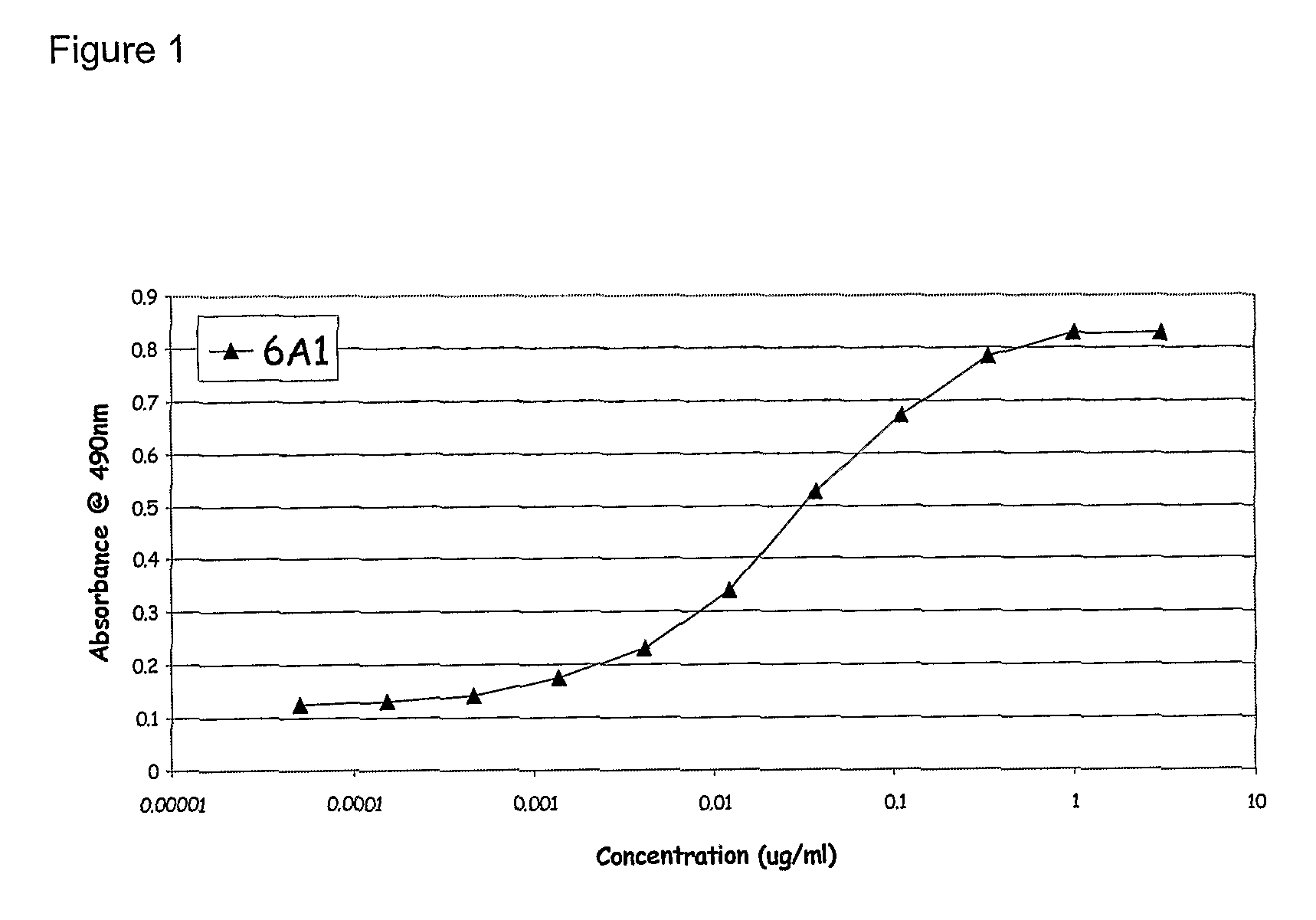
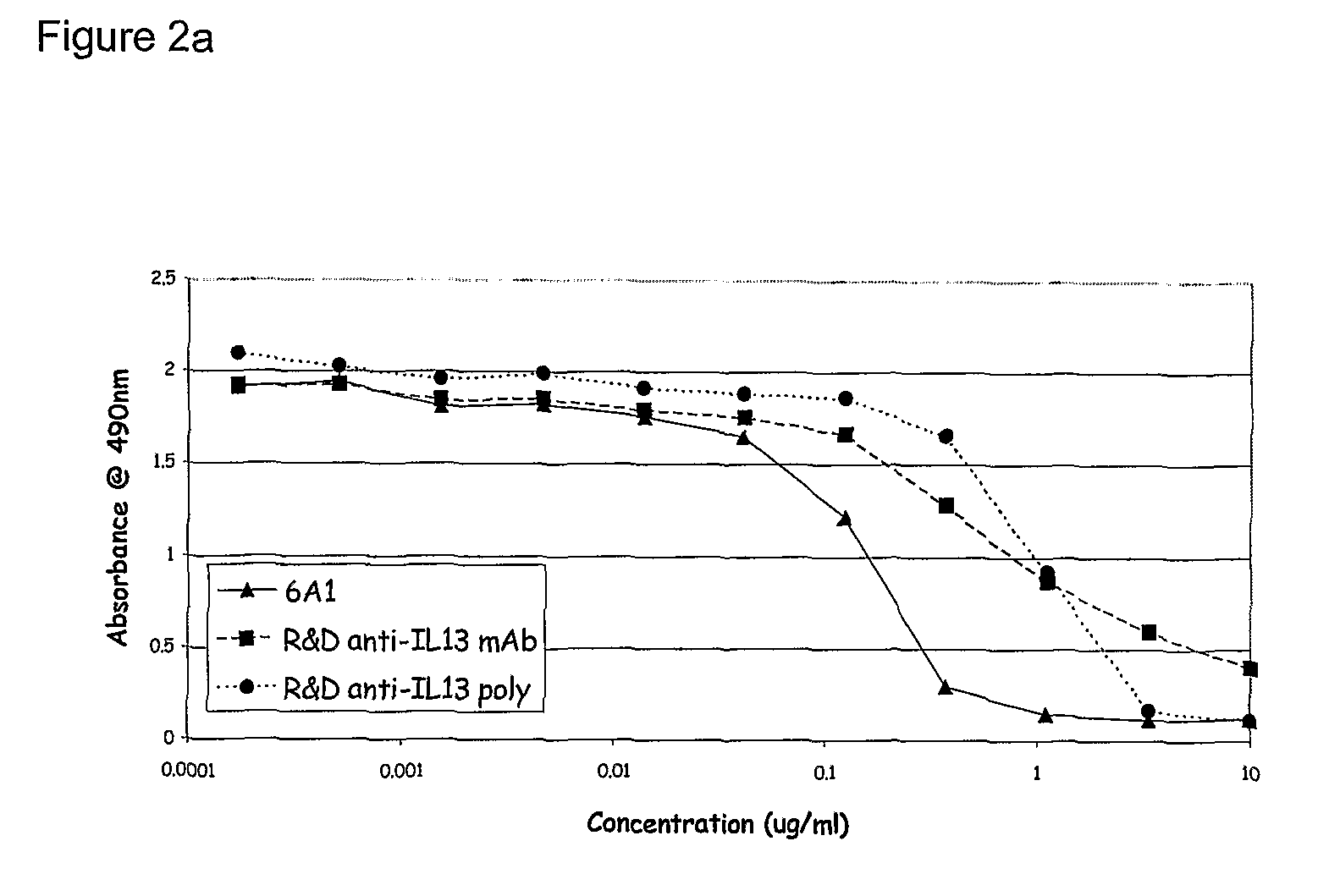
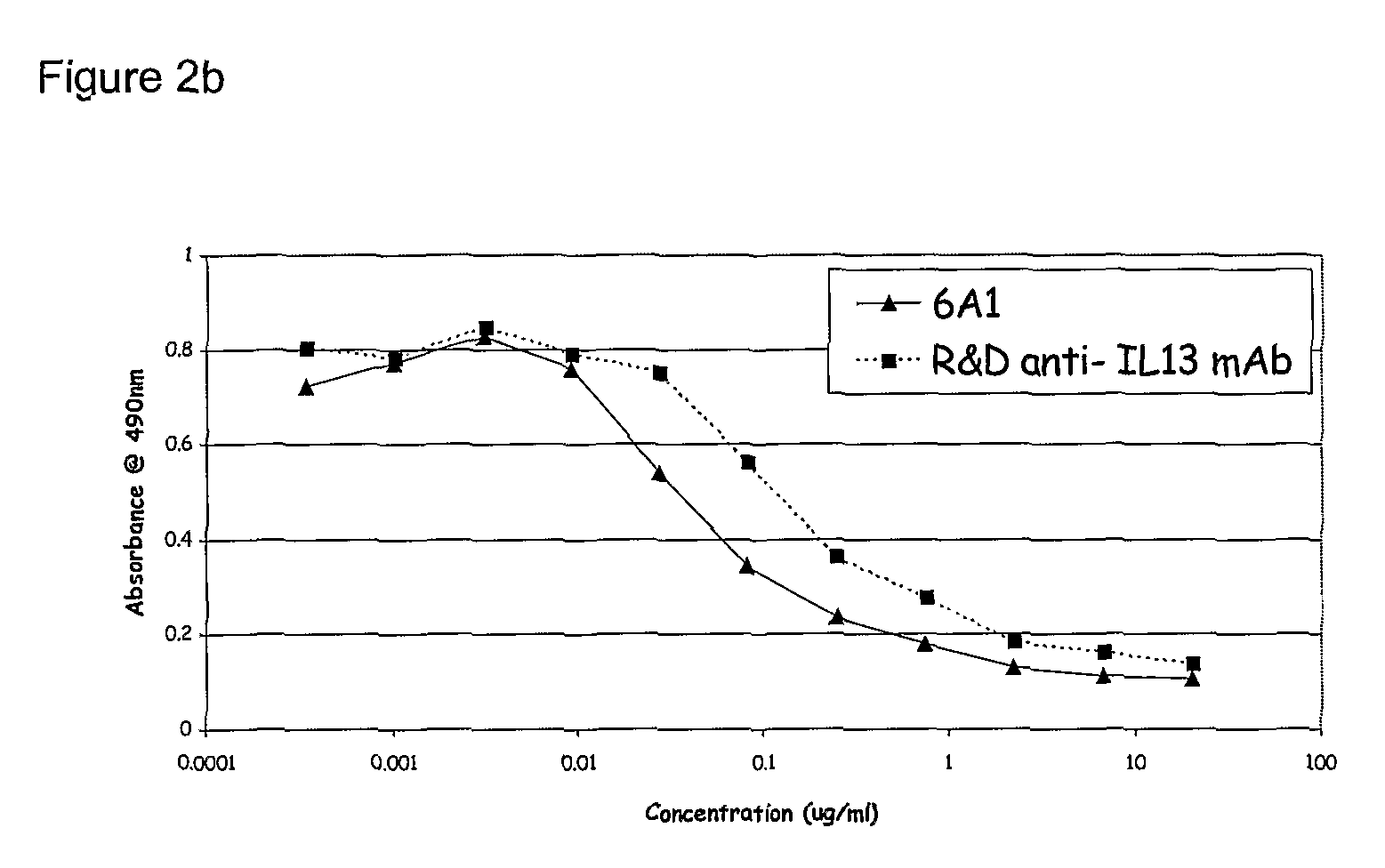
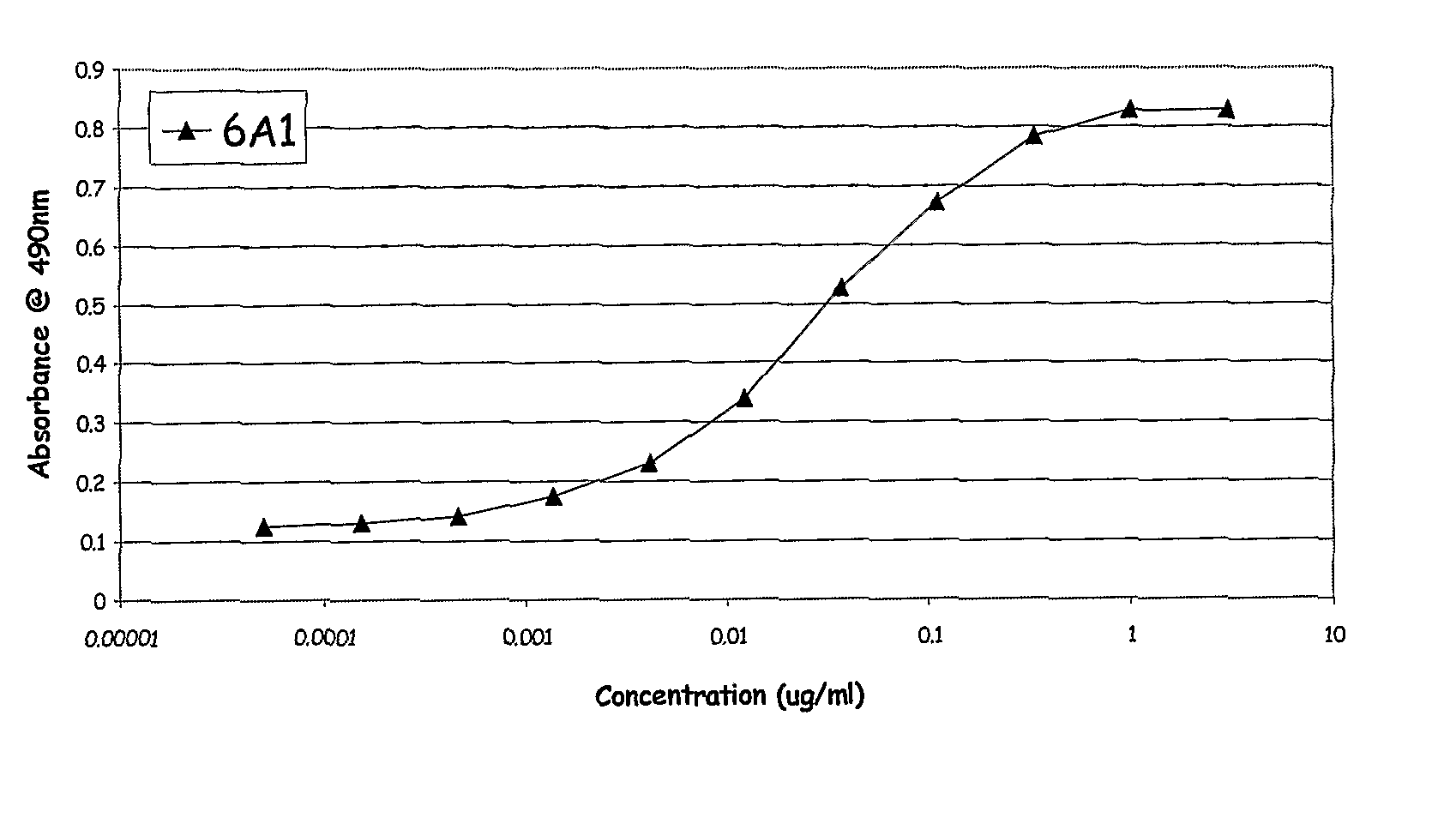
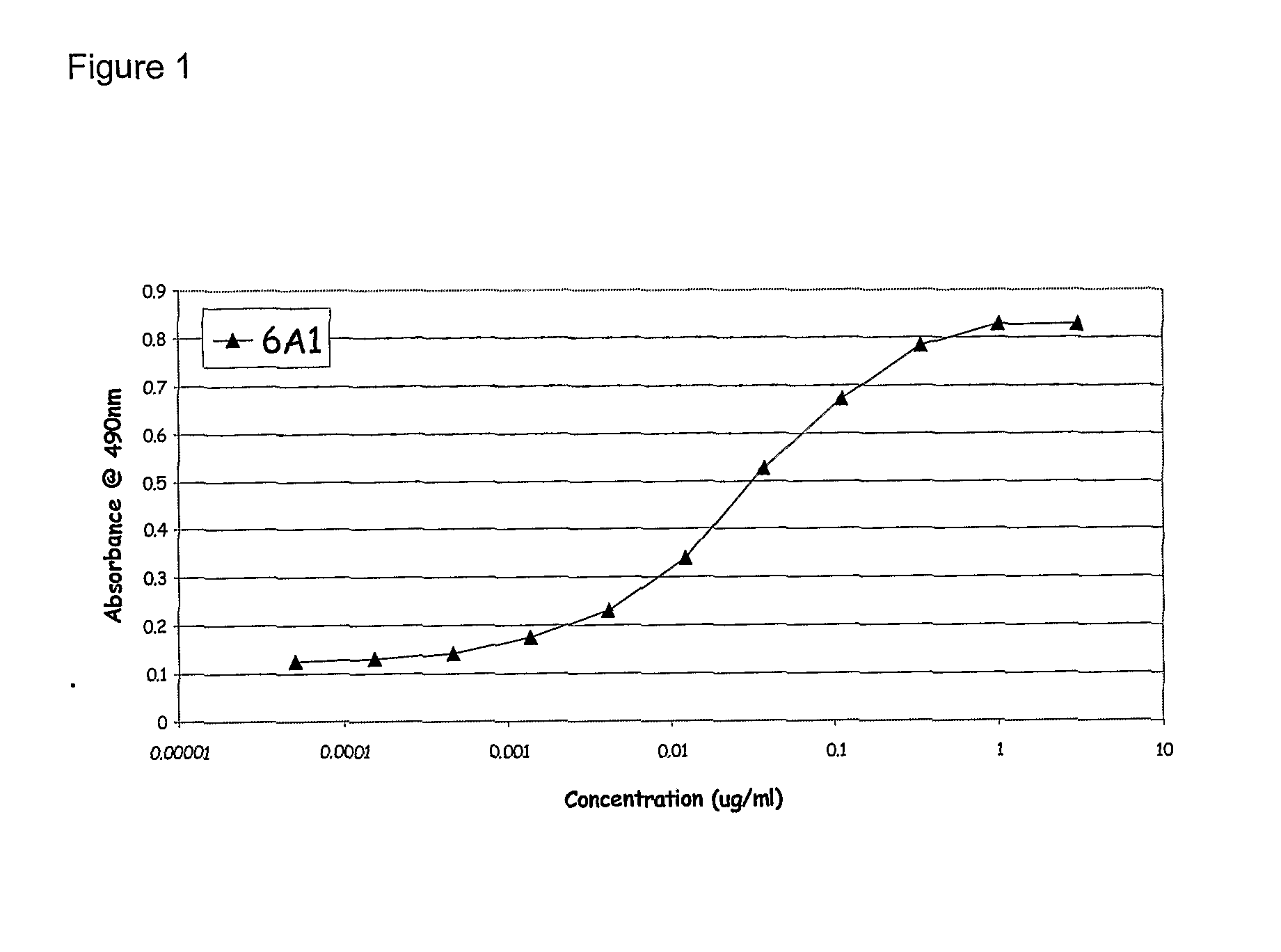
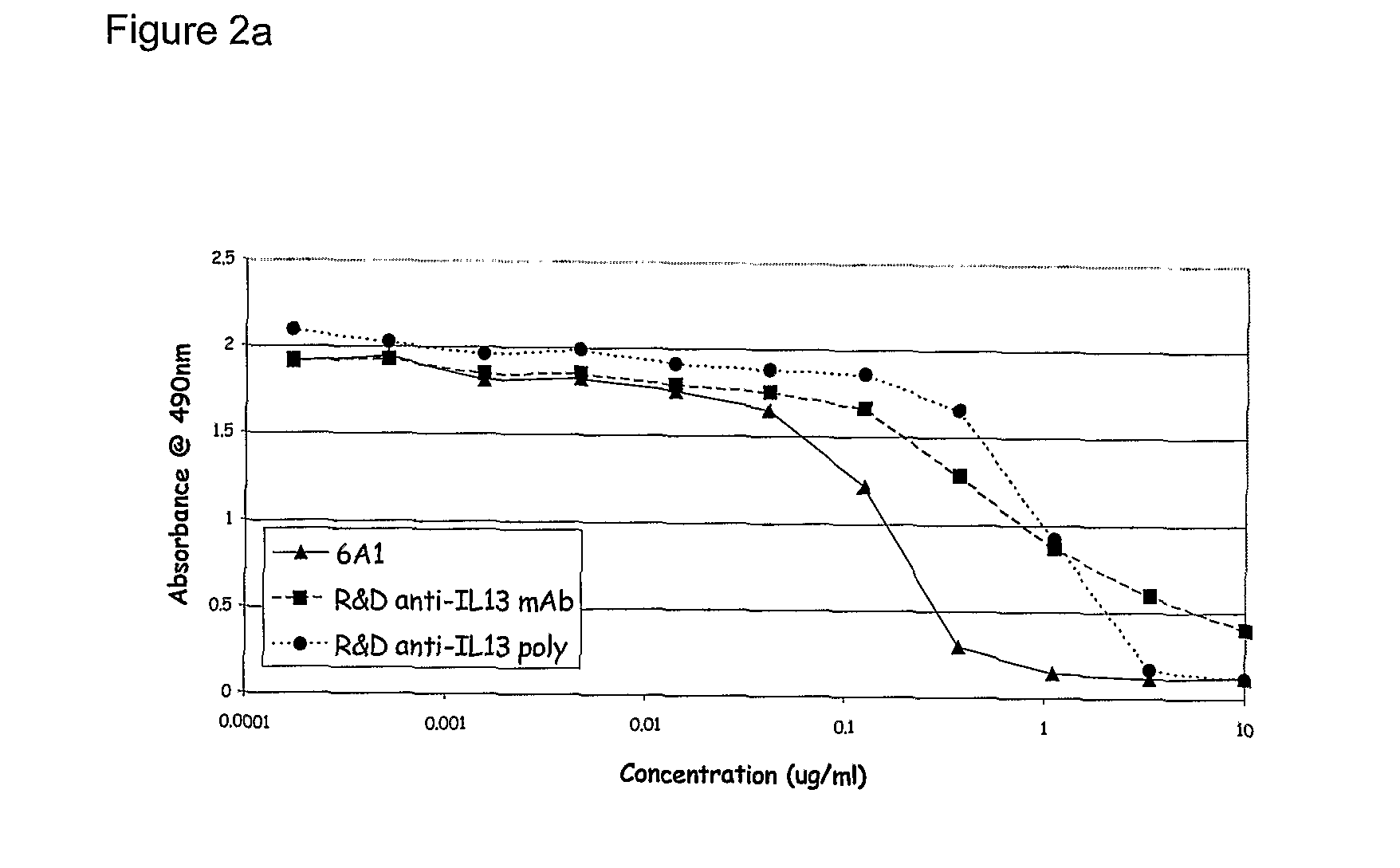
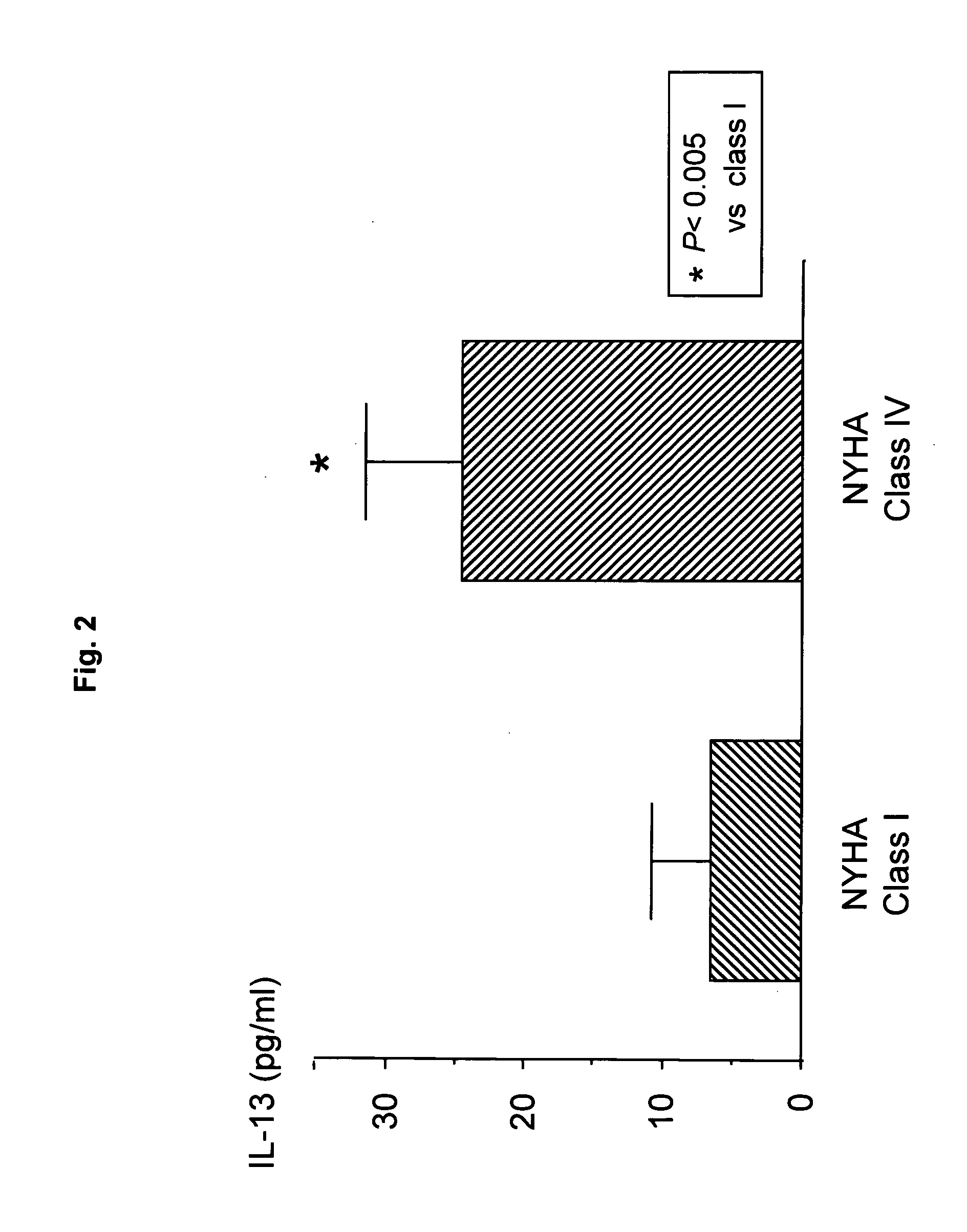
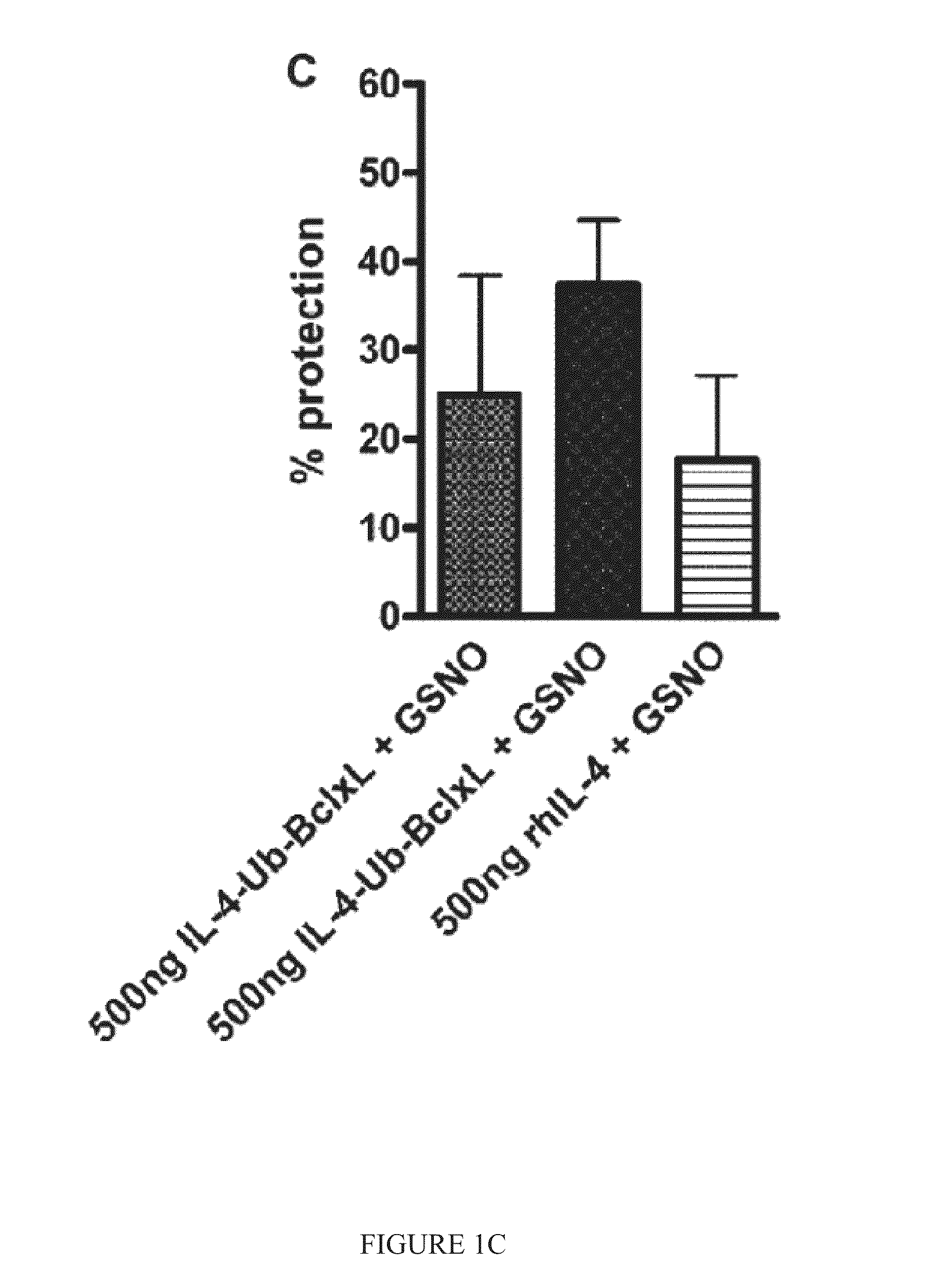
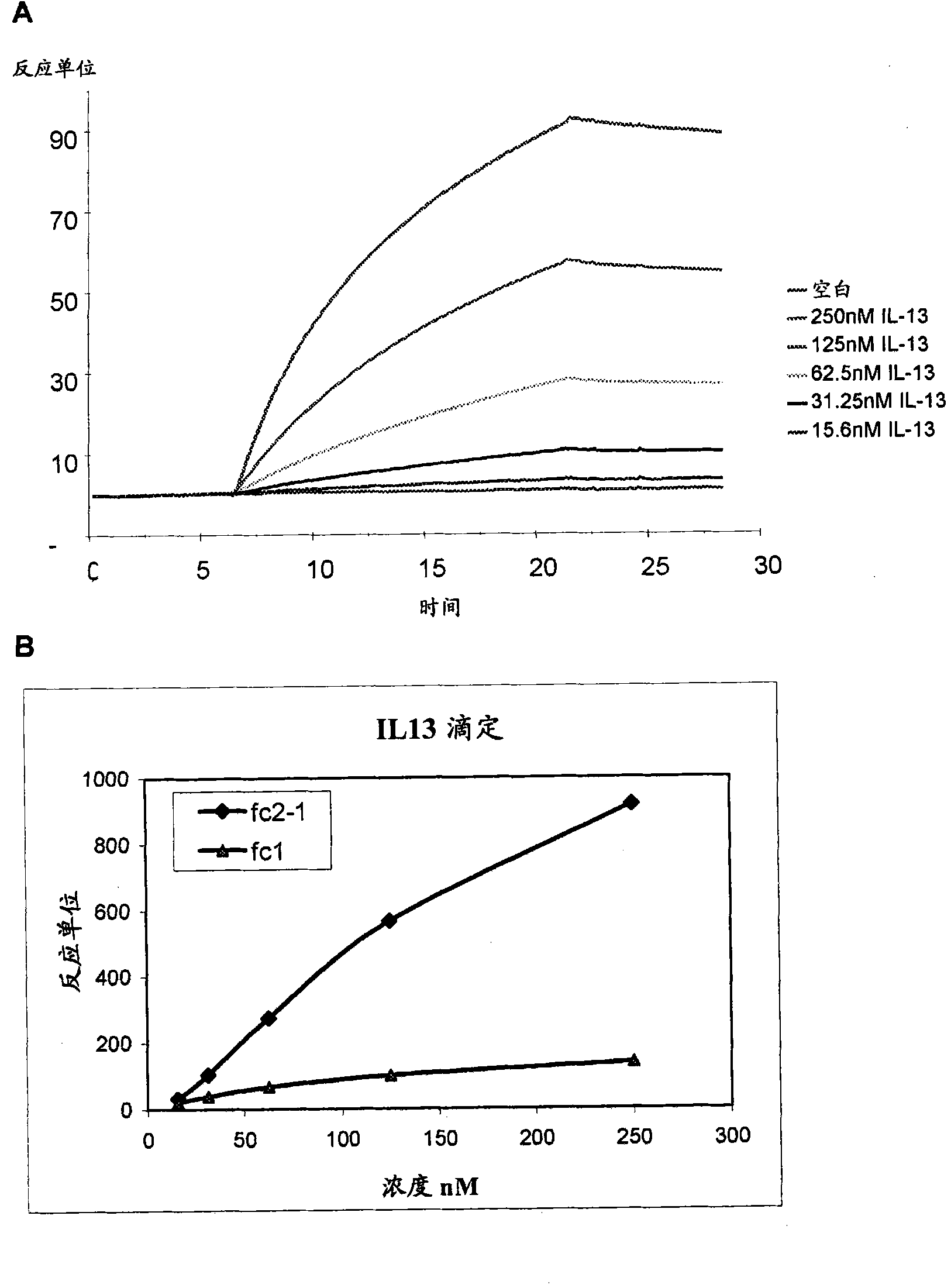
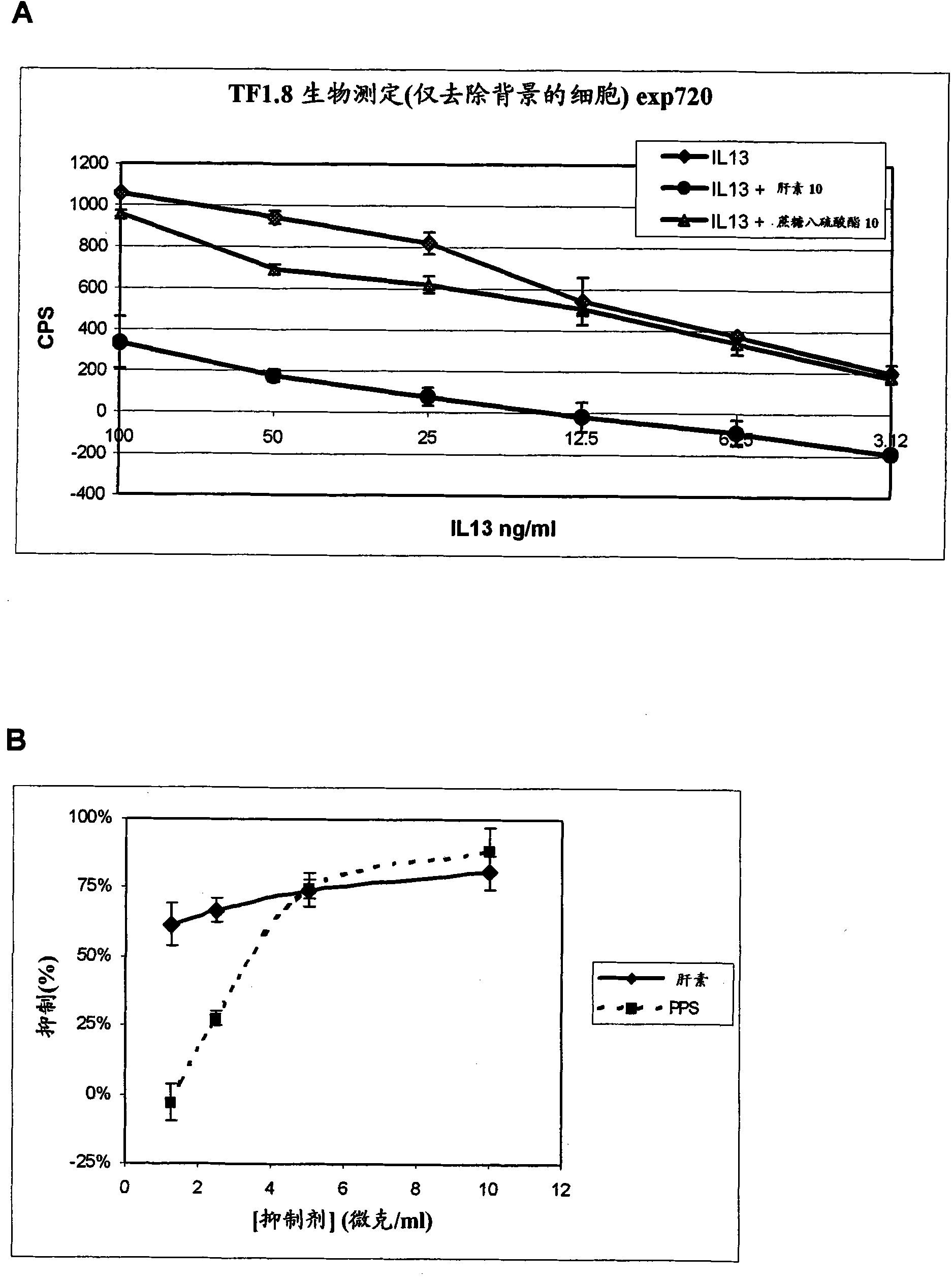
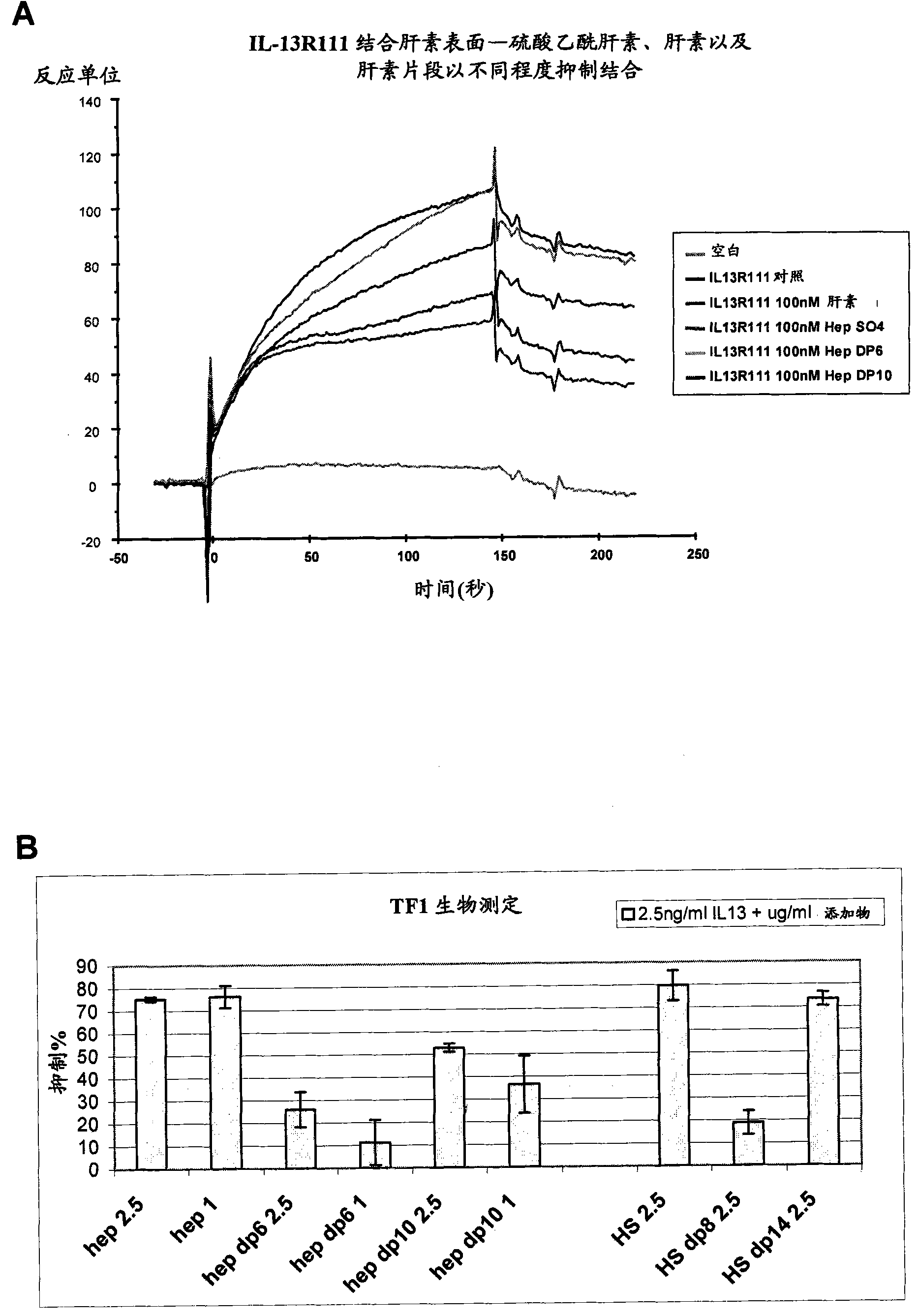
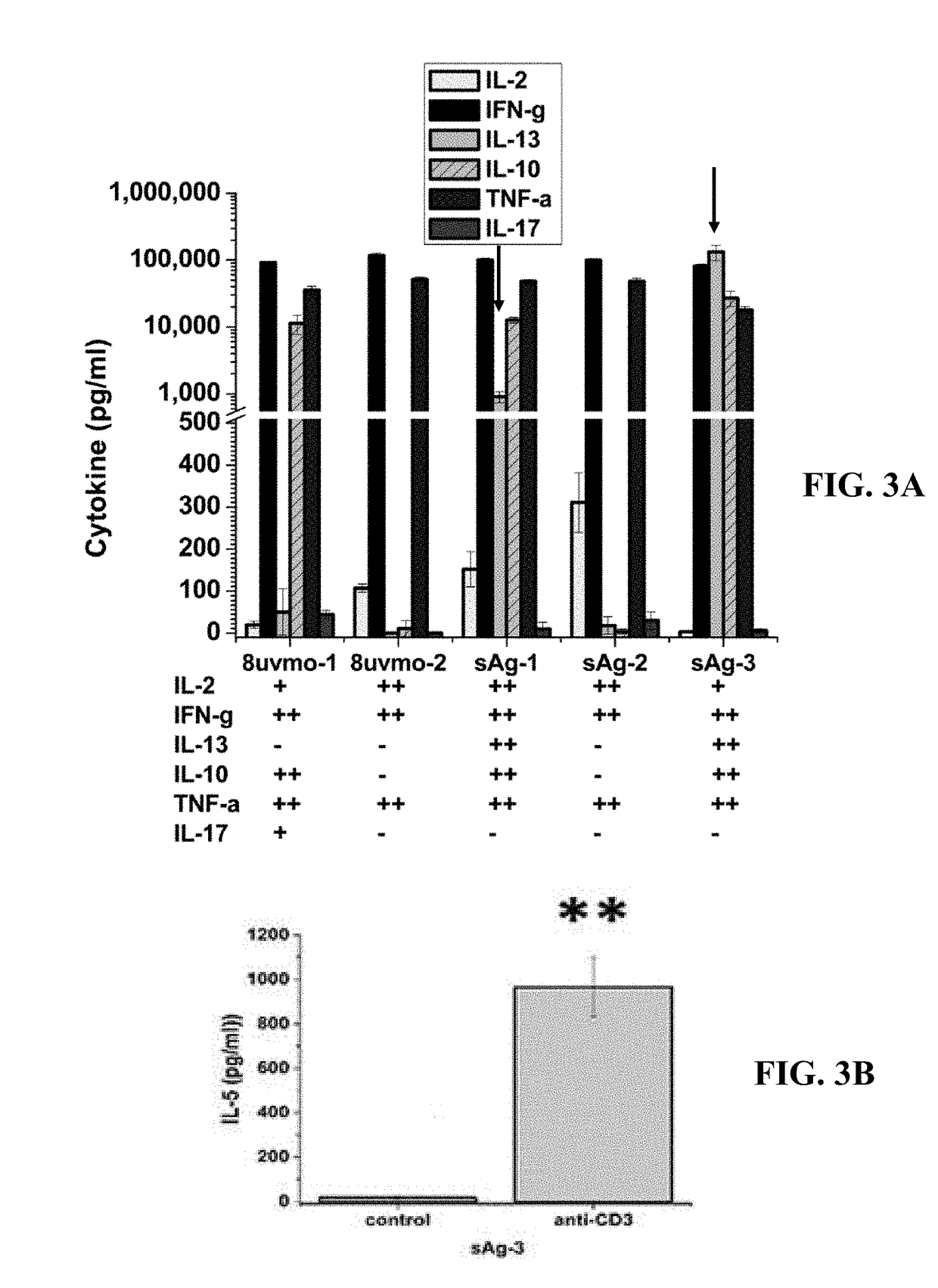
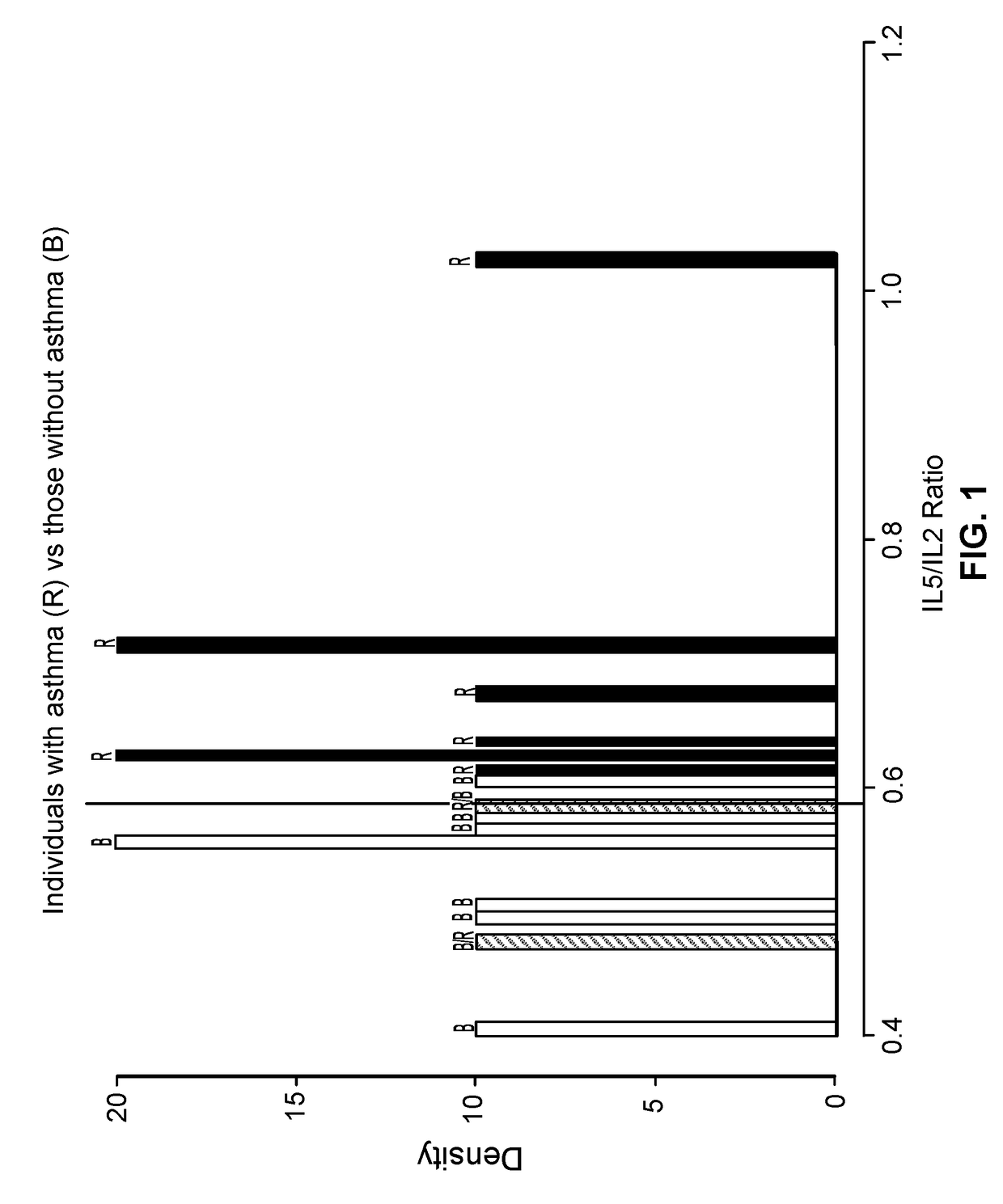
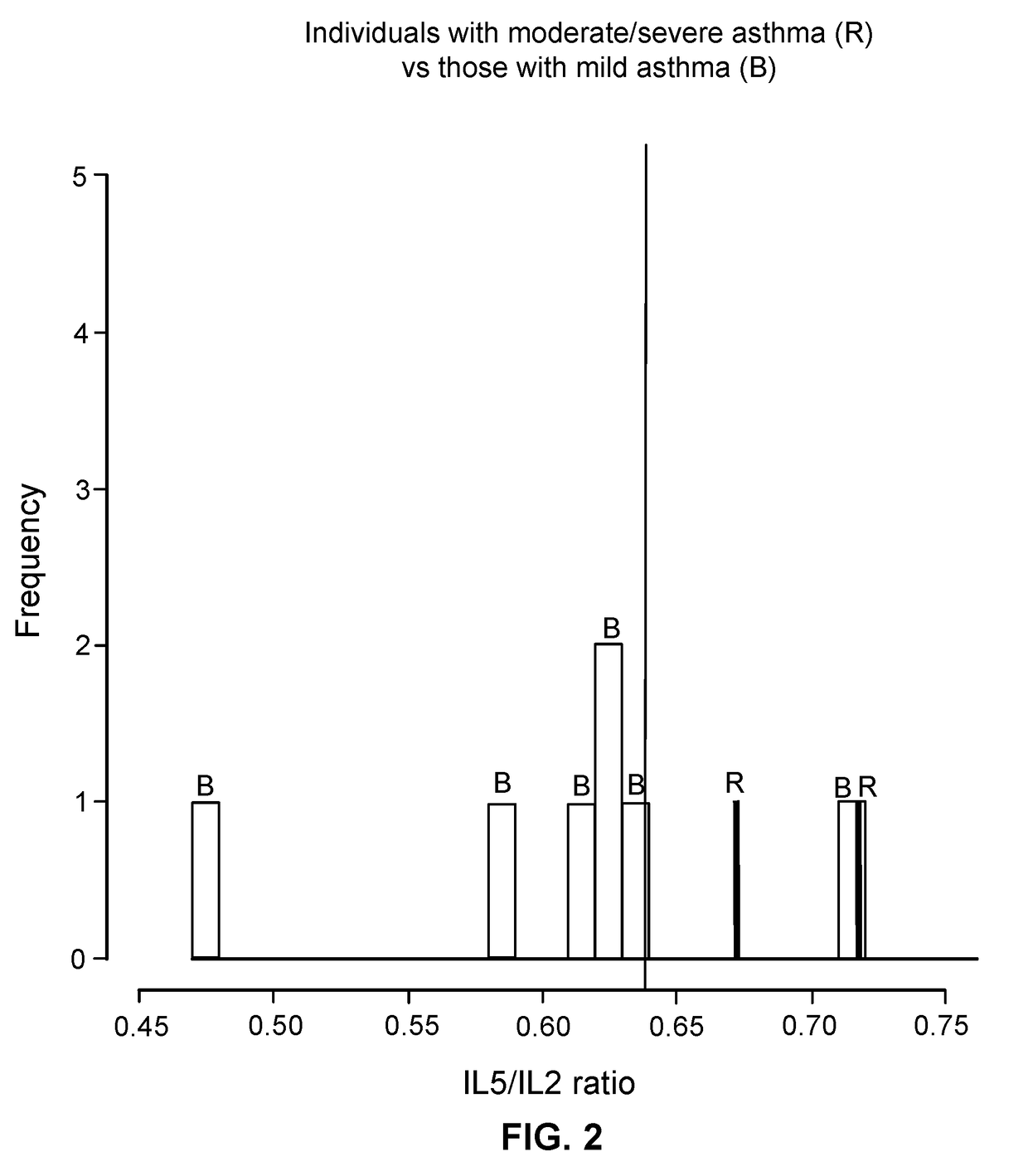
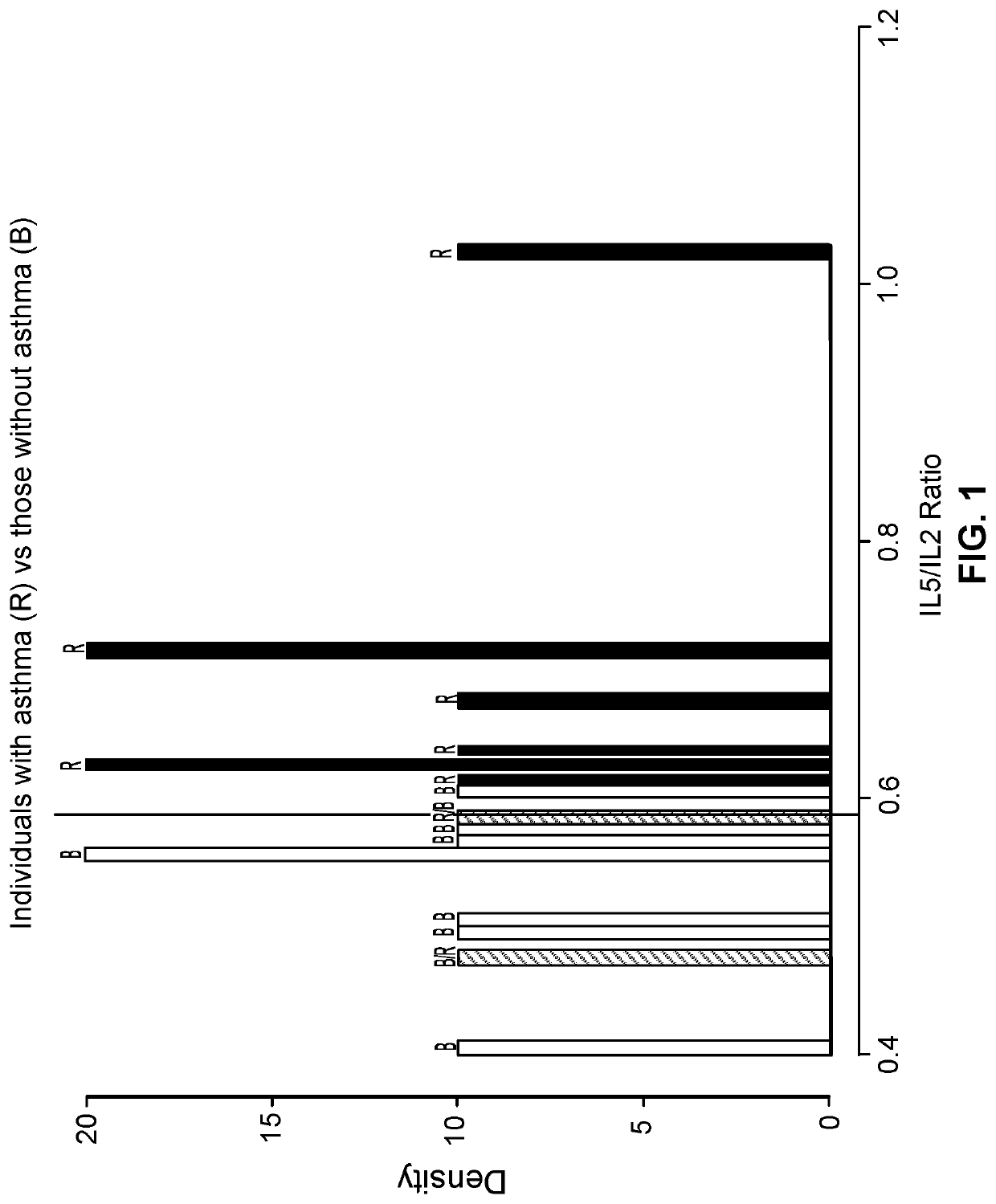
