Application of pulvis fellis suis-combined type cholic acid in preparing drug for treating bronchial asthma
A combination of pig bile powder and combination technology, which can be used in drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., can solve problems that have not been reported, and achieve the effect of regulating immune response and blocking damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
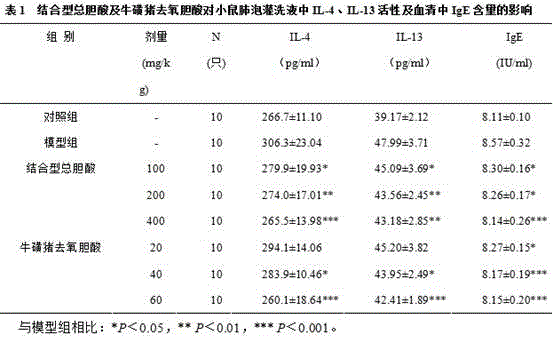
[0021] Taking pig gall powder 100 g as an example, the preparation method of pig gall powder-bound total cholic acid and taurohyodeoxycholic acid is as follows:
[0022] 1. Preparation of pig bile powder-bound total bile acid
[0023] Take 100 g of pig gall powder medicinal material, add 10 times its volume of ethanol with a mass fraction of 95%, ultrasonically extract to completely dissolve pig gall powder, let it stand for 12-14 hours, filter, and recover the solvent to obtain a brown-black extract. Take 5 g of extract, add 10 g of 100-160 mesh column chromatography silica gel to mix the sample, evaporate the solvent, grind into 80 mesh fine powder, and dry the fine powder and neutral alumina at a mass ratio of 1:15 for chromatography In the column, elute with ethanol with a mass fraction of 95%, collect the eluate, and spot the sample on a silica gel G thin-layer plate with a capillary tube. The volume ratio of ethyl acetate to methanol, water, and glacial acetic acid is 10...
Embodiment 2
[0027] The medicinal preparation porcine bile powder-bound total bile acid is prepared into tablets, taking the production of 1000 tablets as an example, the raw materials and auxiliary materials and their quality ratios are as follows:
[0028] Pork Gall Powder Bound Total Cholic Acid 50g
[0029] Dextrin 50g
[0031] Add starch to 300g
[0032] Its preparation method is carried out according to the conventional method of pharmacy tablets, each tablet weighs 0.3 g, and each tablet contains 50 mg of porcine bile powder-bound total cholic acid.
[0033] The medicinal preparation taurohyodeoxycholic acid is prepared into tablets, taking the production of 1000 tablets as an example, the raw materials and auxiliary materials and their quality ratios are as follows:
[0034] Taurohyodeoxycholic Acid 10g
[0035] Dextrin 50g
[0037] Add starch to 300g
[0038] Its preparation method is carried out according to the...
Embodiment 3
[0040] The medicinal preparation porcine bile powder-bound total bile acid is prepared into capsules, taking the production of 1000 capsules as an example, the raw materials and auxiliary materials and their quality ratios are as follows:
[0041] Pork Gall Powder Bound Total Cholic Acid 50g
[0042] Microcrystalline Cellulose 20g
[0044] Add starch to 300g
[0045] Its preparation method is carried out according to the conventional method of pharmacy capsules, each capsule weighs 0.3g, and each capsule contains 50mg of pig bile powder-bound total cholic acid.
[0046] The medicinal preparation taurohyodeoxycholic acid is prepared into capsules, taking the production of 1000 capsules as an example, the raw materials and auxiliary materials and their quality ratios are as follows:
[0047] Taurohyodeoxycholic Acid 10g
[0048] Microcrystalline Cellulose 20g
[0049] Magnesium Stearate 1g
[0050] Add starch to 300g
[0051] Its preparation m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com