Chimeric and humanised monoclonal antibodies against interleukin-13
A humanized antibody and humanized technology, applied in the field of immunoglobulin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
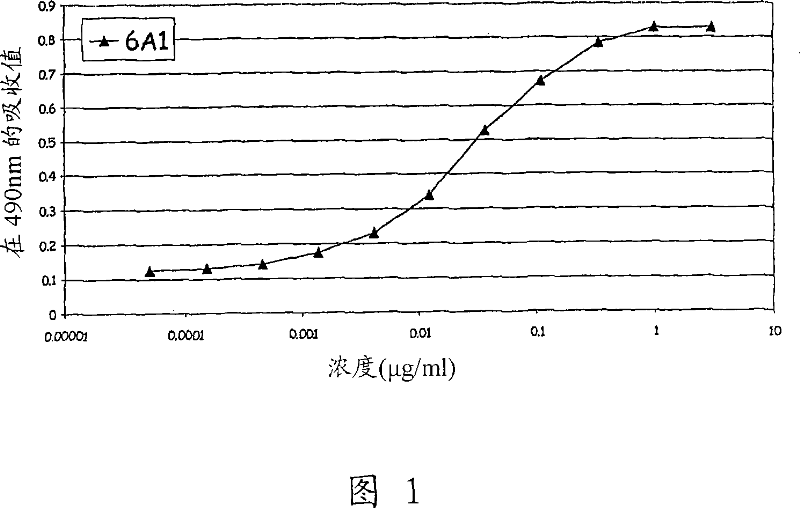
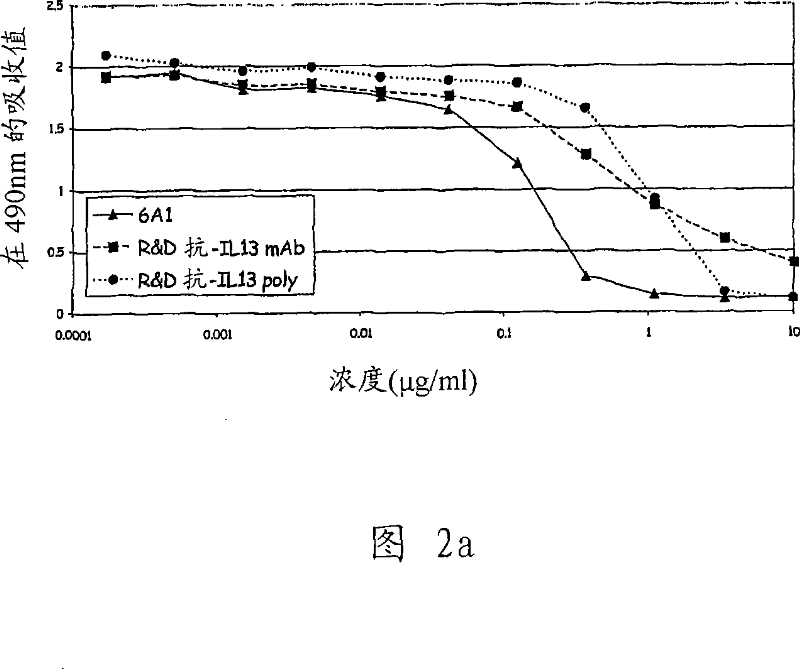
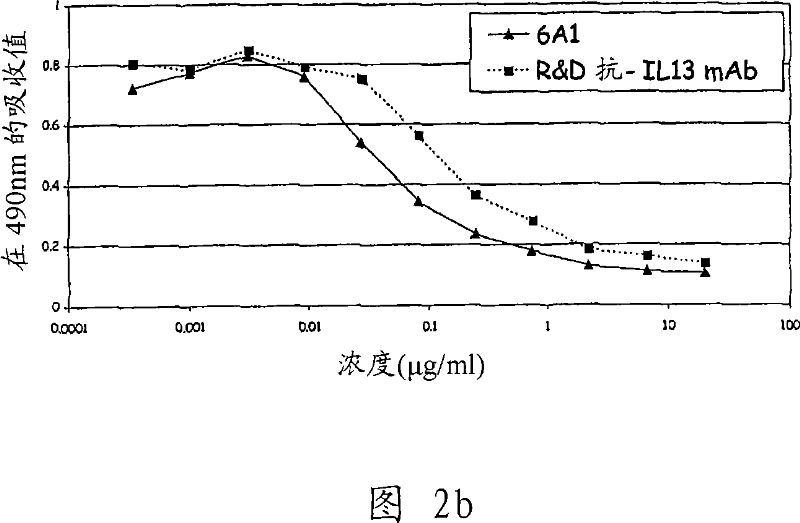
[0339] 1. Preparation of monoclonal antibody and study of mouse monoclonal antibody 6A1
[0340] Monoclonal antibodies (mAbs) are generally prepared from hybridoma cells as taught by E Harlow and D Lane in Antibodies a Laboratory Manual, Cold Spring Harbor Laboratory, 1988 . As a result, the fusion of mouse myeloma cells and mouse B lymphocytes immunized with the target antigen was obtained. Hybridoma cells acquire the ability to proliferate indefinitely with the help of myeloma fusion partners, while the ability to produce antibodies is provided by B lymphocytes.
[0341] Five SJL mice were immunized by intraperitoneal injection of 2 μg each of E. coli-derived recombinant human IL-13 (Cambridge Bioscience, Cat. No. CH-013). The vaccination procedure used produced high titer anti-human IL-13 antibody immune responses in mice. After 5 inoculations within 64 days, mice were sacrificed and splenocytes were collected. Splenocytes from three mice were removed, and B lymphocytes ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com