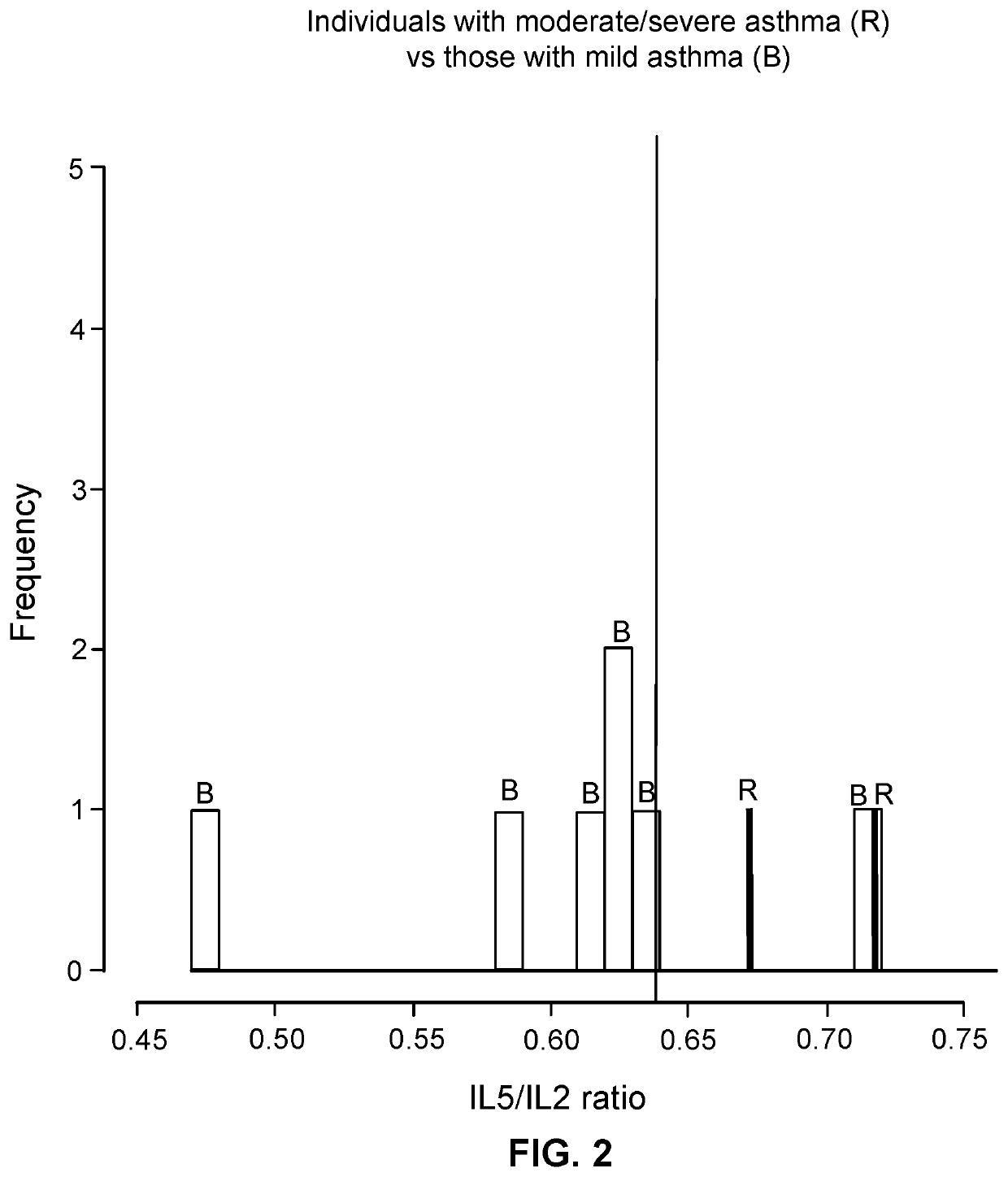
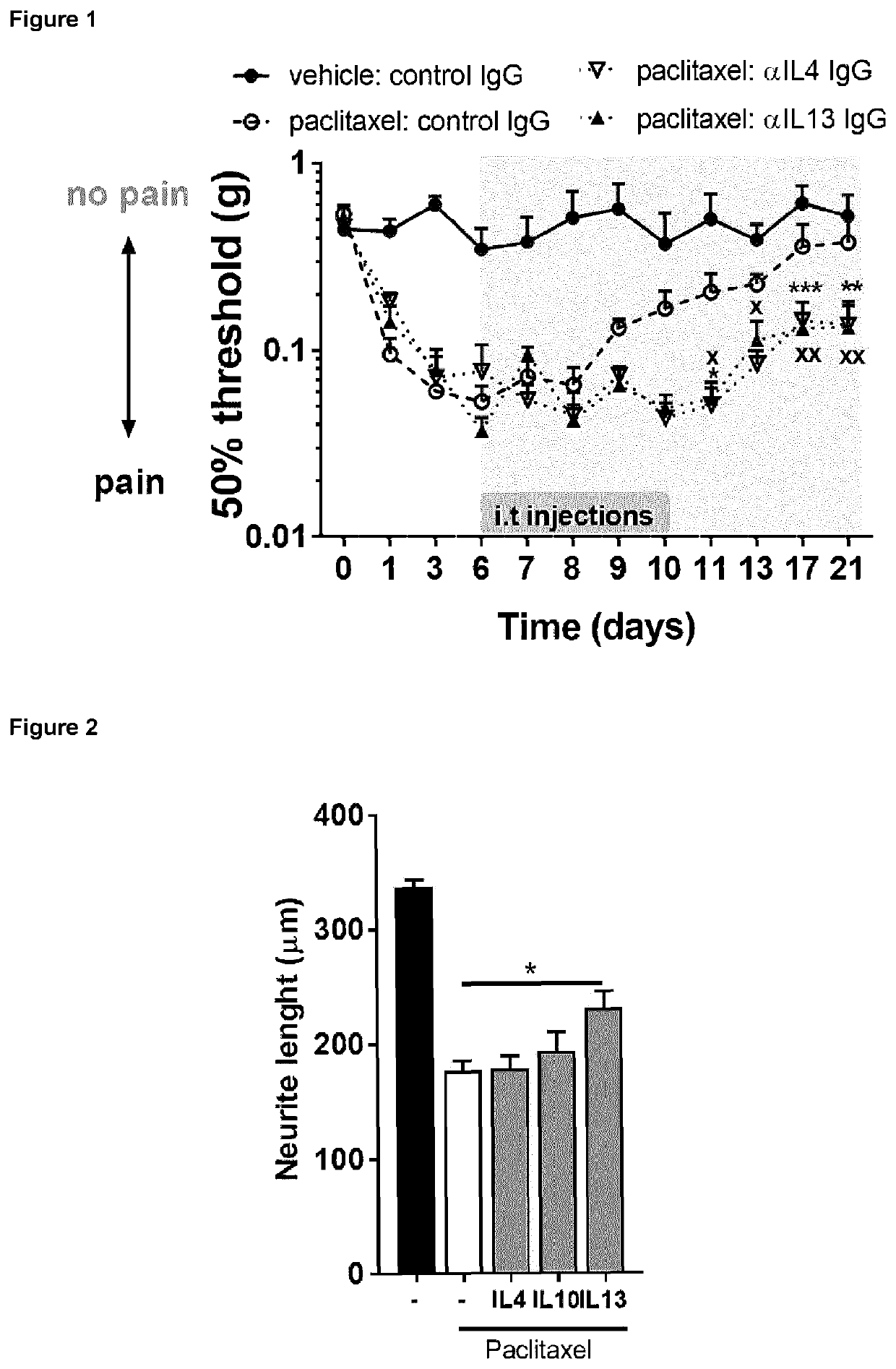
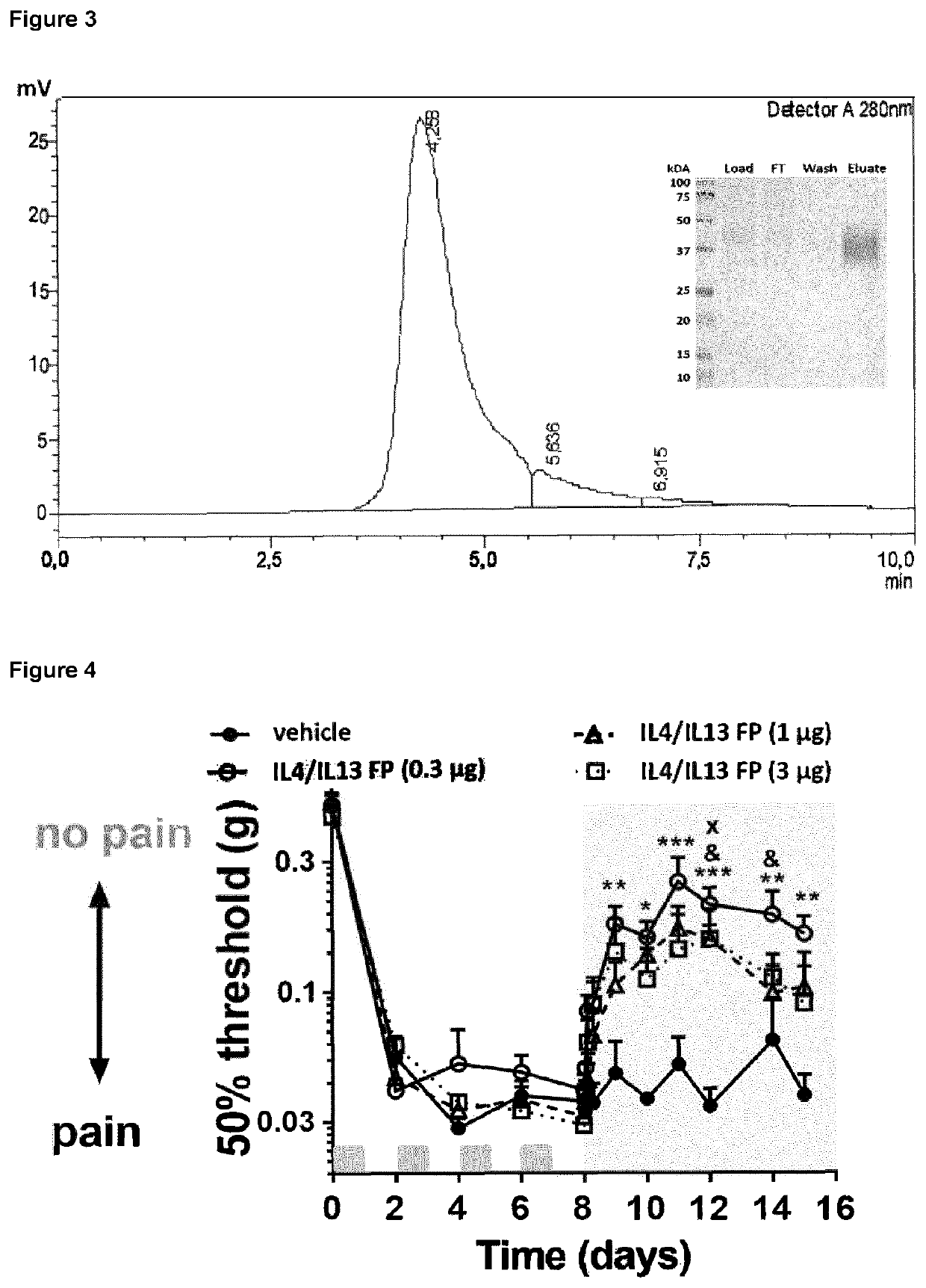
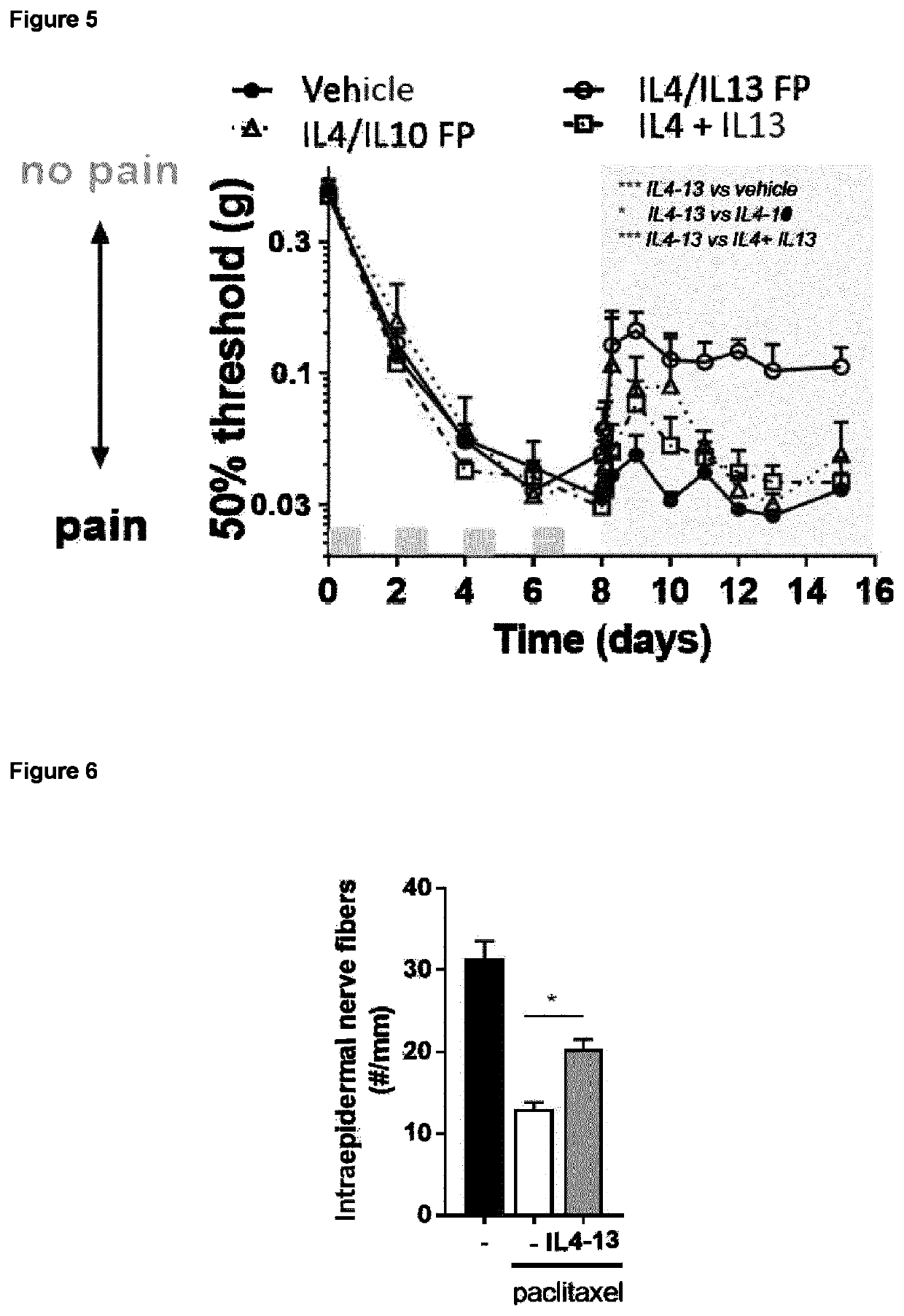
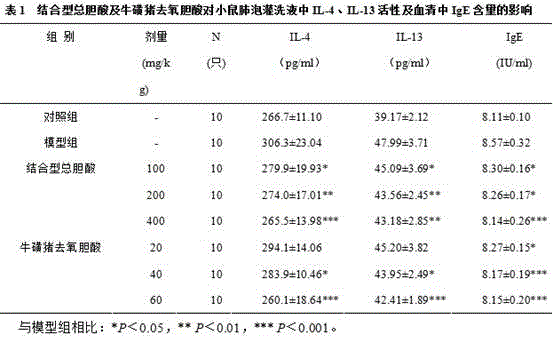
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "Interleukin 13" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
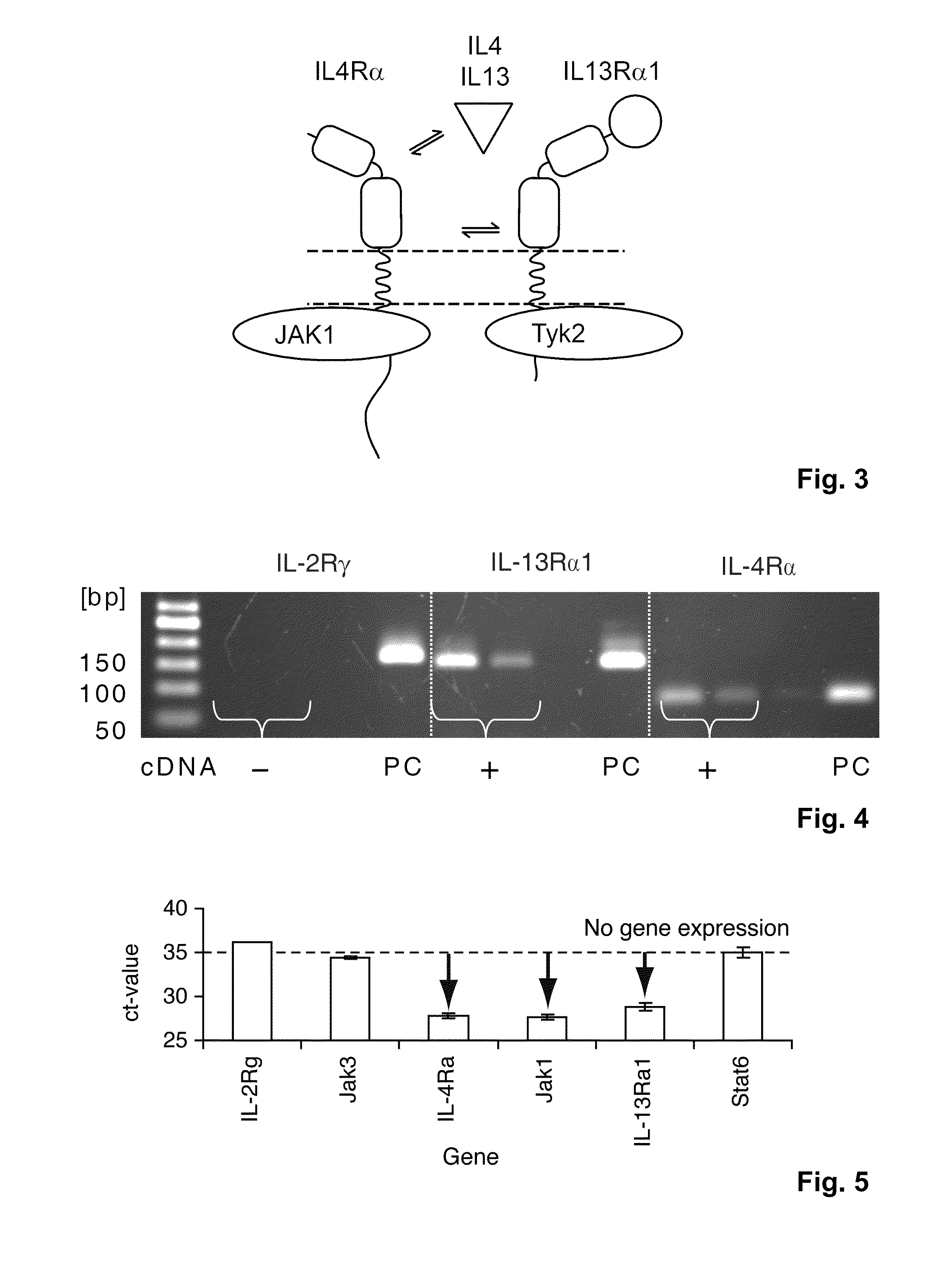
Interleukin 13 (IL-13) is a protein that in humans is encoded by the IL13 gene. IL-13 was first cloned in 1993 and is located on chromosome 5q31 with a length of 1.4kb. It has a mass of 13 kDa and folds into 4 alpha helical bundles. The secondary structural features of IL-13 are similar to that of Interleukin 4 (IL-4); however it only has 25% sequence homology to IL-4 and is capable of IL-4 independent signaling. IL-13 is a cytokine secreted by T helper type 2 (Th2) cells, CD4 cells, Natural killer T cell, Mast cell, Basophil cells, Eosinophil cells and Nuocyte cells. Interleukin-13 is a central regulator in IgE synthesis, goblet cell hyperplasia, mucus hypersecretion, airway hyperresponsiveness, fibrosis and chitinase up-regulation. It is a mediator of allergic inflammation and different diseases including asthma.
