Design and selection of medicaments that modulate the function and activity of interleukin 13
A technology selected from and equivalent to, applied in the direction of interleukin, drug combination, molecular design, etc., can solve problems such as chemical obstacles of carbohydrate building blocks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
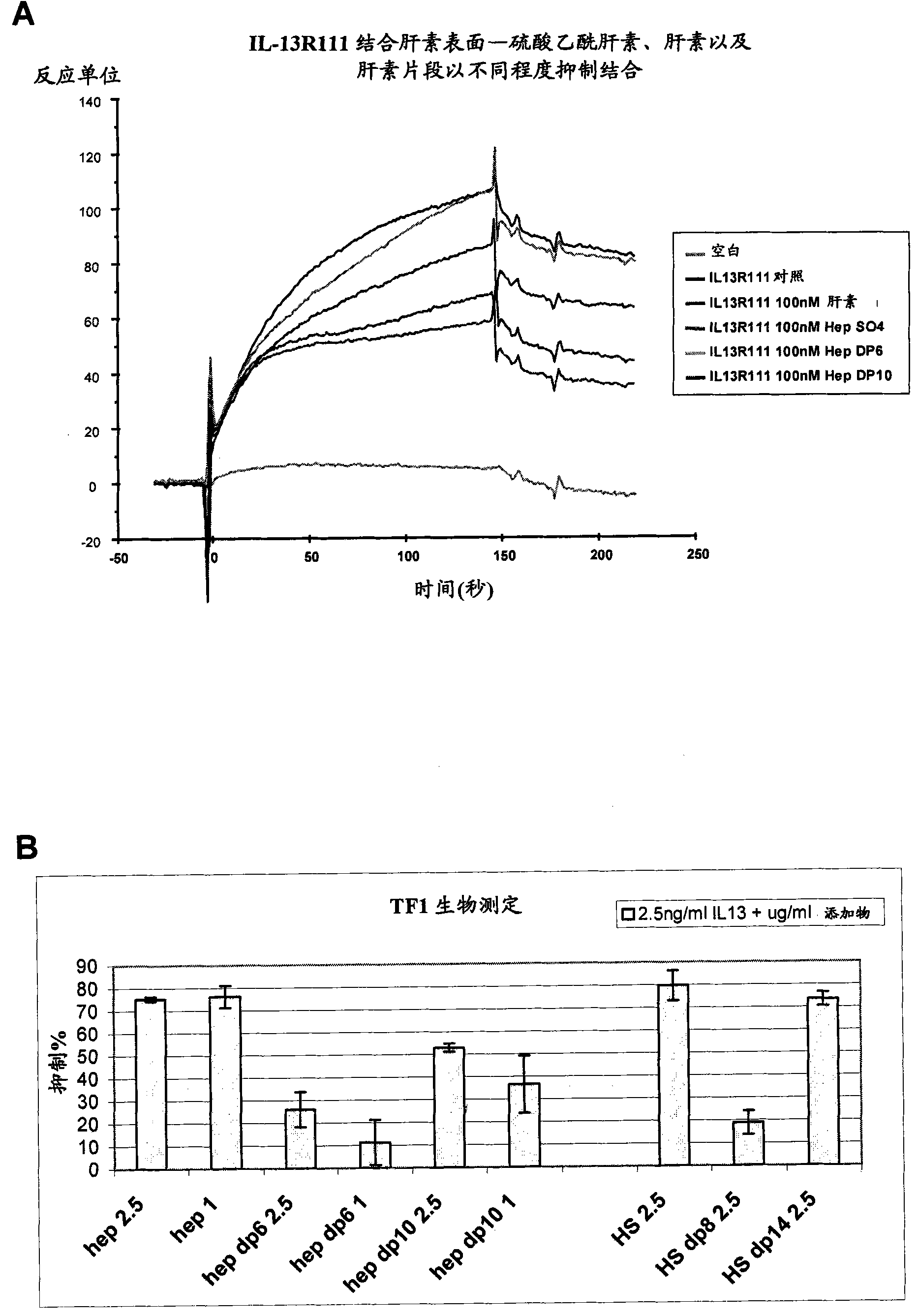
[0237] Sequence and structure of heparin and heparan sulfate
[0238]The most well-studied and best-understood sequence of heparin monosaccharide residues is the specific pentasaccharide as the minimum condition for high affinity to antithrombin. It is the sequence that accounts for the high anticoagulant potency of heparin and thus its use as an anticoagulant; the basic pentasaccharide has been made synthetically and is itself used as a drug (Petitou and van Boeckel, Angew. Chem. Int. Edit. 43:3118, 2004). When it became clear that heparin, as a model compound for heparan sulfate, was capable of important physiological interactions with other protein classes such as fibroblast growth factor (Mohammadi et al., Curr. Opin. Struct. Biol. 15:506, 2005), antithrombin Examples of binding sequences lead to the search for other equally specific sequences in heparin or heparan sulfate that confer a specific affinity for any given binding partner. The search for high-level specificit...
