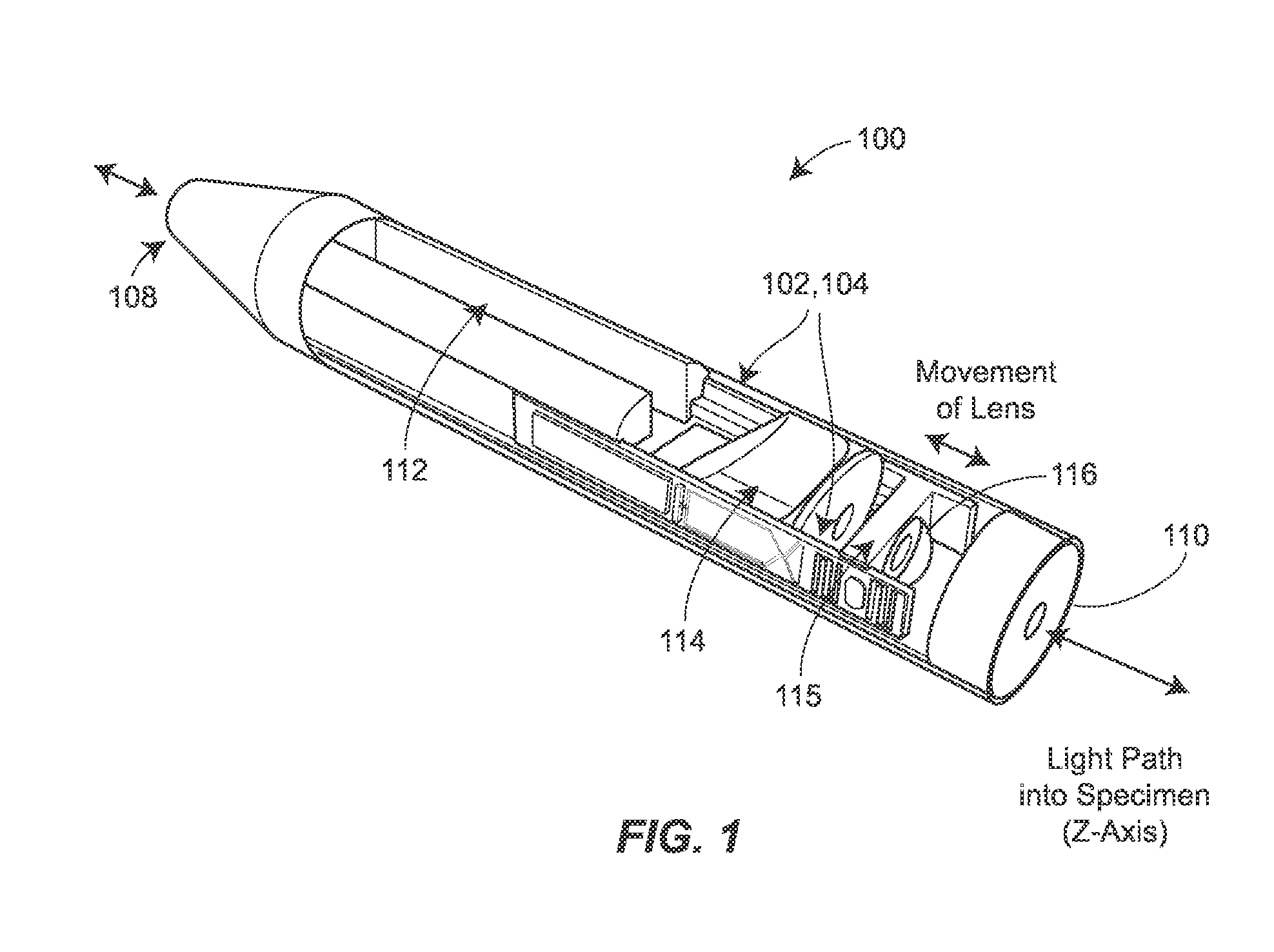
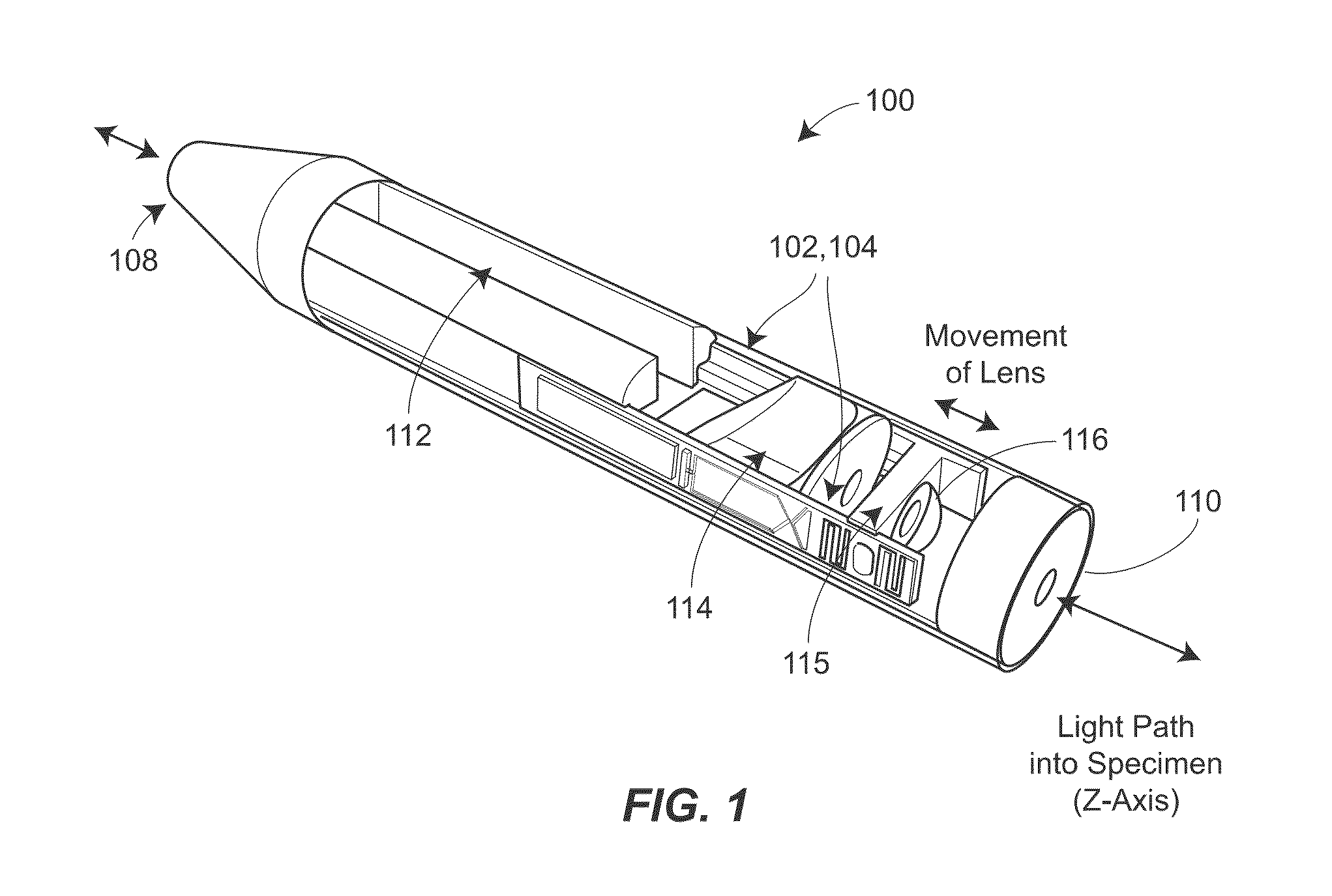
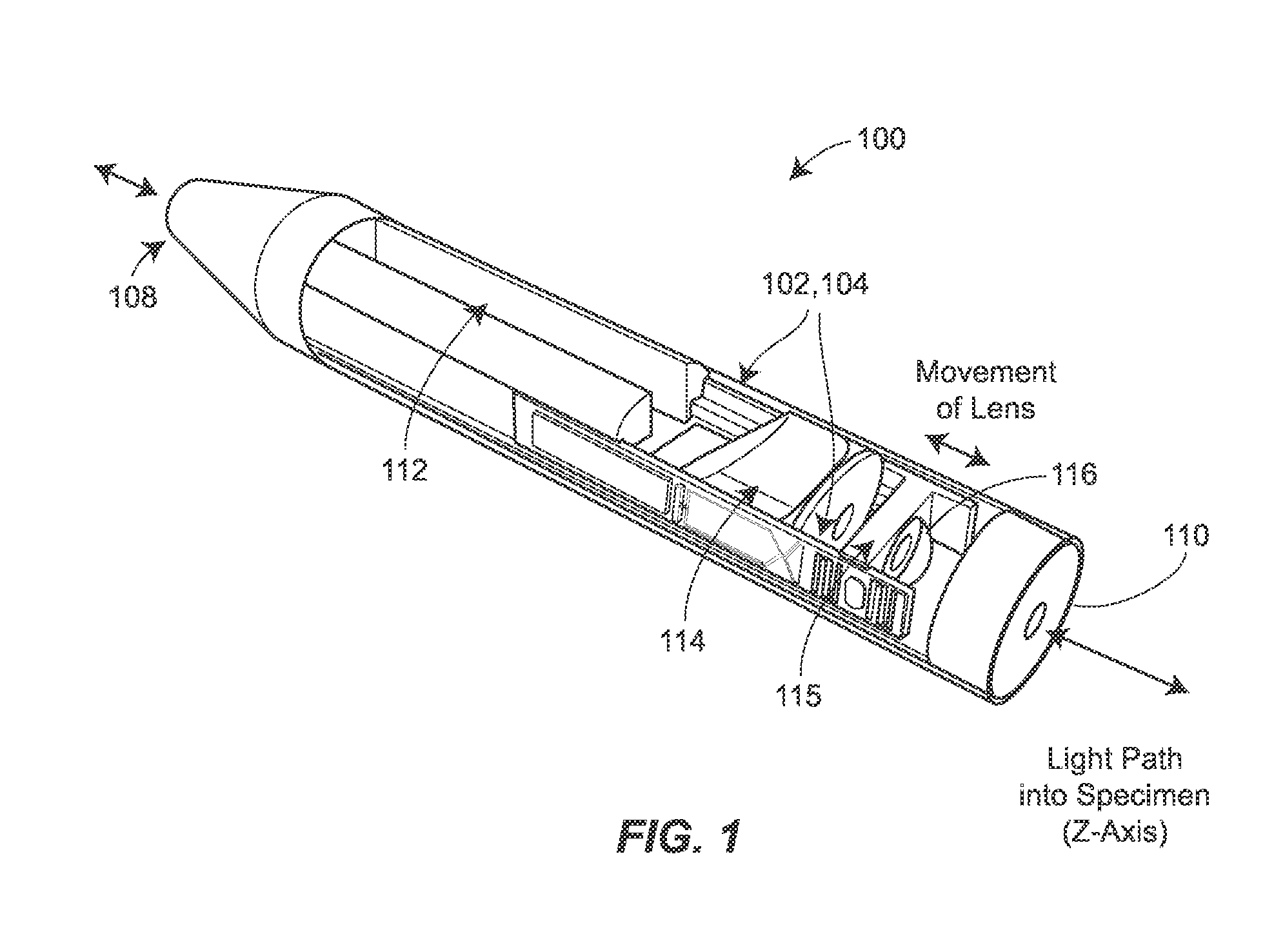
Two-photon endoscopic scanning assembly for inflammatory disease detection
ActiveUS8807801B2Minimize total cross-sectional area and volumeOesophagoscopesMicrobiological testing/measurementDiseasePiezoelectric microactuator
Owner:RGT UNIV OF MICHIGAN
Two-photon endoscopic scanning assembly for inflammatory disease detection
ActiveUS20140255985A1Minimize total cross-sectional area and volumeOesophagoscopesPreparing sample for investigationPiezoelectric microactuatorDisease detection
An endscopic imaging device is described that achieves longitudinal axis (z-axis) scanning into a tissue or sample, using a piezoelectric microactuator. In some configurations, additional lateral (xy-plane) scanning is also achieved, to allow for the creation of full three-dimensional imaging, ex vivo or in vivo. The techniques may be used to image and diagnosis allergic rhinitis and eosinophilic esophagitis in tissue.
Owner:THE RGT UNIV OF MICHIGAN
Two-Photon Endoscopic Scanning Assembly for Inflammatory Disease Detection
ActiveUS20120270256A1Large stroke lengthMinimize total cross-sectional area and volumeOesophagoscopesMicrobiological testing/measurementPiezoelectric microactuatorDisease detection
An endscopic imaging device is described that achieves longitudinal axis (z-axis) scanning into a tissue or sample, using a piezoelectric microactuator. In some configurations, additional lateral (xy-plane) scanning is also achieved, to allow for the creation of full three-dimensional imaging, ex vivo or in vivo. The techniques may be used to image and diagnosis allergic rhinitis and eosinophilic esophagitis in tissue.
Owner:RGT UNIV OF MICHIGAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com