Chimeric and humanised monoclonal antibodies against Interleukin-13
The present invention concerns immunoglobulins, particularly antibodies which specifically bind human Interleukin 13 (hIL-13). Antibodies of the invention may be used in the treatment of a variety of diseases or disorders responsive to modulation of the interaction between hIL-13 and the human IL-13 receptor. Such diseases include severe asthma, atopic dermatitis, COPD and various fibrotic diseases. Pharmaceutical compositions comprising said antibodies and methods of manufacture are also disclosed.
Owner:GLAXO GRP LTD
Interleukin-13 antagonist powders, spray-dried particles, and methods
A powder includes IL-13 antagonist, wherein the powder has a mass median aerodynamic diameter (MMAD) of less than about 10 μm. A composition includes a spray-dried particle including IL-13 antagonist. A method of administering IL-13 antagonist to the lungs of a subject includes: dispersing a dry powder composition involving IL-13 antagonist to form an aerosol; and delivering the aerosol to the lungs of the subject by inhalation of the aerosol by the subject, thereby ensuring delivery of the IL-13 antagonist to the lungs of the subject. A method of treating an IL-13-related condition includes: pulmonarily administering a therapeutically effective amount of a dry powder including IL-13 antagonist. A method of preparing IL-13 antagonist-containing powder involves: combining IL-13 antagonist, optional excipient, and solvent to form a mixture or solution; and spray drying the mixture or solution to obtain the powder.
Owner:NOVARTIS FARMA
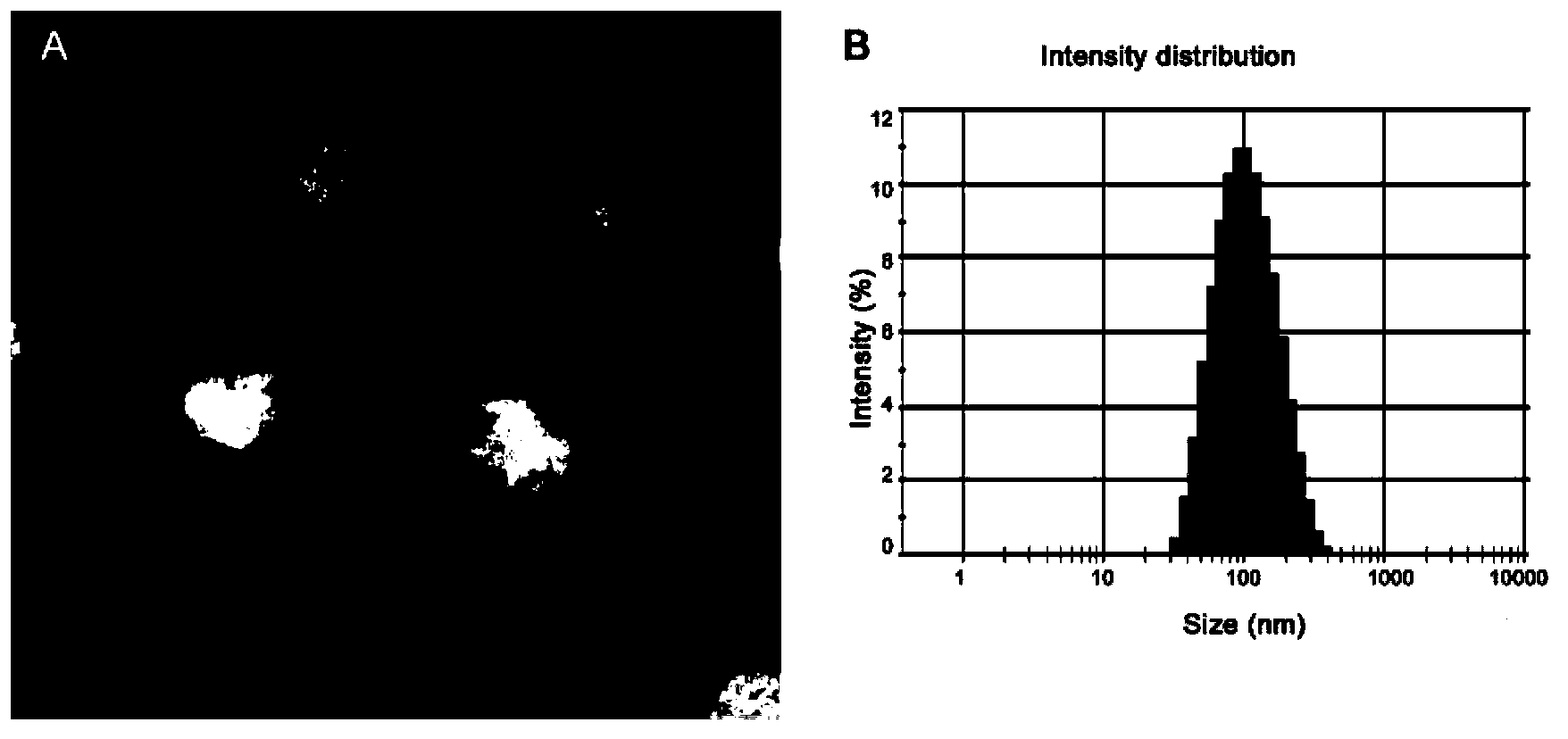
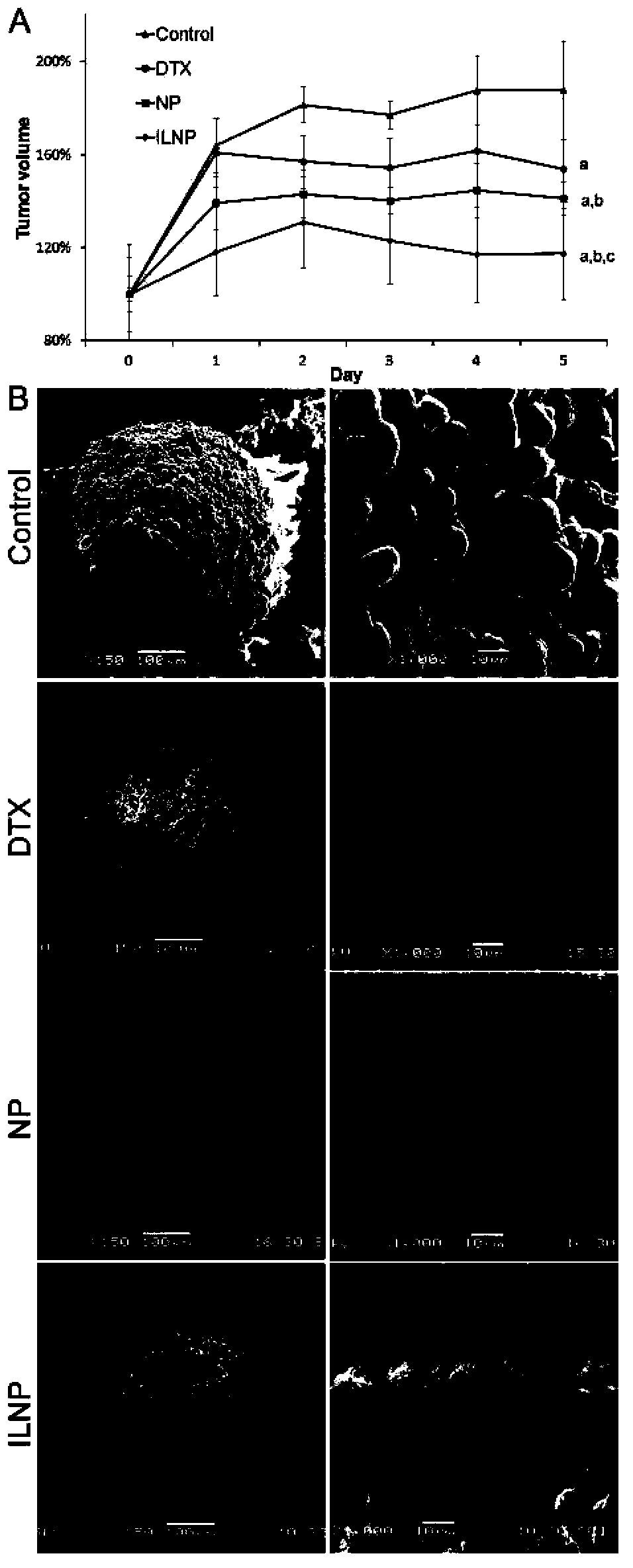
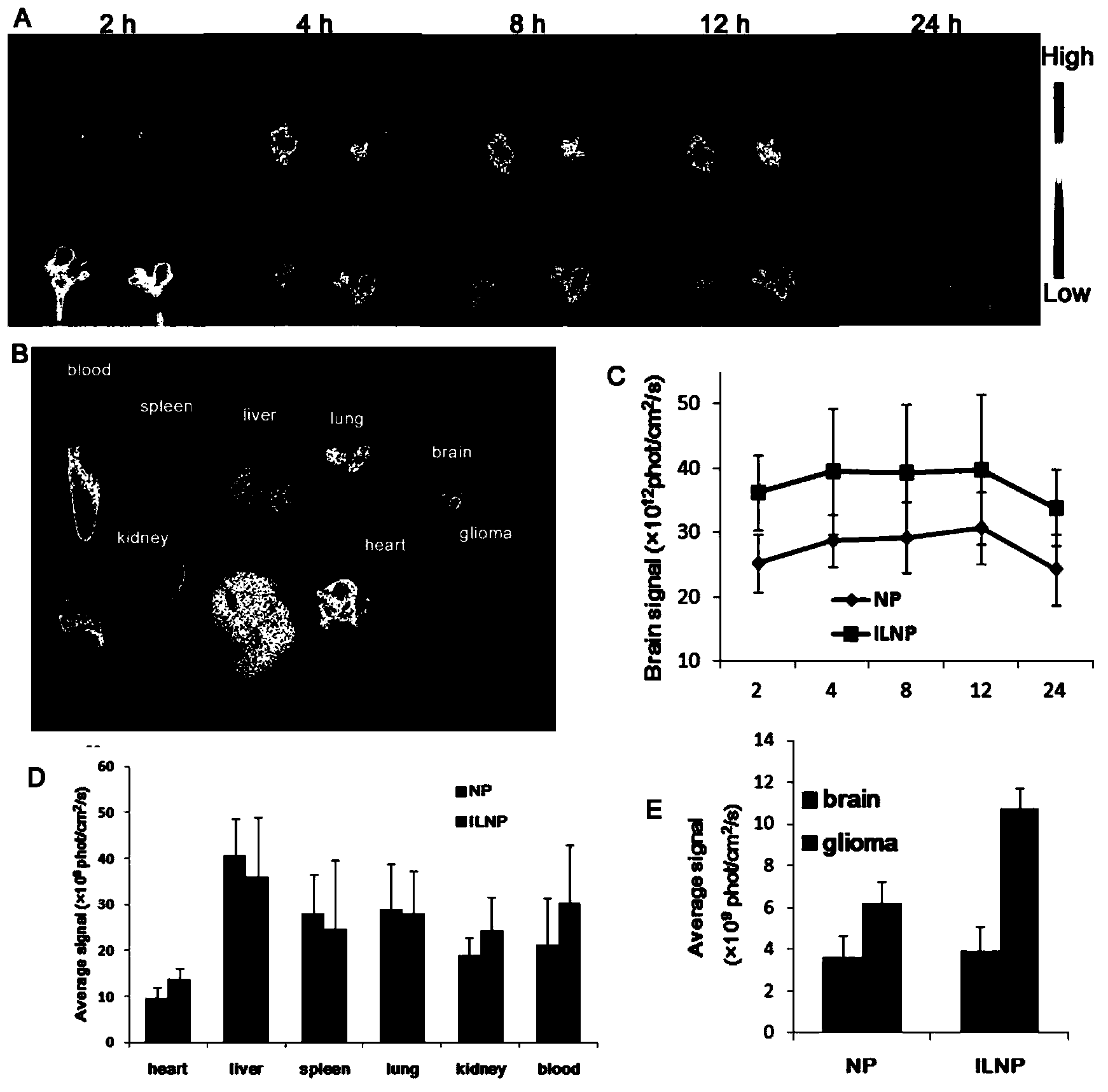
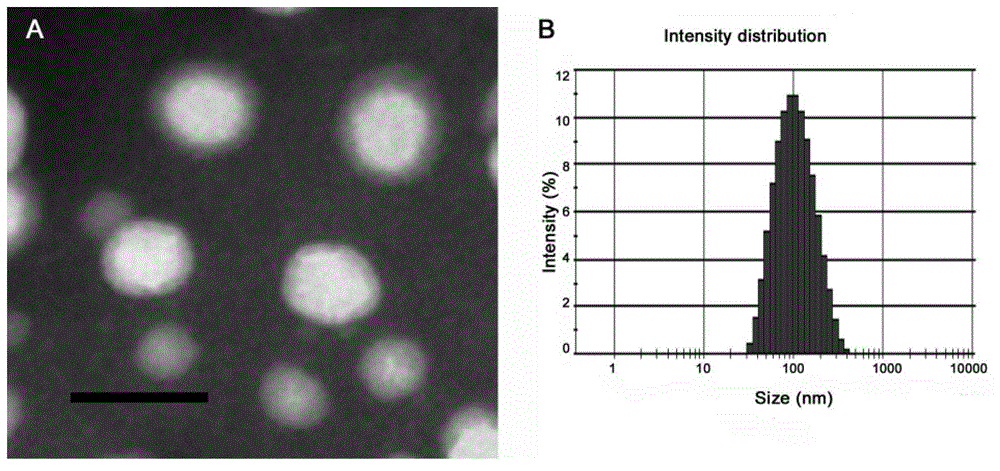
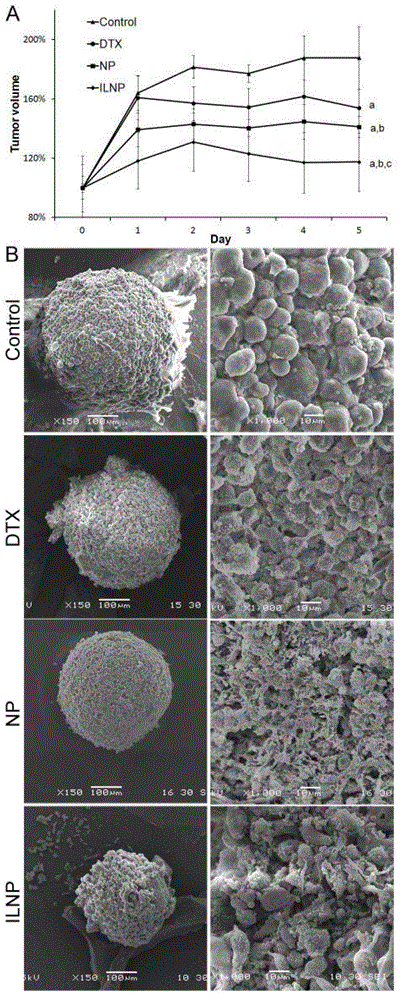
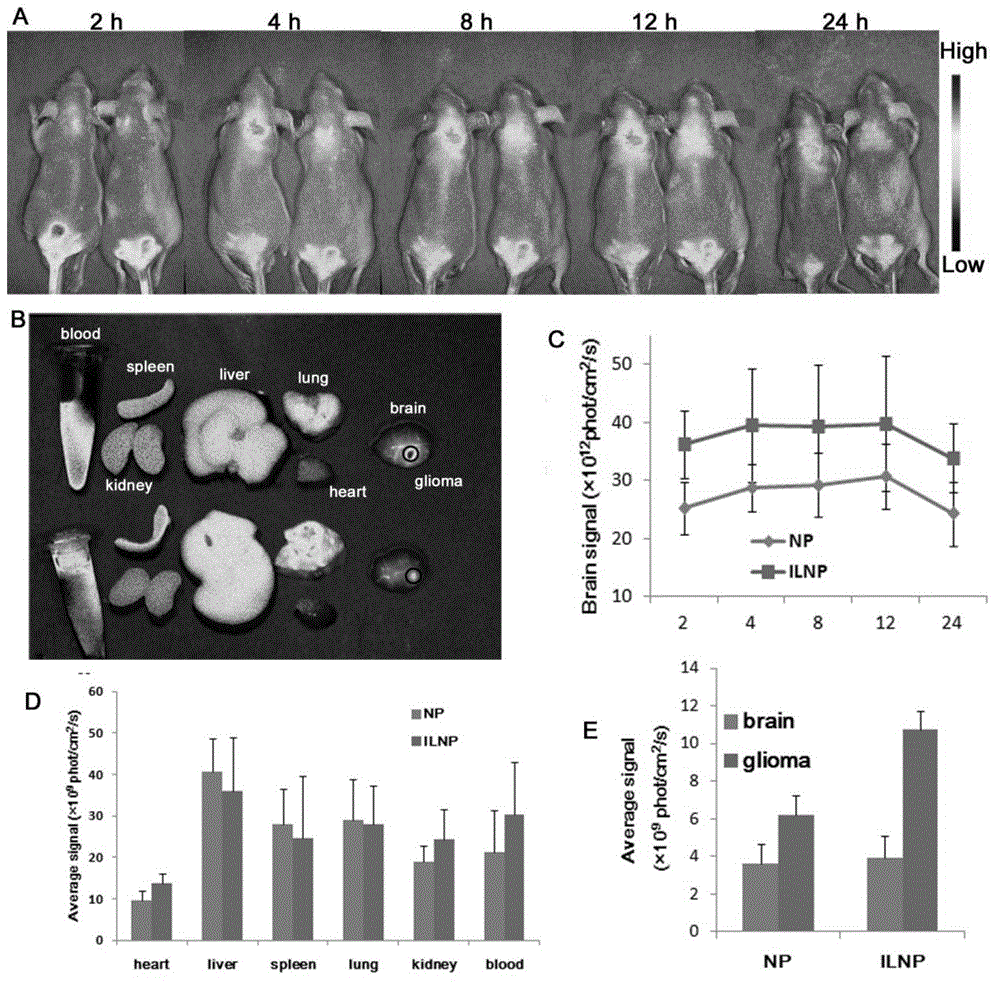
Targeting nanometer drug delivery system aiming at glioma
ActiveCN103622915AAchieving Targeted TherapyIncrease intakePowder deliveryMacromolecular non-active ingredientsNanocarriersPolyethylene glycol
The invention belongs to the field of medicine preparation, and in particular to a targeting nanometer drug delivery system aiming at glioma, and a preparation method and application thereof. The drug delivery system comprises target functional molecules, a drug and nano carriers. The target functional molecules are from short chain polypeptide from interleukin 13; the drug is a micromolecular anti-glioma drug; the nano carriers are liposome with surface modified by polyethylene glycol, nanoparticles, polymeric vesicles, polymer micelles and solid lipid nanoparticles; and the drug is enveloped in the nano carriers in an enveloping or covalent connection manner, and the short chain polypeptide is connected with the polyethylene glycol on the surfaces of the nanoparticles through covalent connection. The drug delivery system can promote uptake of the glioma cells by mediated effect of an interleukin 13 receptor alpha 2 on the surfaces of the glioma cells, so as to improve the effect of anti-glioma chemotherapeutics.