Embodiment 2
[0241] Drug Development Technology
[0242] Molecular modeling techniques, especially those in which small molecules are docked into their binding sites, are used in the design of new drugs.
[0243] A routine application of theoretical techniques used in new drug design methods is to take a specific protein such as an enzyme and observe the detailed experimental structure of a complex between the protein and its ligand such as a substrate or inhibitor. On the basis of the structural details of IL-13 and its binding site for GAGs, new compounds were proposed. Through molecular modeling, compounds are screened using a technique called docking computation, which studies the many mutual orientations of proteins and ligands to find out how best to accommodate the ligand in the binding site. Compounds with the best affinity for proteins generate theoretical complexes with suitably low interaction energies.
Embodiment 3
[0245] BIAcore screening test
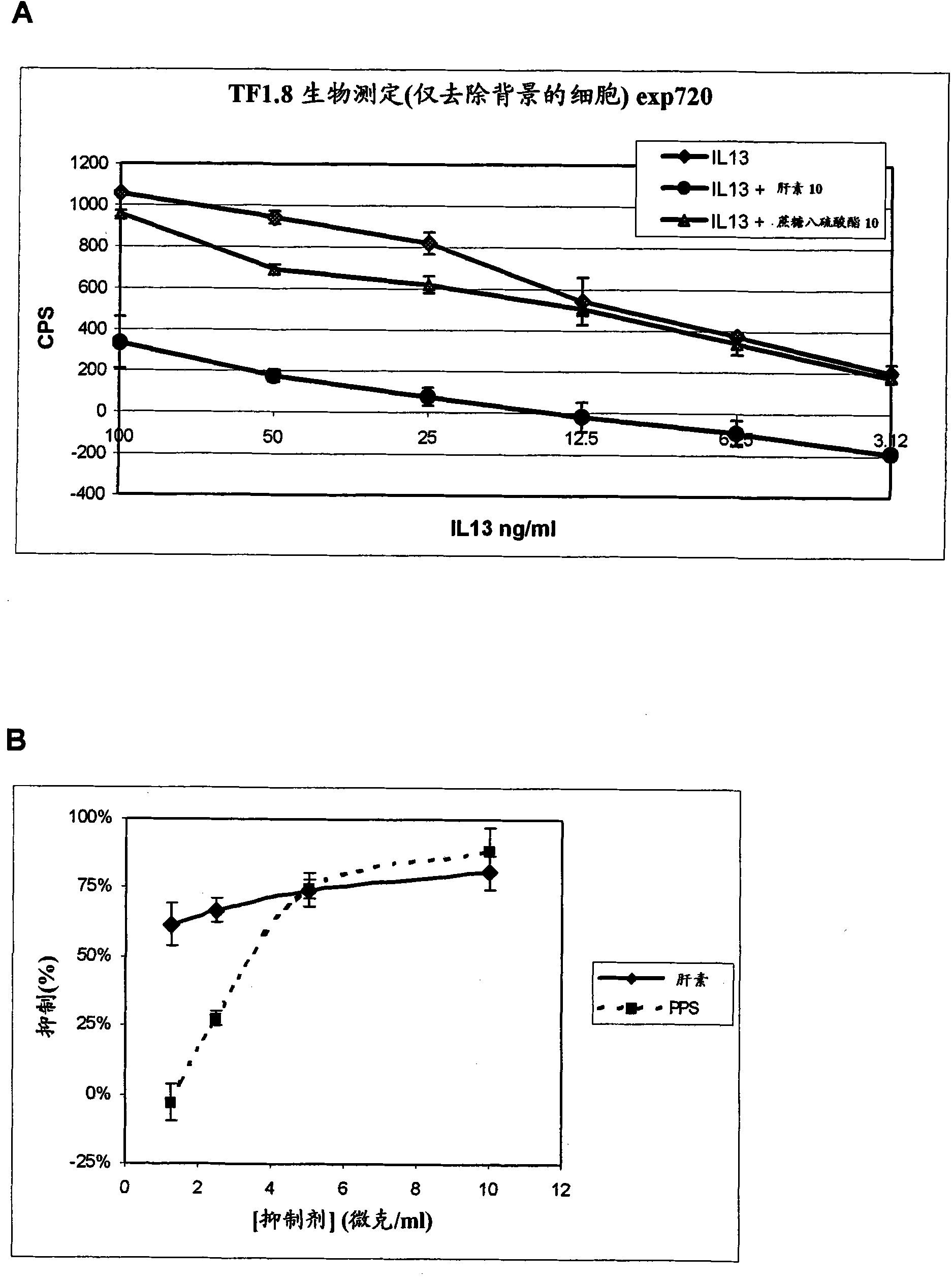
[0246] The optical phenomenon of surface plasmon resonance is used to monitor physical interactions between molecules. Real-time binding of the protein ligand to the immobilized target is monitored by passing a solution of the potential protein ligand (eg, IL-13) over the sensor surface to which the target (eg, heparin) is coupled. Detection is achieved by measuring changes in the refractive index very close to the sensor surface. When the refractive index changes, the angle at which plasmon resonance occurs changes and this change is directly related to the amount of protein interacting with the surface. Use BIAcore 2000 conveniently. It is very sensitive and its microfluidics ensures that only small amounts of material are required.