Owner:FUDAN UNIV
Method and composition for treatment of renal disease with antibodies and their equivalents
InactiveUS20070218063A1Improve the quality of lifeMore pathogenetic treatmentPeptide/protein ingredientsImmunoglobulins against animals/humansInterleukin 6Interleukin 10
A method for treating renal disease includes administering to a patient suffering from renal disease an effective amount of antibody or of a functional equivalent thereof to at least two of urea, creatinine, tumor necrosis factor alpha, interferon gamma, interleukin 6 and interleukin 1 beta. Soluble cytokine receptors also can be employed. Antibody, functional equivalent thereof or soluble cytokine receptor to interleukin 10 or interleukin 13 also can be administered. The method can be used as a supplement to or as partial or complete replacement for dialysis. A pharmaceutical composition includes antibody or functional equivalent thereof to urea, creatinine, or both; antibody, functional equivalent or soluble cytokine receptor to tumor necrosis factor alpha, interferon gamma, interleukin 6, interleukin 1 beta or any combination thereof; and, optionally, antibody, functional equivalent or soluble cytokine receptor to interleukin 10, interleukin 13 or both. The composition can be included in a kit.
Owner:SKURKOVICH BORIS +2
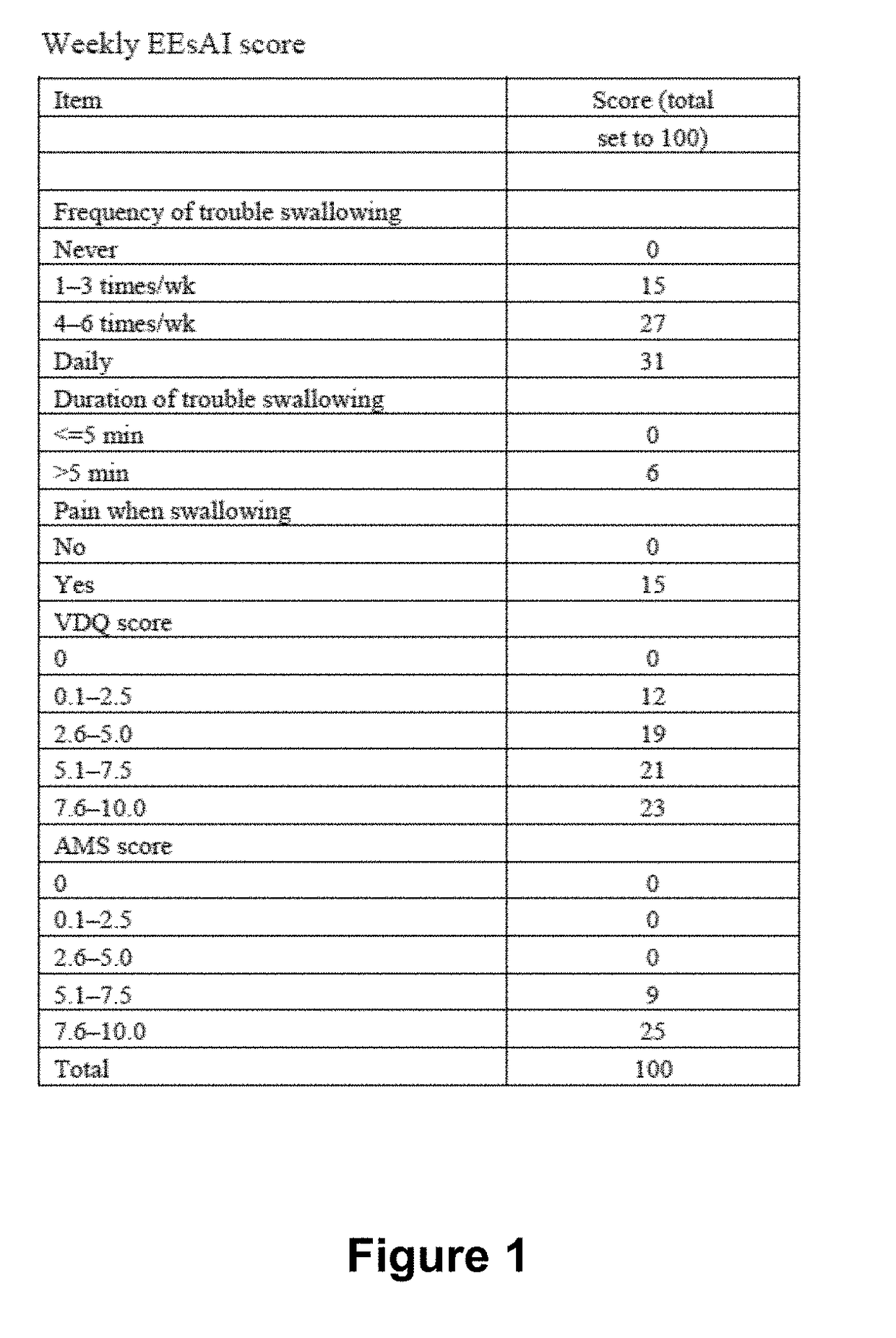
Methods for treating active eosinophilic esophagitis
ActiveUS20190040126A1Improve acceleration performanceImprovement of biomarker levelHybrid immunoglobulinsDigestive systemWhite blood cellInterleukin 13
The present invention provides methods for treating, preventing or reducing the severity of active eosinophilic esophagitis. In certain embodiments, the present invention provides methods of increasing esophageal distensibility. The methods of the present invention comprise administering to a subject in need thereof a therapeutic composition comprising an interleukin-4 / interleukin-13 (IL-4 / IL-13) pathway inhibitor such as an anti-IL-4R antibody.
Owner:REGENERON PHARM INC +1
Methods and Compositions for Reducing Interleukin-4 or Interleukin-13 Signaling
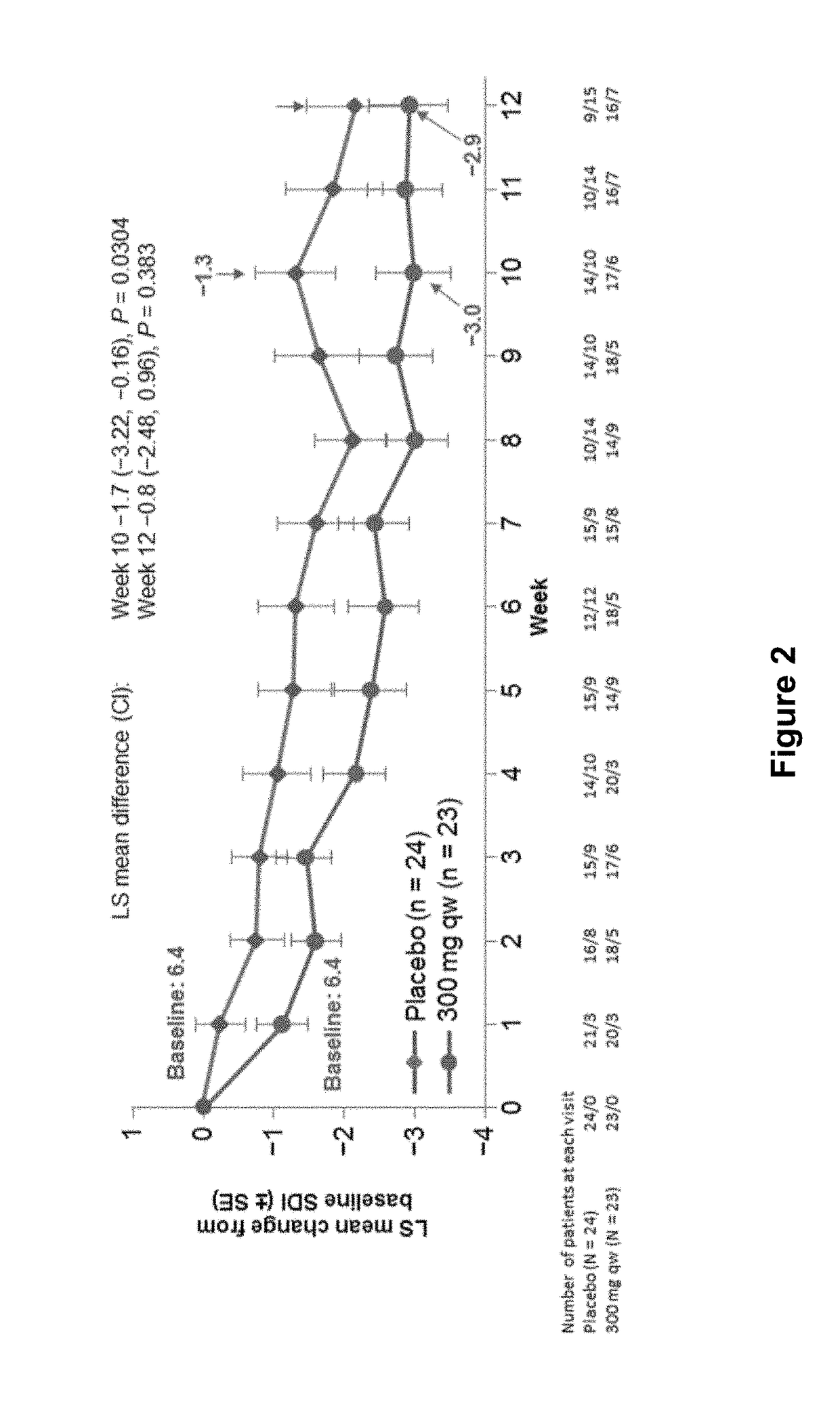
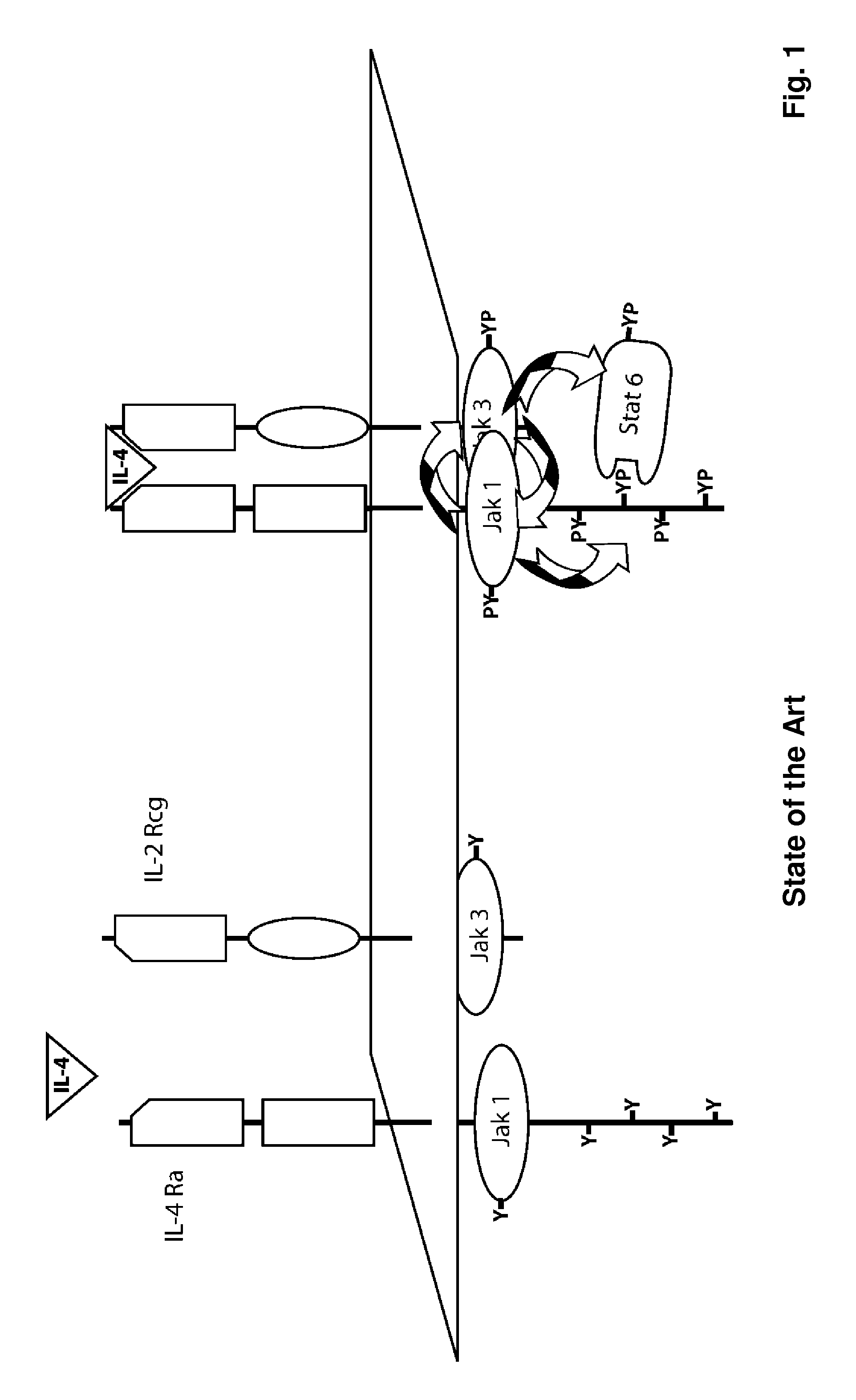
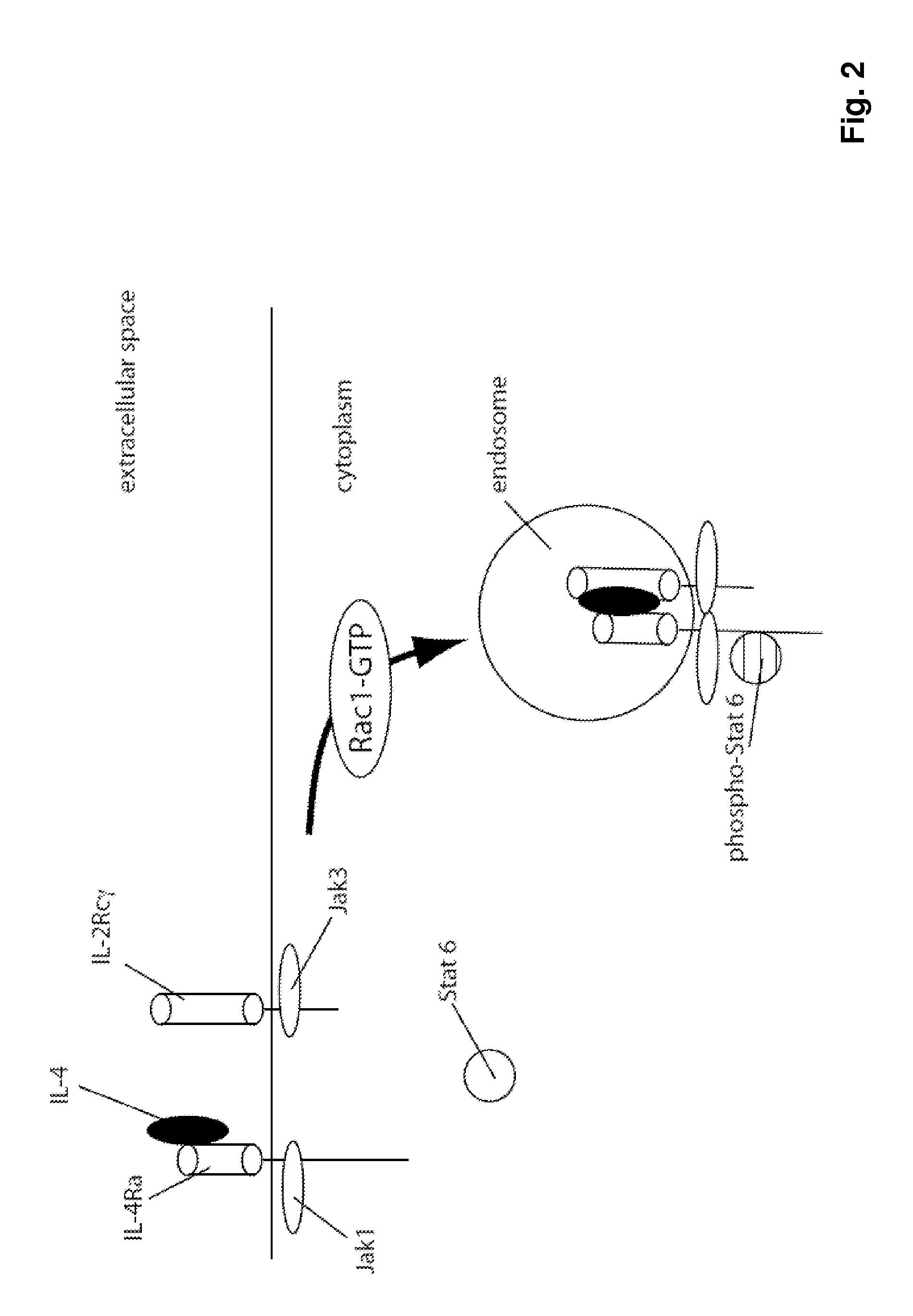
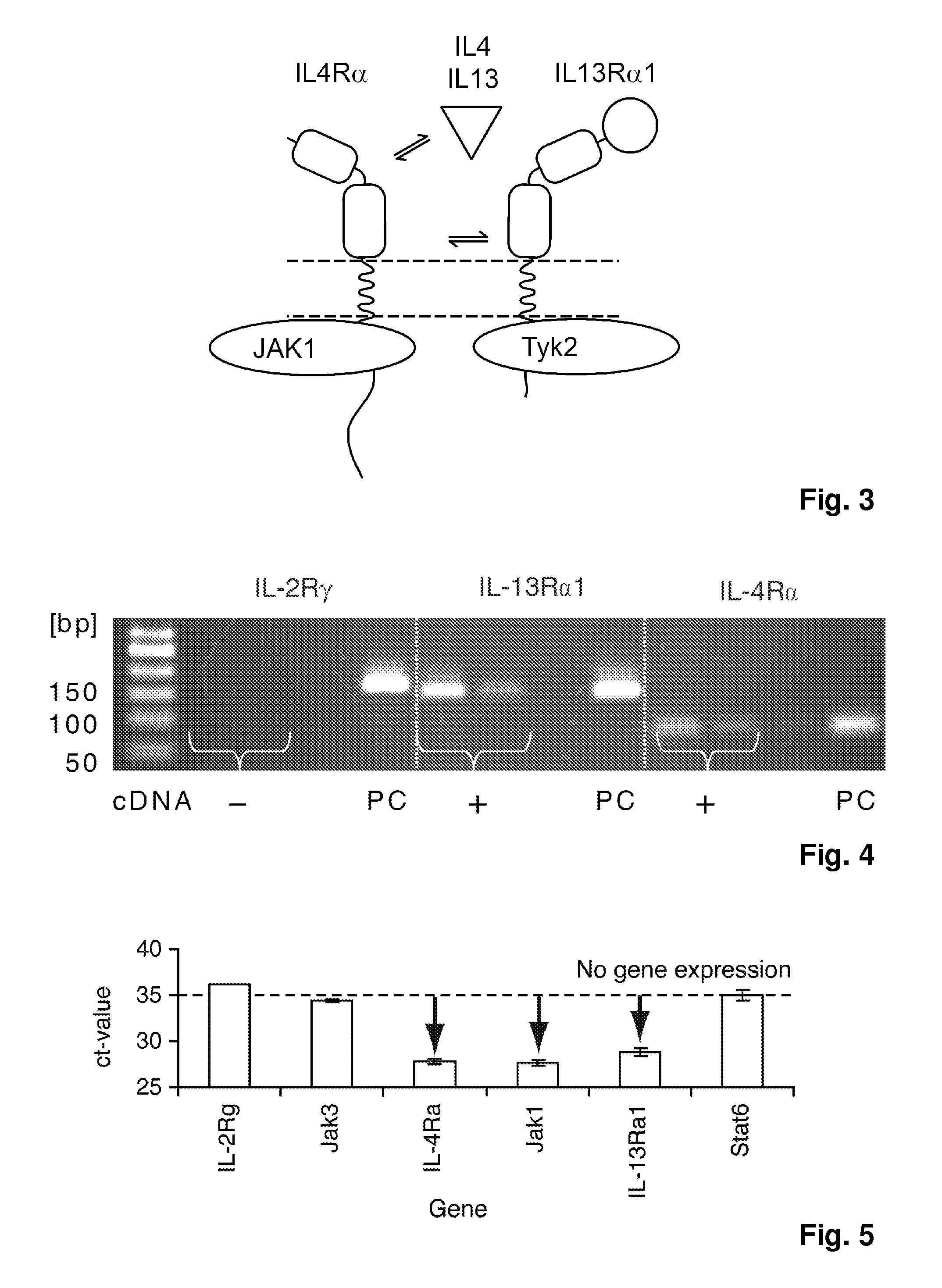
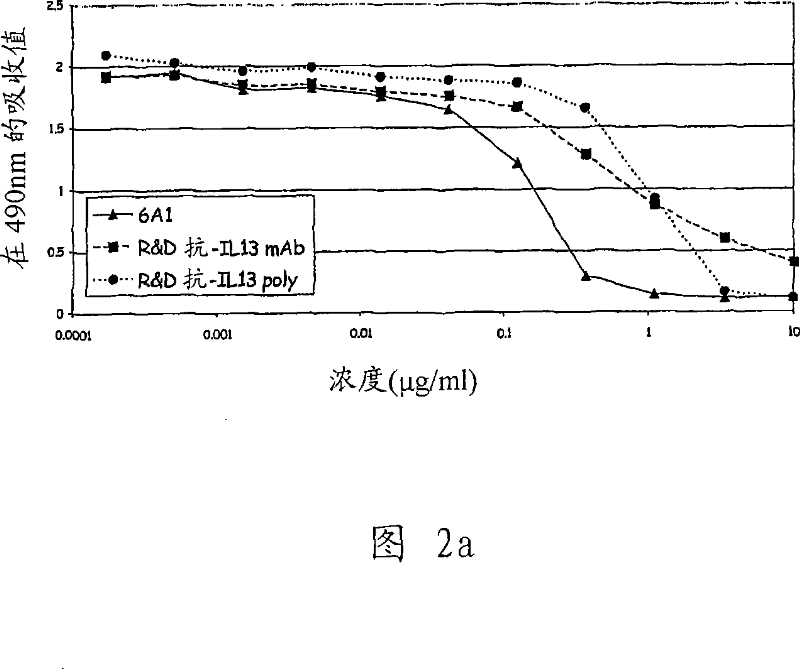
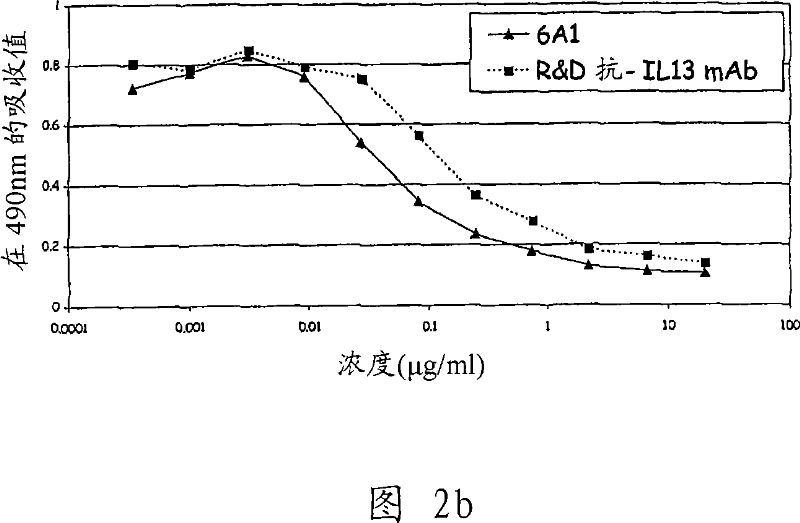
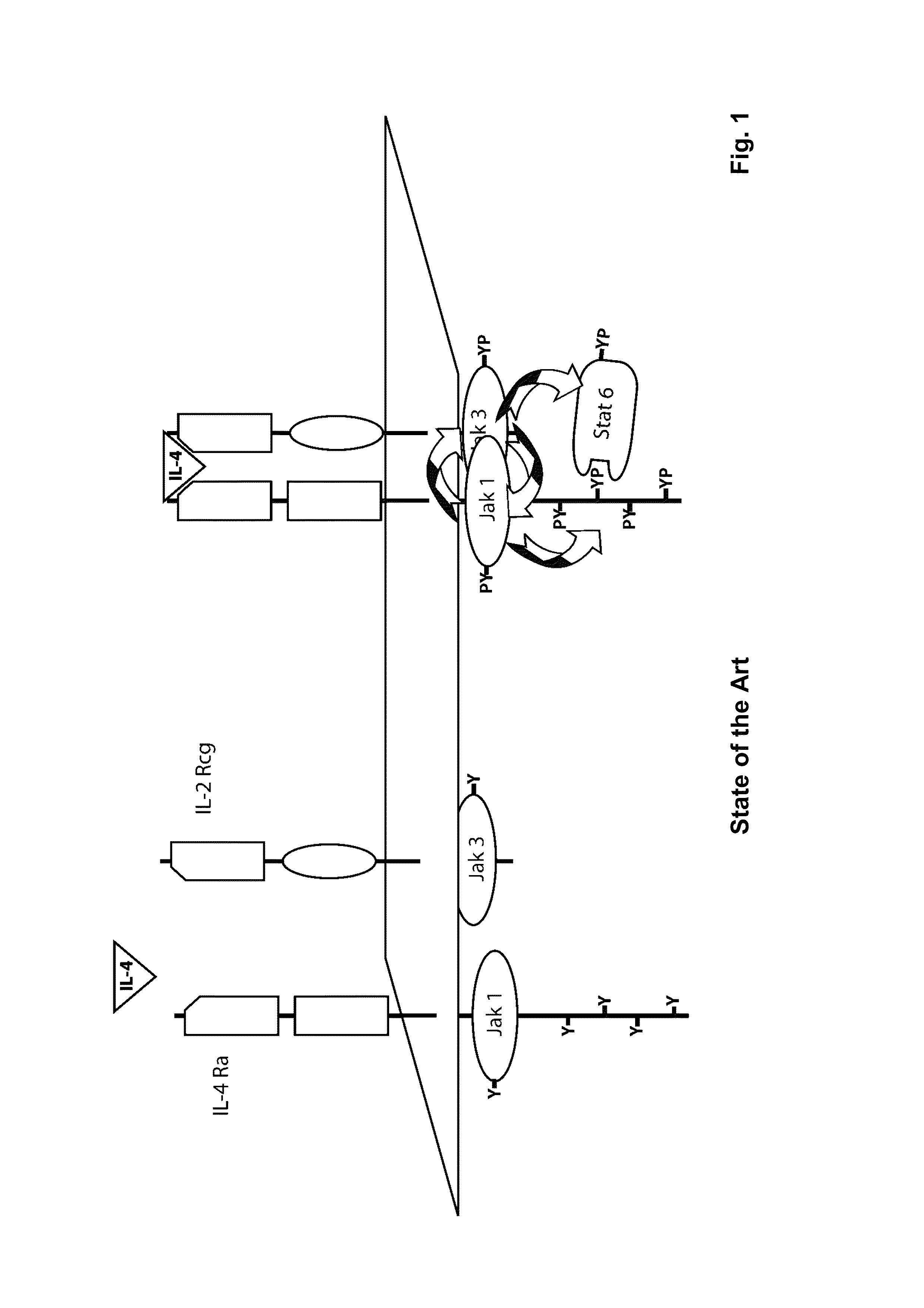
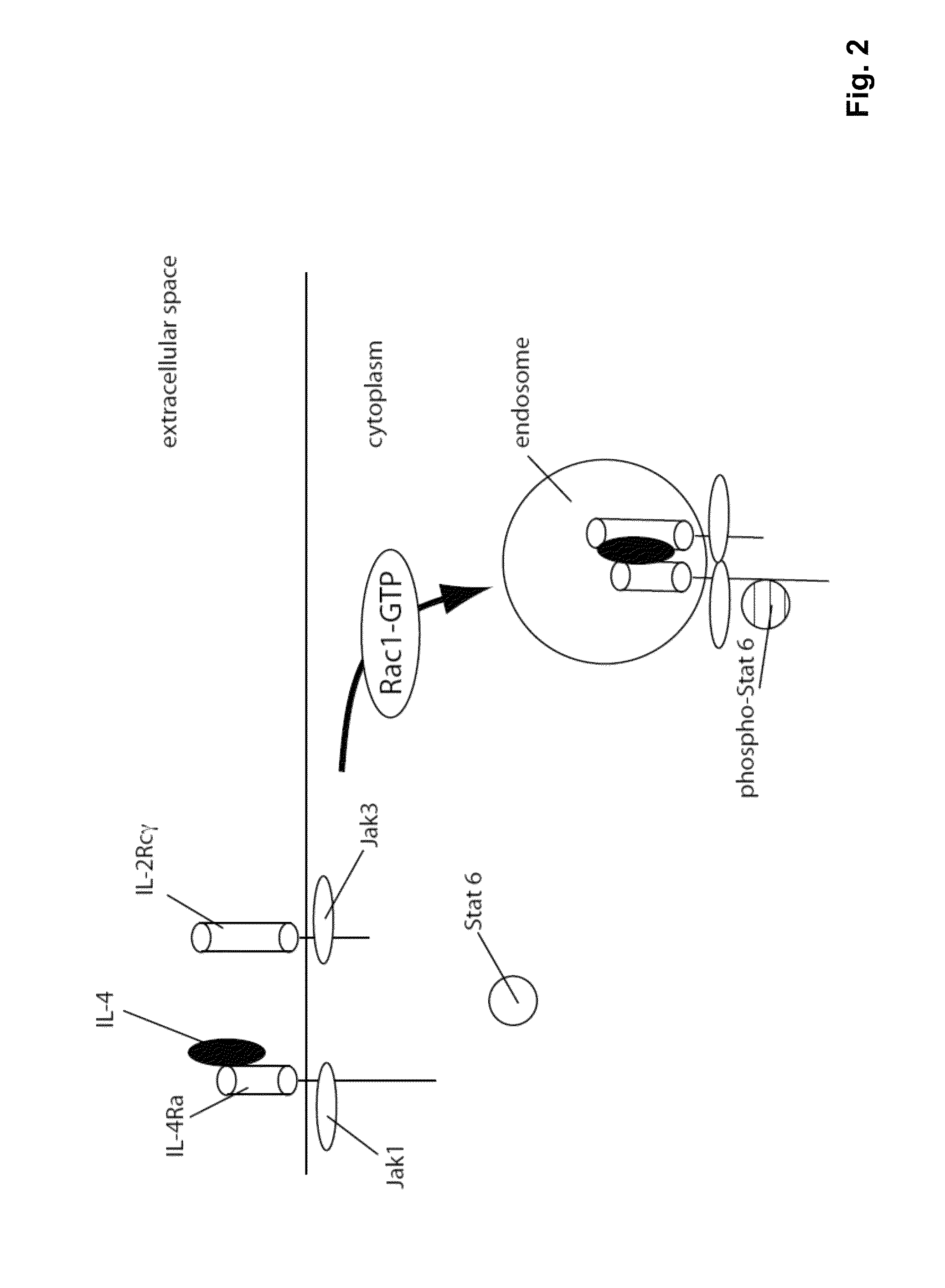
The present invention relates generally to methods and compositions for reducing Interleukin-4 or Interleukin-13 signaling, in particular to treat asthma and atopic dermatitis. The inventors have found that Rac / PAK mediated endocytosis of the ligand bound type I (IL-4R with the chains IL-4Ra and IL-2-Rg) and / or type II receptor (IL-13R with the chains IL-4Ra and IL-13Ra1) is needed for the IL-4 and / or IL-13 mediated activation of downstream signalling events including phosphorylation of Stat family transcrition factors. These discoveries enable new methods of screening compounds that modulate Interleukin-4 and Interleukin-13 signalling, as well as new methods for treating conditions characterized by increased Interleukin-4 and Interleukin-13 levels. These conditions include inflammatory conditions, asthma bronchiale, atopic dermatitis, allergies, atopic syndromes, allergic rhinitis, and th2-induced conditions.
Owner:DRESDEN UNIVERSITY OF TECHNOLOGY
Chimeric and Humanised Monoclonal Antibodies Against Interleukin-13
The present invention concerns immunoglobulins, particularly antibodies which specifically bind human Interleukin 13 (hIL-13). Antibodies of the invention may be used in the treatment of a variety of diseases or disorders responsive to modulation of the interaction between hIL-13 and the human IL-13 receptor. Such diseases include severe asthma, atopic dermatitis, COPD and various fibrotic diseases. Pharmaceutical compositions comprising said antibodies and methods of manufacture are also disclosed.
Owner:GLAXO GROUP LTD
Interleukin-13 as a cardiovascular disease marker
InactiveUS20060188504A1Parenterally administerDosing level be limitImmunoglobulins against cytokines/lymphokines/interferonsAntibody ingredientsInterleukin 13Antibody
The present invention relates to use of interleukin-13 as a cardiovascular disease marker. The therapeutic composition and diagnostic composition of the invention for cardiovascular diseases are characterized by comprising an antibody to an interleukin-13 receptor and / or an antibody to interleukin-13.
Owner:SAGA UNIVERSITY
CANINE IgG NUCLEIC ACID MOLECULES
The invention relates to canine immunoglobulin G (IgG) and canine interleukin-13 receptors (IL-13R) as well as fusion proteins containing canine IgG and / or canine IL-13R. In particular, the present invention discloses nucleic acid molecules encoding canine IgG, including species-specific regions of the heavy chain of canine IgG, and canine IL-13R alpha chain (IL-13Rα) proteins, particularly canine interleuken receptor alpha 1 (IL-13Rα1) and canine interleuken receptor alpha 2 (IL-13Rα2) proteins. Also included are canine IgG and IL-13Rα proteins, antibodies having selectivity for such proteins, inhibitors of such proteins and / or nucleic acid molecules, cells transformed with said nucleic acid molecules, assays employing such cells, nucleic acids molecules, proteins, antibodies and / or inhibitors, and therapeutic compositions comprising said nucleic acids molecules, proteins, antibodies and / or inhibitors. Also included are kits containing said molecules or chimera thereof, including their use to evaluate and regulate an immune response in an animal.
Owner:MCCALL CATHERINE A +1
Medicine for treating asthma
PendingCN105617356AReduce airway hyperresponsivenessRelieve spasmsOrganic active ingredientsInorganic active ingredientsInterleukin 5Interferon alpha
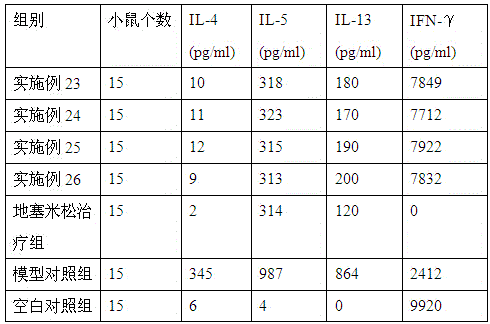
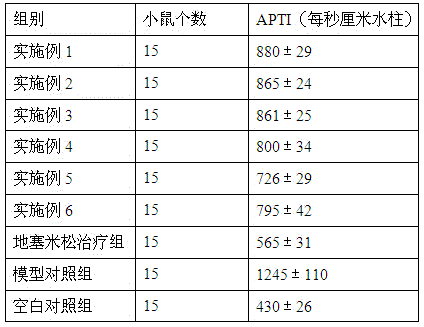
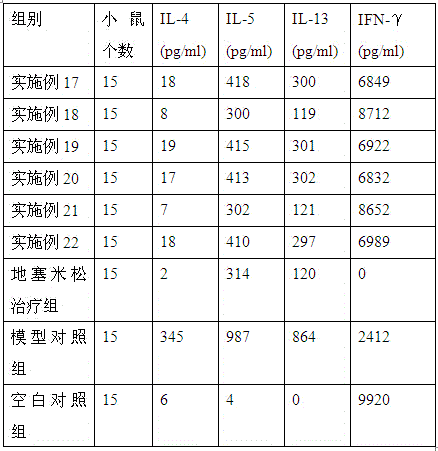
The invention provides medicine for treating asthma. The medicine is glycoprotein, mixture of polysaccharide and protein, polypeptide or protein. The medicine has the advantages that the bronchial hyperresponsiveness of a mouse asthma model can be effectively lowered, the tracheospasm can be relieved, and airway pressure-time index (APTI) is 726-880 per second centimeter water column; the inflammation in the lung can be treated effectively, inflammatory cell infiltration can be reduced, and the percentage of eosinophilic granulocyte is 14.6-16.6%; the gamma-interferon (IFN-gamma) and antibody which are capable of inhibiting asthmatic attack can be increased, and the contents of interleukin-4 (IL-4), interleukin-5 (IL-5) and interleukin-13 (IL-13) which can induce the asthma can be lowered; the medicine is safe, efficient, free of side effect, quick in action during asthma preventing and treatment, capable of relieving chest distress and short of breath and evident in curative effect on the asthma.
Owner:程潜
Low Molecular Weight Immune-Modulators As Adjuvants for Specific Immunotherapy
InactiveUS20160271257A1Lasting immunological toleranceControl developmentOintment deliveryPharmaceutical non-active ingredientsAntigenDisease
The invention relates to a pharmaceutical composition for local modulation of T cell and B cell responses at the site of allergen or antigen presentation, made of one or more preparations comprising one or more antigens or allergens, and a therapeutically effective dose of two or more low molecular weight immune modulators selected from four groups of tolerance-inducing therapeutics comprising inhibitors of complement-mediated functions, inhibitors of tumor necrosis factor receptor 1 (TNFR1)-mediated functions, inhibitors of interleukin 4- and interleukin 13-mediated functions, and therapeutic agents suitable for vitamin D3 supplementation wherein preferably two or more low molecular weight immune modulators and one or more antigens or allergens are coated or adsorbed on or embedded in a matrix, wherein the matrix is selected as to enable sustained release of one or more antigens or allergens and two or more immune modulators for the treatment of T cell-mediated diseases.
Owner:PLS DESIGN +2
Chimeric and humanised monoclonal antibodies against interleukin-13
InactiveCN101039960AImmunoglobulins against cytokines/lymphokines/interferonsImmunological disordersDiseaseCOPD
The present invention concerns immunoglobulins, particularly antibodies which specifically bind human Interleukin 13 (hIL-13). Antibodies of the invention may be used in the treatment of a variety of diseases or disorders responsive to modulation of the interaction between hIL-13 and the human IL-13 receptor. Such diseases include severe asthma, atopic dermatitis, COPD and various fibrotic diseases. Pharmaceutical compositions comprising said antibodies and methods of manufacture are also disclosed.
Owner:GLAXO GROUP LTD
Methods and compositions for reducing interleukin-4 or interleukin-13 signaling
The present invention relates generally to methods and compositions for reducing Interleukin-4 or Interleukin-13 signaling, in particular to treat asthma and atopic dermatitis. The inventors have found that Rac / PAK mediated endocytosis of the ligand bound type I (IL-4R with the chains IL-4Ra and IL-2-Rg) and / or type II receptor (IL-13R with the chains IL-4Ra and IL-13Ra1) is needed for the IL-4 and / or IL-13 mediated activation of downstream signalling events including phosphorylation of Stat family transcrition factors. These discoveries enable new methods of screening compounds that modulate Interleukin-4 and Interleukin-13 signalling, as well as new methods for treating conditions characterized by increased Interleukin-4 and Interleukin-13 levels. These conditions include inflammatory conditions, asthma bronchiale, atopic dermatitis, allergies, atopic syndromes, allergic rhinitis, and th2-induced conditions.
Owner:DRESDEN UNIVERSITY OF TECHNOLOGY
A method for diagnosing tuberculous meningitis
InactiveCN106461674AMicrobiological testing/measurementBiological material analysisVascular endotheliumVascular endothelial growth factor
A method for diagnosing tuberculous meningitis (TBM) is described herein. A fluid sample from a subject suspected of having TBM is tested for the presence of at least two of Interleukin-13 (IL-13), Vascular endothelial growth factor (VEGF), Cathelicidin (LL-37), IL- 7, IL-12p70, IFN-gamma, IL-6, IL-10, IL-13, IP-10, MIP-1a, MIP-1b, RANTES and GM-CSF. Increased levels of at least any two of these biomarkers in the sample compared to levels in subjects without TBM are indicative that the subject has TBM.