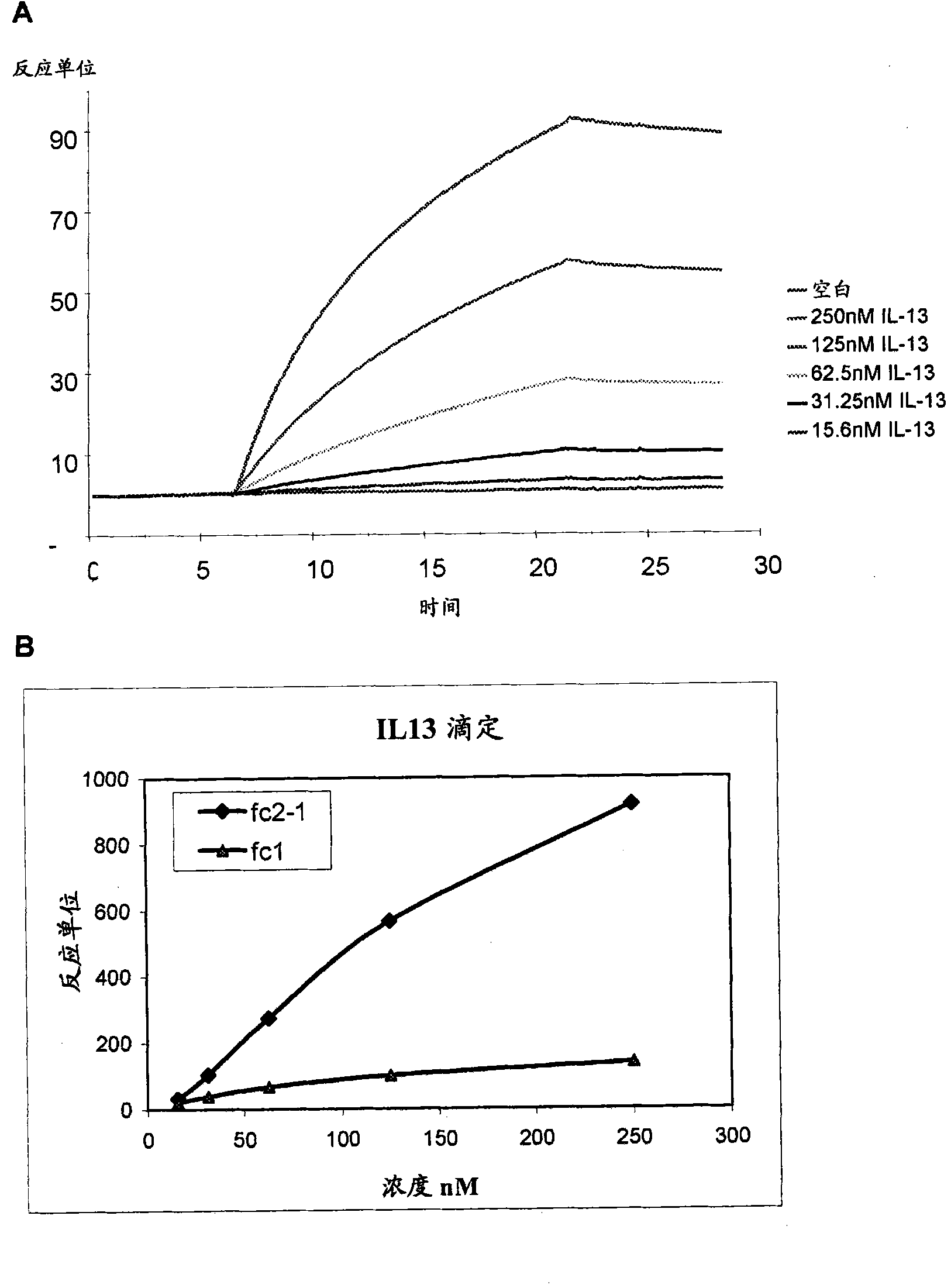
[0247] Biotinylated heparin was immobilized on the biosensor chip. Biotinylation proceeds with sulfo-NHS-biotin via the amino group or the reducing end modified with ammonia by reductive amination. A solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com