Owner:STELLENBOSCH UNIVERSITY
Compositions and methods for treatment of airway hypersecretion
InactiveUS20070082865A1Convenient treatmentGenetic material ingredientsAntibody ingredientsDiseaseMedicine
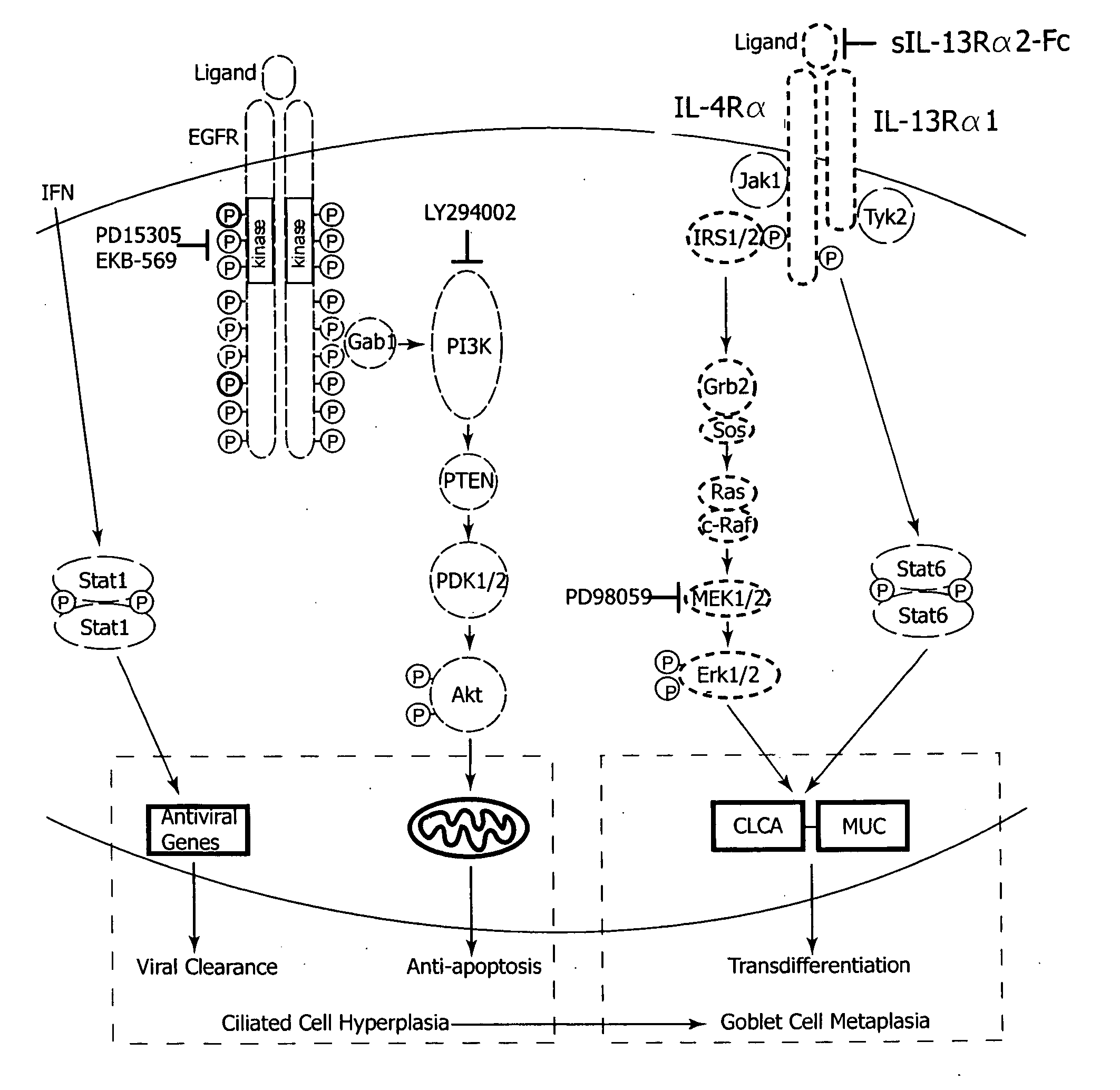
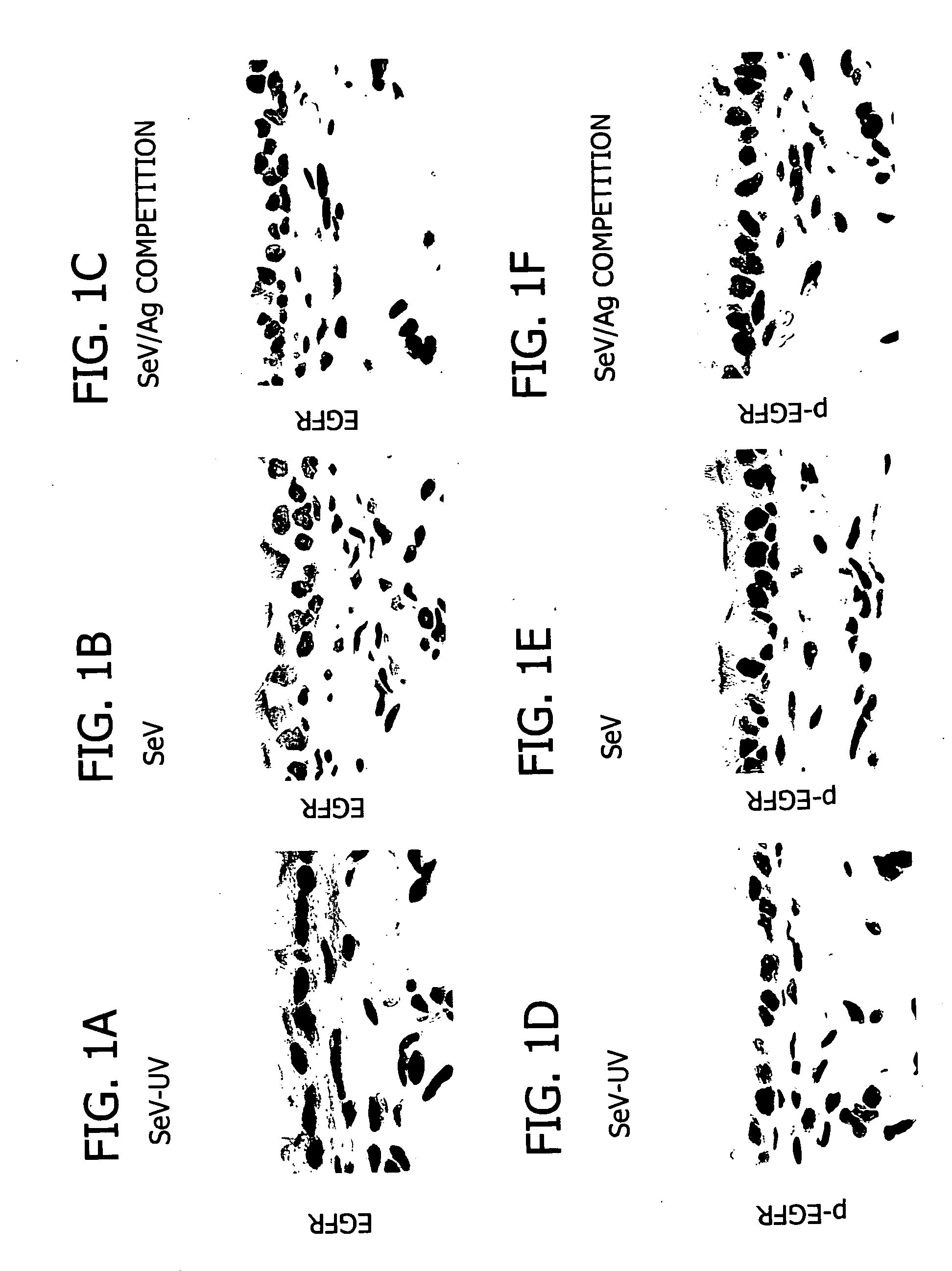
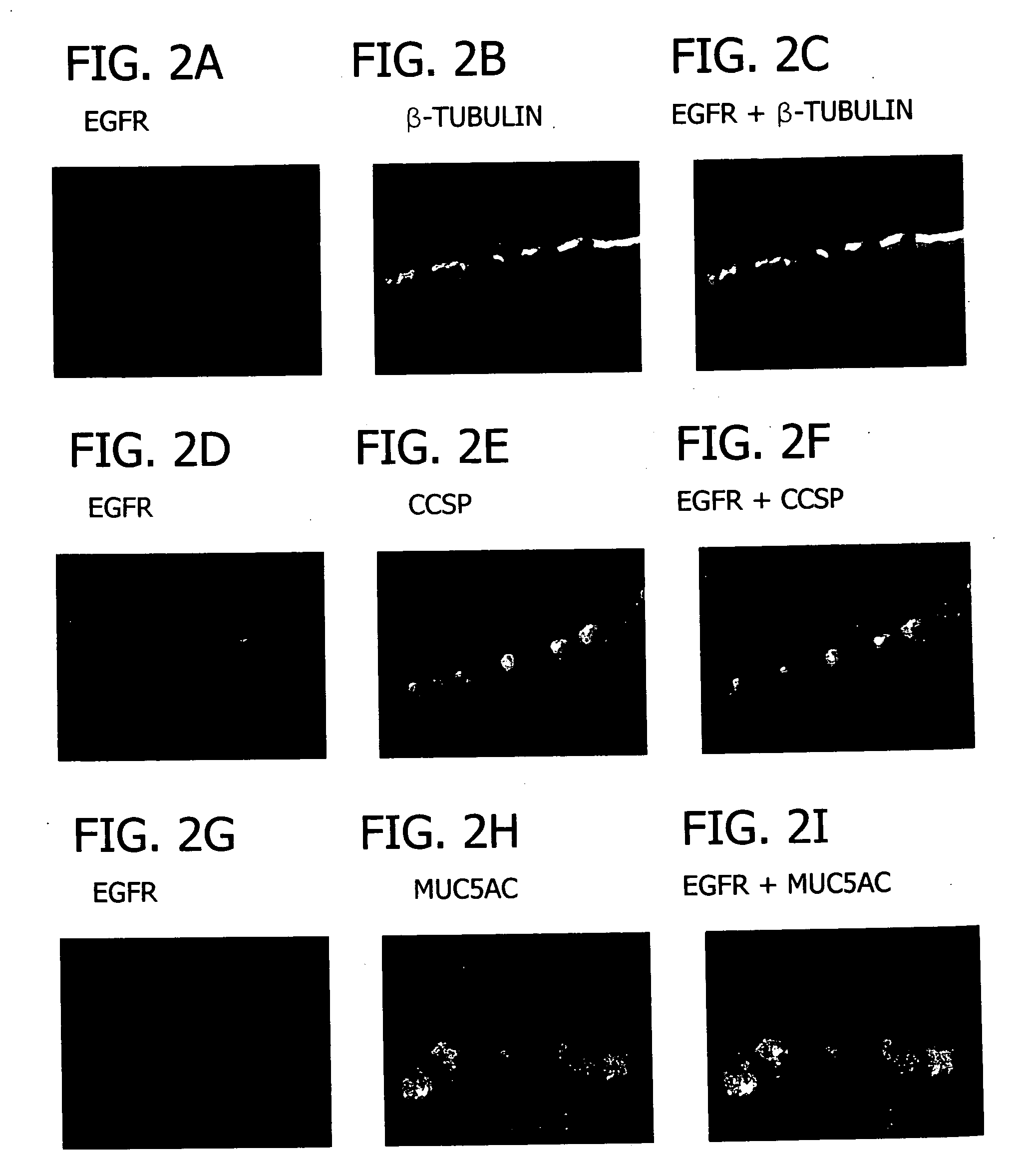
The invention relates generally to the field of treating pulmonary diseases. More specifically, the invention relates to the treatment of airway hypersecretion by the administration of an inhibitor of the epidermal growth factor receptor (EGFR) signaling pathway in combination with an inhibitor of the interleukin-13 (IL-13) signaling pathway, as well as compositions thereof.
Owner:WASHINGTON UNIV IN SAINT LOUIS
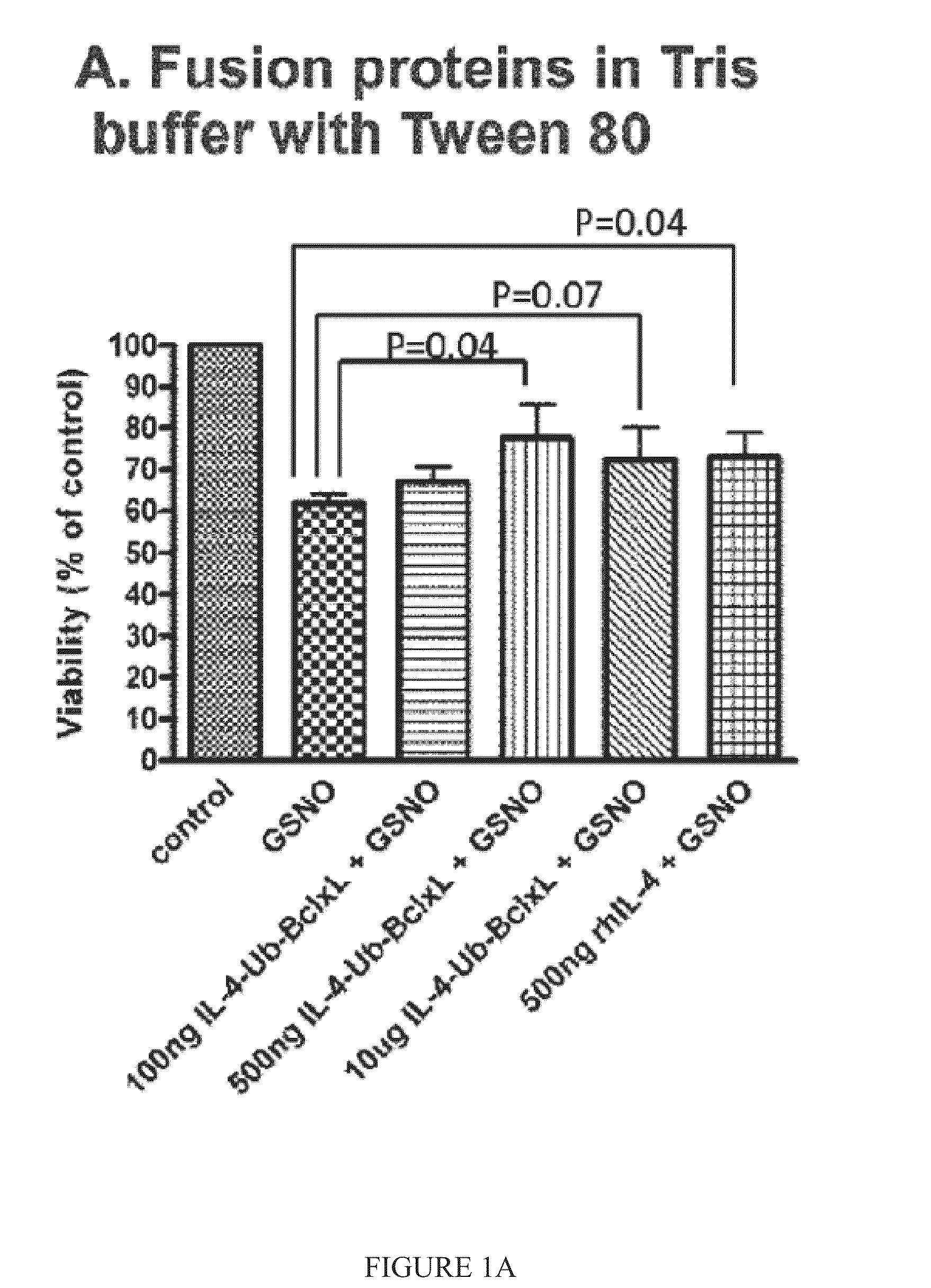
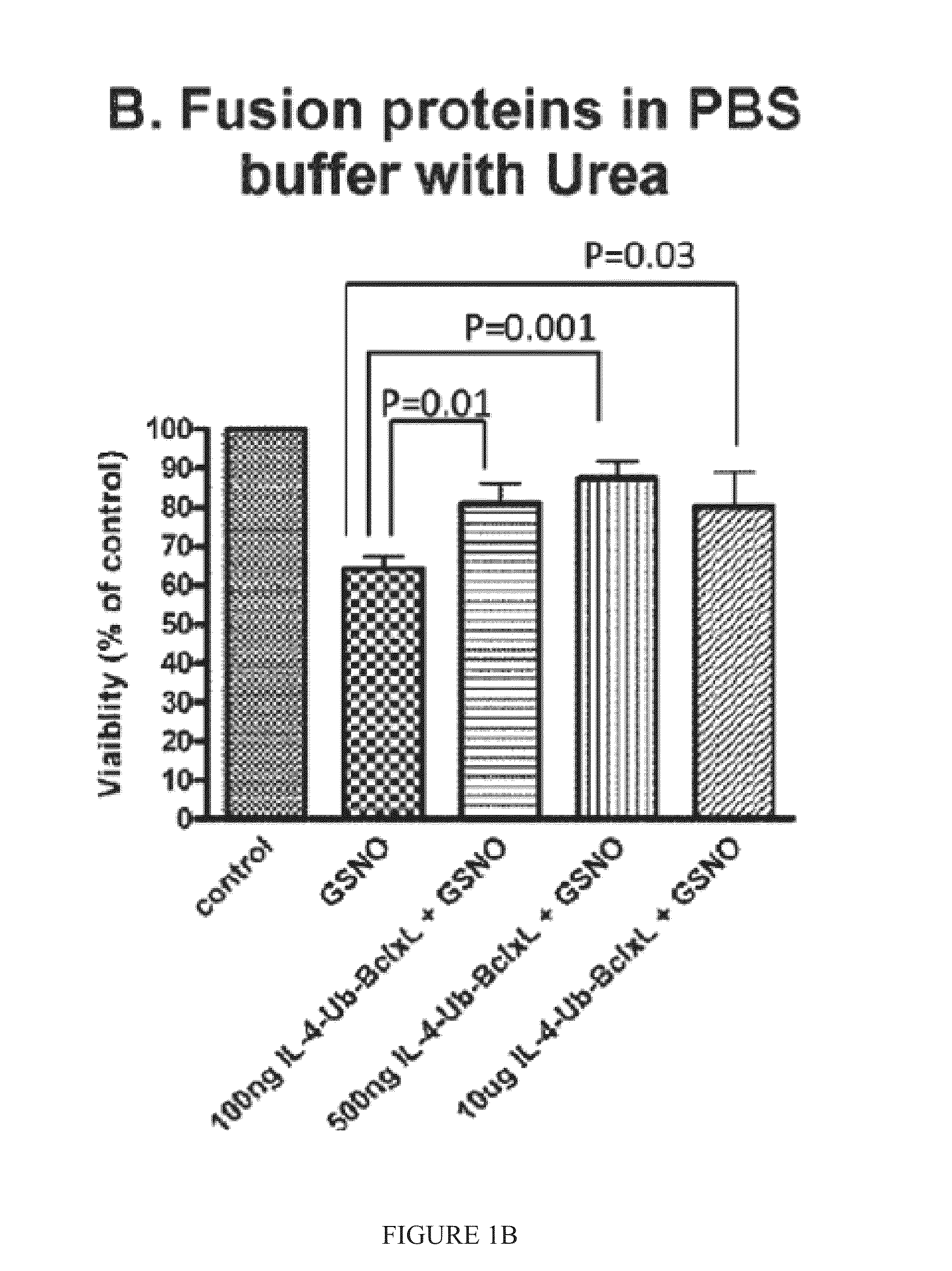
Interleukin-4 receptor-binding fusion proteins and uses thereof
ActiveUS20160237135A1Enhance cell viabilityIncreased activationSenses disorderNervous disorderWhite blood cellInterleukin 5
The present invention relates to interleukin-4 receptor-binding fusion proteins. More specifically, the invention provides, in part, fusion proteins that include an interleukin-4 or interleukin-13 protein moiety joined to an anti-apoptotic Bcl-2 family member protein moiety.
Owner:MEDICENNA THERAPEUTICS +1
Design and selection of medicaments that modulate the function and activity of interleukin 13
The present invention relates generally to the field of medicaments in the form of therapeutic molecules including inflammatory modulators and their design and selection. More specifically, the present invention relates to a target site on Interleukin 13 (IL- 13) by which a GAG molecule or polyanionic glycoconjugate or anionic polysaccharide modulates IL-13 activity or function, said target site selected from the list consisting of amino acids located in the AB loops and / or helix D of human IL- 13 or its homolog or derivative, and the use of said IL- 13 target site to design a medicament for modulating physiological processes. Therapeutic and prophylactic compositions comprising the designed medicaments are also contemplated.
Owner:格莱坎生物科学股份有限公司
Fusion protein comprising il13
ActiveUS20210214411A1Chronic neuropathic painNervous disorderPeptide/protein ingredientsInterleukin 10White blood cell
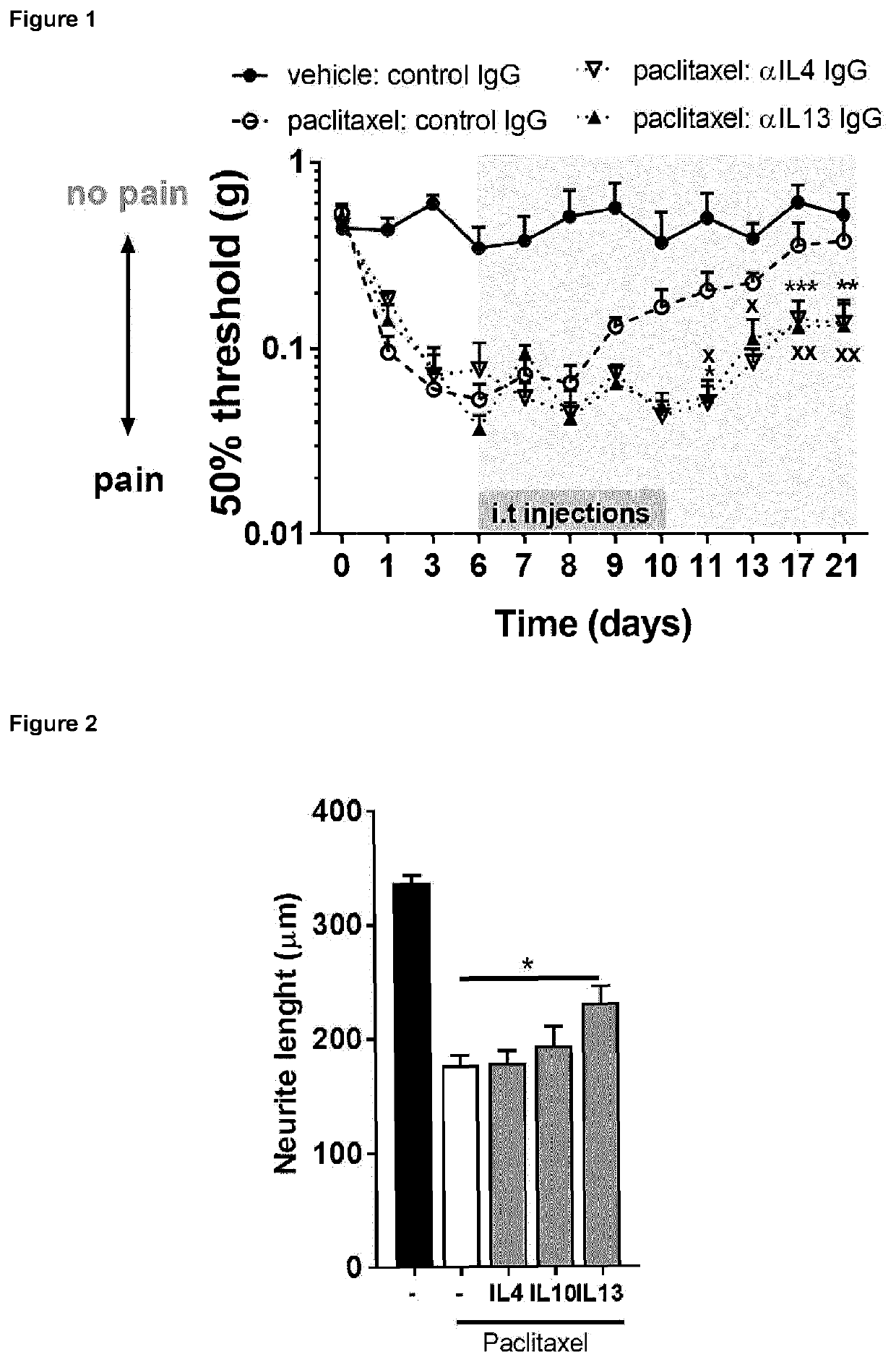
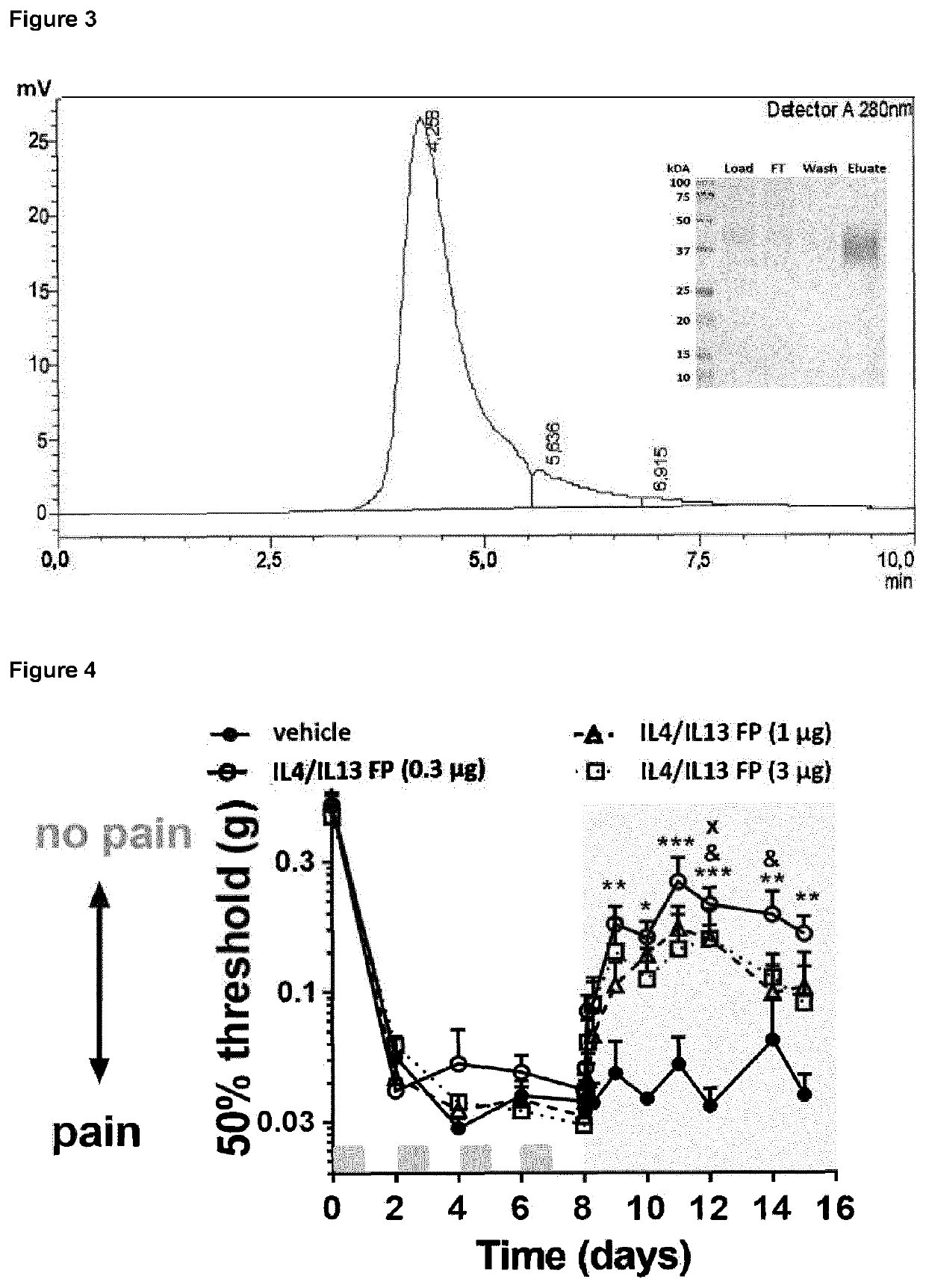
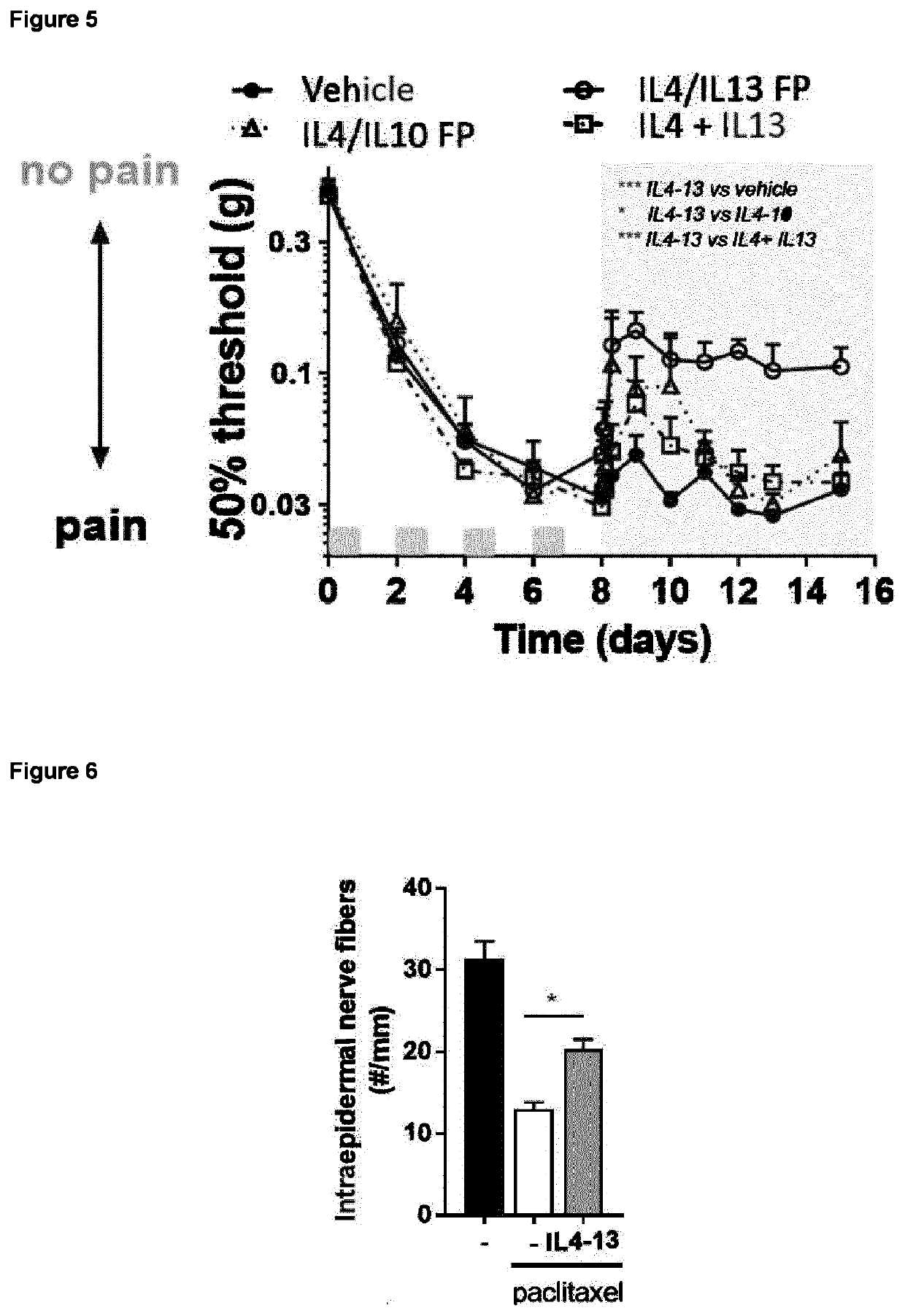
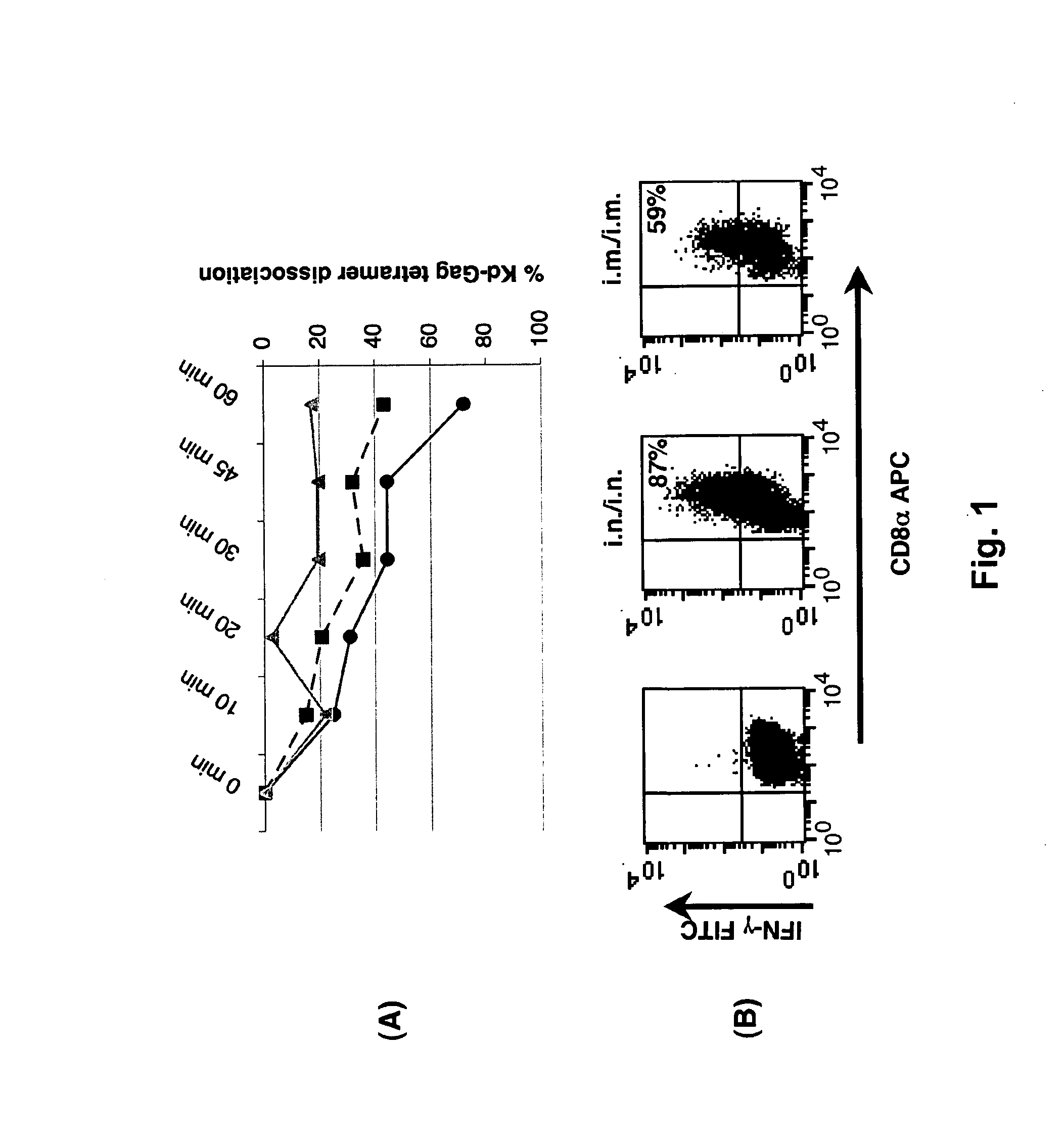
The invention is concerned with a fusion protein comprising interleukin 13 and a regulatory cytokine, for example, an interleukin chosen from interleukin 4, interleukin 10, interleukin 27, interleukin 33, transforming growth factor beta 1, transforming growth factor beta 2, and interleukin 13, a nucleic acid molecule encoding such fusion protein, a vector comprising such nucleic acid molecule, and a host cell comprising such nucleic acid molecule or such vector. The invention further pertains to a method for producing such fusion protein. The fusion protein or a gene therapy vector encoding the fusion protein may be used in the prevention or treatment of a condition characterized by pathological pain, chronic pain, neuro-inflammation and / or or neurodegeneration.
Owner:SYNERKINE PHARMA BV
Skin care product for promoting RNA transcription and preparation process of skin care product
PendingCN111773139APrevent agingIncrease contentCosmetic preparationsToilet preparationsEthylhexyl palmitateMessenger RNA
The invention discloses a skin care product for promoting RNA transcription. The skin care product comprises the following components in parts by mass: 2.3 to 5.1 parts of triglyceride decanoate, 4 to6 parts of squalane, 2 to 3.6 parts of ethylhexyl palmitate, 5 to 7.3 parts of isopropyl myristate, 3.2 to 4.4 parts of mink oil, 1.3 to 3.3 parts of PEG-7 glyceryl cocoate, 20 to 40.8 parts of deionized water, 5 to 7 parts of seaweed oligosaccharin, 7 to 10 parts of glycerol, 2.3 to 3.6 parts of microorganism C, 5.5 to 8.6 parts of retinol, 3.8 to 6.5 parts of peptide, 3.3 to 4.5 parts of coenzyme Q10, 20 to 30 parts of recombinant human III type human-like collagen and 1.5 to 3.4 parts of interleukin 13. The invention further provides a preparation process of the skin care product for promoting RNA transcription. A preparation method comprises the following steps of S1, selecting 2.3 to 5.1 parts of triglyceride decanoate, 4 to 6 parts of squalane, 2 to 3.6 parts of ethylhexyl palmitate, 5 to 7.3 parts of isopropyl myristate, 3.2 to 4.4 parts of mink oil and 1.3 to 3.3 parts of PEG-7 glyceryl cocoate, and starting steam for heating; S2; S3; S4; and S5. The skin care product is reasonable in structure; transcription of type III collagen genes can be accelerated; the higher the transcription number is, the more generated messenger RNA is; and the content of skin collagen is greatly increased accordingly.
Owner:上海兰葹生物科技有限公司
Ligands that bind il-4 and/or il-13
InactiveCN101578298AGood tissue permeabilityImmunoglobulins against cytokines/lymphokines/interferonsAntibody ingredientsAllergic asthmaInterleukin 13
Disclosed are ligands that have binding specificity for interleukin-4 (IL-4), for interleukin-13 (IL-13), or for IL-4 and IL-13. Also disclosed are methods of using these ligands. In particular, the use of these ligands for treating allergic asthma is described.
Owner:DORMANTIS LTD
Immunomodulating compositions comprising interleukin 13 inhibitors and uses therefor
InactiveUS20120177668A1Stimulate immune responsePeptide/protein ingredientsViral antigen ingredientsDiseaseWhite blood cell
This invention relates generally to compositions and methods for modulating immune responses. More particularly, the present invention relates to the co-expression, co-location or co-presentation on host cells (e.g. antigen-presenting cells, leukocytes, etc) of an inhibitor of IL-13 function and an immune stimulator that stimulates an immune response to a target antigen in compositions and methods for stimulating protective or therapeutic immune responses to the target antigen. The compositions and methods of the present invention are particularly useful in the prophylaxis and / or treatment of a range of diseases or conditions including pathogenic infections and cancers.
Owner:AUSTRALIEN NAT UNIV
Methods and antisera for isolating and identifying subsets of cd8 t cells
ActiveUS20170363626A1Strong specificityImprove utilizationMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsSerum igePresent method
Methods for identifying and isolating CD8 T cells that produce interleukin-13 upon activation are provided. The present methods leverage one or more newly-identified biomarkers to identify such CD8 T cells and, in certain cases, sort the same. Certain methods comprise obtaining a sample from a mammal, quantifying a level of expression of one or more biomarkers therein, and determining if the level of expression is elevated as compared, wherein an elevated expression level is indicative of an active disease state. Antisera and antibodies are also provided. In particular, an anti-C10orf128 antiserum formulated against a particular peptide is provided, such anti-C10orf128 antiserum characterized in that it identifies a subset of CD8 T cells that produce interleukin-13 upon activation.
Owner:JOHNSON RAYMOND M
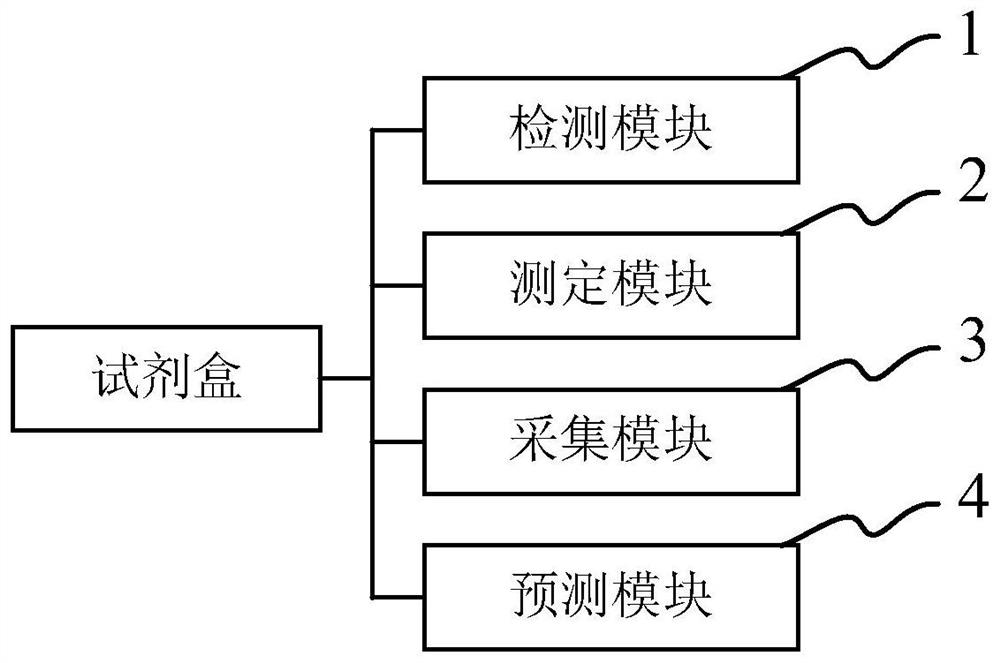
Kit for predicting lung injury clinical progress of patient with chest tumor after radiotherapy
PendingCN111912973AEffective clinical interventionBiological testingInterleukin 6Pulmonary infection
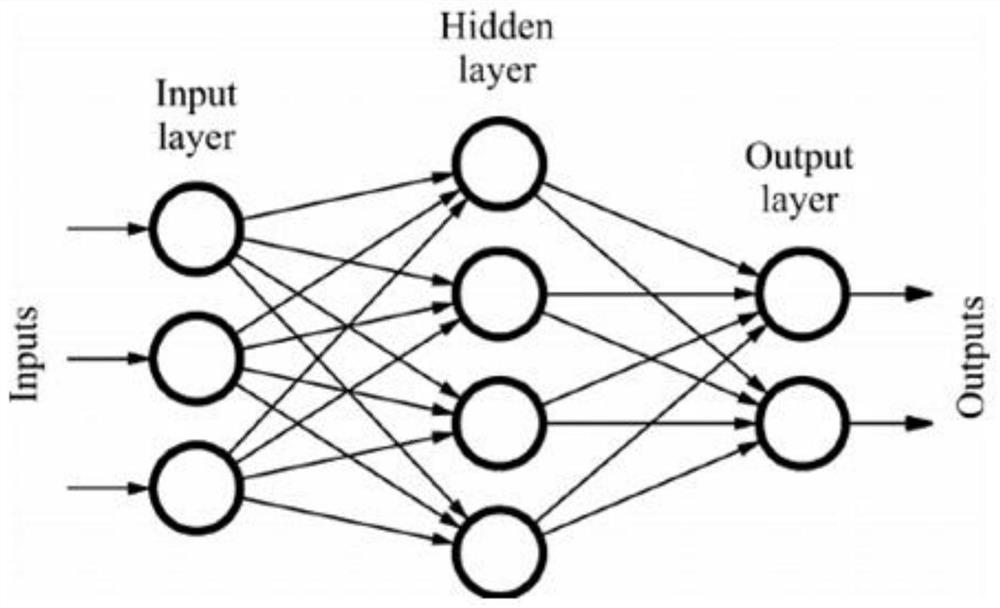
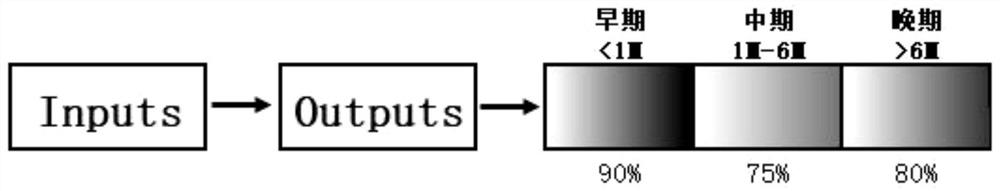
The invention discloses a kit for predicting the lung injury clinical progress of a patient with chest tumor after radiotherapy. A detection module detects the number and proportion of seven immune cells in peripheral blood of the patient with chest tumor, and the seven immune cells comprise NK cells, lymphocytes, granulocytes, monocytes, DC cells, T lymphocytes and B lymphocytes; a measuring module is used for measuring the concentrations of five cytokines in peripheral blood, and the five cytokines comprise interleukin 4, interleukin 6, interleukin 8, interleukin 13 and gamma interferon; anacquisition module acquires the clinical information of the patient withchest tumor, and the clinical information comprises age, gender, weight, hypertension, diabetes, pulmonary infection, radiationfield and radiation dose; and a prediction module inputs the number and proportion of the seven immune cells, the concentrations of the five cell factors and the clinical information into a feedforward neural network for prediction, and predicts the probability of occurrence and severity of lung injury at different stages after radiotherapy of the patient with chest tumor .
Owner:上海焕一生物科技有限公司
Method for diagnosing sleep apnea
ActiveUS20170059587A1Readily apparentDisease diagnosisBiological testingBetatrophinBrain-derived neurotrophic factor
The method for diagnosing sleep apnea includes measuring concentrations of biomarkers in a patient's bodily sample. To determine whether a patient suffers from sleep apnea, or has a predisposition for developing sleep apnea, a sample from the patient is analyzed. If one or more of the following biomarker concentrations are found in the patient's sample, then the patient may be diagnosed as suffering from sleep apnea or having a predisposition for developing sleep apnea: between approximately 992.8 pg / mL and approximately 1309.6 pg / mL of adipsin; between approximately 1,640 pg / mL and approximately 2,900 pg / mL of betatrophin; between approximately 8,090.82 pg / mL and approximately 11,829.07 pg / mL of brain-derived neurotrophic factor (BDNF); between approximately 11.82 pg / mL and approximately 88.26 pg / mL of interleukin-13 (IL-13); between approximately 49.45 pg / mL and approximately 103.29 pg / mL of tumor necrosis factor-α (TNF-α); and between approximately 16.55 pg / mL and approximately 29.76 pg / mL of the protein encoded by Human DNAJC27.
Owner:ALTERKI ABDULMOHSEN EBRAHIM
Methods and materials for treating medical conditions
ActiveUS20170183402A1Rapidly and reliably identifiedReduce severityImmunoglobulins against cytokines/lymphokines/interferonsDisease diagnosisDiseaseInterleukin 5
This document provided methods and materials involved in treating medical conditions. For example, methods and materials for using anti-Interleukin 4, anti-Interleukin 5, and / or anti-Interleukin 13 antibodies to treat asthma in a mammal identified as having a Th2 immune response using a whole blood cell-based cytokine whole blood cell-based cytokine assay are provided.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Canine IL-13 receptor alpha-1 subunit nucleic acid molecules
The invention relates to canine immunoglobulin G (IgG) and canine interleukin-13 receptors (IL-13R) as well as fusion proteins containing canine IgG and / or canine IL-13R. In particular, the present invention discloses nucleic acid molecules encoding canine IgG, including species-specific regions of the heavy chain of canine IgG, and canine IL-13R alpha chain (IL-13Rα) proteins, particularly canine interleuken receptor alpha 1 (IL-13Rα1) and canine interleuken receptor alpha 2 (IL-13Rα2) proteins. Also included are canine IgG and IL-13Rα proteins, antibodies having selectivity for such proteins, inhibitors of such proteins and / or nucleic acid molecules, cells transformed with said nucleic acid molecules, assays employing such cells, nucleic acids molecules, proteins, antibodies and / or inhibitors, and therapeutic compositions comprising said nucleic acids molecules, proteins, antibodies and / or inhibitors. Also included are kits containing said molecules or chimera thereof, including their use to evaluate and regulate an immune response in an animal.
Owner:HESKA
Methods for treating medical conditions by anti-type 2 therapy
ActiveUS10654923B2Immunoglobulins against cytokines/lymphokines/interferonsDisease diagnosisDiseaseAntiendomysial antibodies
This document provided methods and materials involved in treating medical conditions. For example, methods and materials for using anti-Interleukin 4, anti-Interleukin 5, and / or anti-Interleukin 13 antibodies to treat asthma in a mammal identified as having a Th2 immune response using a whole blood cell-based cytokine whole blood cell-based cytokine assay are provided.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Fusion protein comprising IL13
The invention is concerned with a fusion protein comprising interleukin 13 and a regulatory cytokine, for example, an interleukin chosen from interleukin 4, interleukin 10, interleukin 27, interleukin 33, transforming growth factor beta 1, transforming growth factor beta 2, and interleukin 13, a nucleic acid molecule encoding such fusion protein, a vector comprising such nucleic acid molecule, and a host cell comprising such nucleic acid molecule or such vector. The invention further pertains to a method for producing such fusion protein. The fusion protein or a gene therapy vector encoding the fusion protein may be used in the prevention or treatment of a condition characterized by pathological pain, chronic pain, neuro-inflammation and / or or neurodegeneration.
Owner:SYNERKINE PHARMA BV
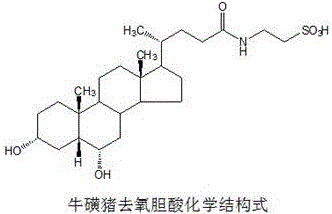
Application of pulvis fellis suis-combined type cholic acid in preparing drug for treating bronchial asthma
InactiveCN106389471AInhibition of secretionAvoid damageOrganic active ingredientsUnknown materialsMedicineHyodeoxycholic acid
The invention discloses the application of a pulvis fellis suis-combined type total cholic acid and taurohyodeoxycholic acid in preparing a drug for treating bronchial asthma. The result of the invention shows that, the pulvis fellis suis-combined type total cholic acid and taurohyodeoxycholic acid has an inhibiting effect on the increased content of immunoglobulin, interleukin 4 and interleukin 13 in a model mouse. Meanwhile, the pulvis fellis suis-combined type total cholic acid and taurohyodeoxycholic acid can inhibit the secretion of proinflammatory cytokine, regulate the immunoreaction and effectively prevent the damage of immunoreactivity to human bodies, so that the bronchial asthma can be treated. The prepared pulvis fellis suis-combined type total cholic acid and taurohyodeoxycholic acid is adopted as a raw material to prepare various types of common preparations, such as tablets, capsules, powders, pills, granules and the like.
Owner:NORTHWEST UNIV(CN)
A targeted nano drug delivery system for glioma
ActiveCN103622915BAchieving Targeted TherapyIncrease intakePowder deliveryMacromolecular non-active ingredientsLipid formationNanocarriers
The invention belongs to the field of medicine preparation, and in particular to a targeting nanometer drug delivery system aiming at glioma, and a preparation method and application thereof. The drug delivery system comprises target functional molecules, a drug and nano carriers. The target functional molecules are from short chain polypeptide from interleukin 13; the drug is a micromolecular anti-glioma drug; the nano carriers are liposome with surface modified by polyethylene glycol, nanoparticles, polymeric vesicles, polymer micelles and solid lipid nanoparticles; and the drug is enveloped in the nano carriers in an enveloping or covalent connection manner, and the short chain polypeptide is connected with the polyethylene glycol on the surfaces of the nanoparticles through covalent connection. The drug delivery system can promote uptake of the glioma cells by mediated effect of an interleukin 13 receptor alpha 2 on the surfaces of the glioma cells, so as to improve the effect of anti-glioma chemotherapeutics.
Owner:FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com