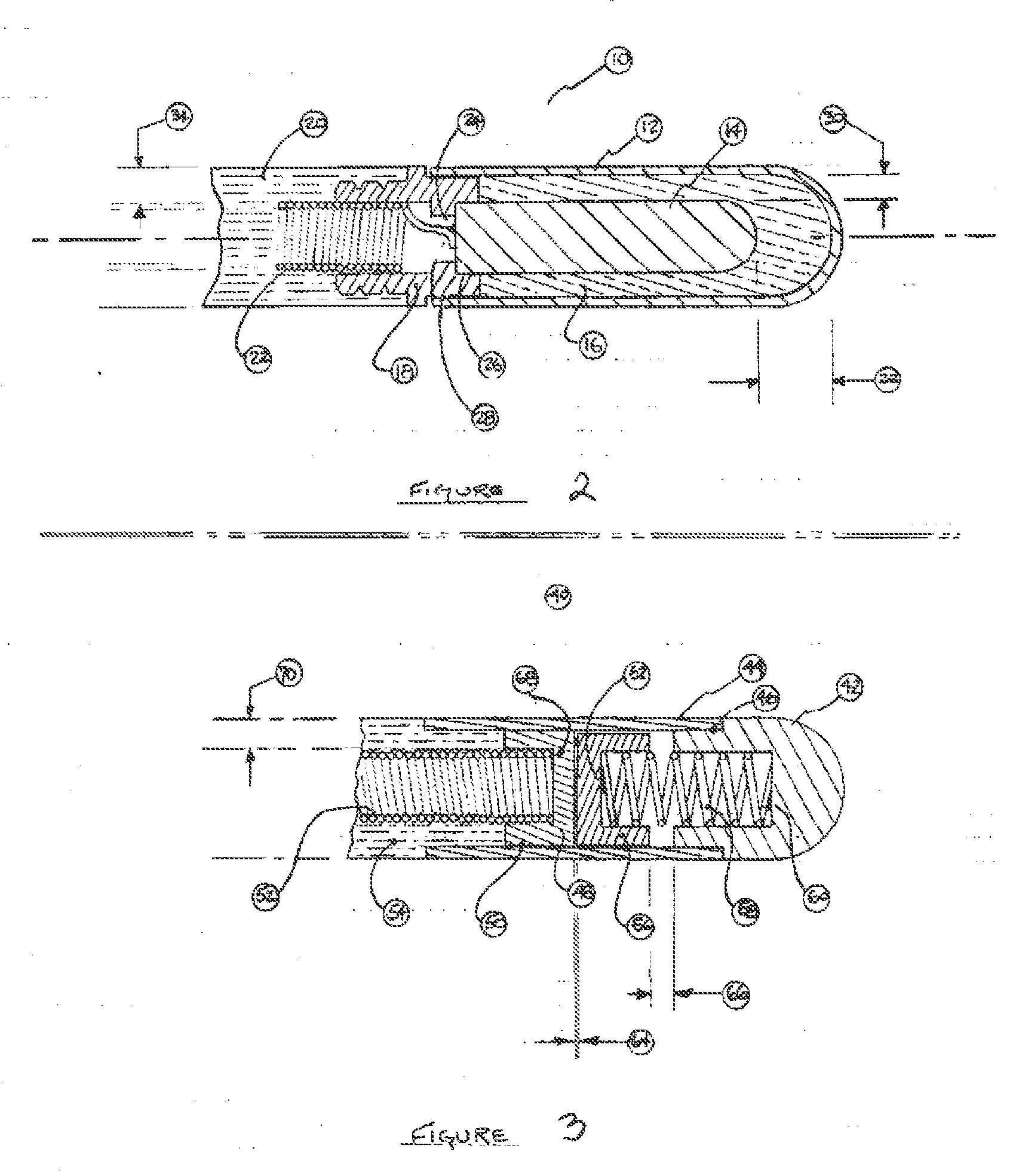
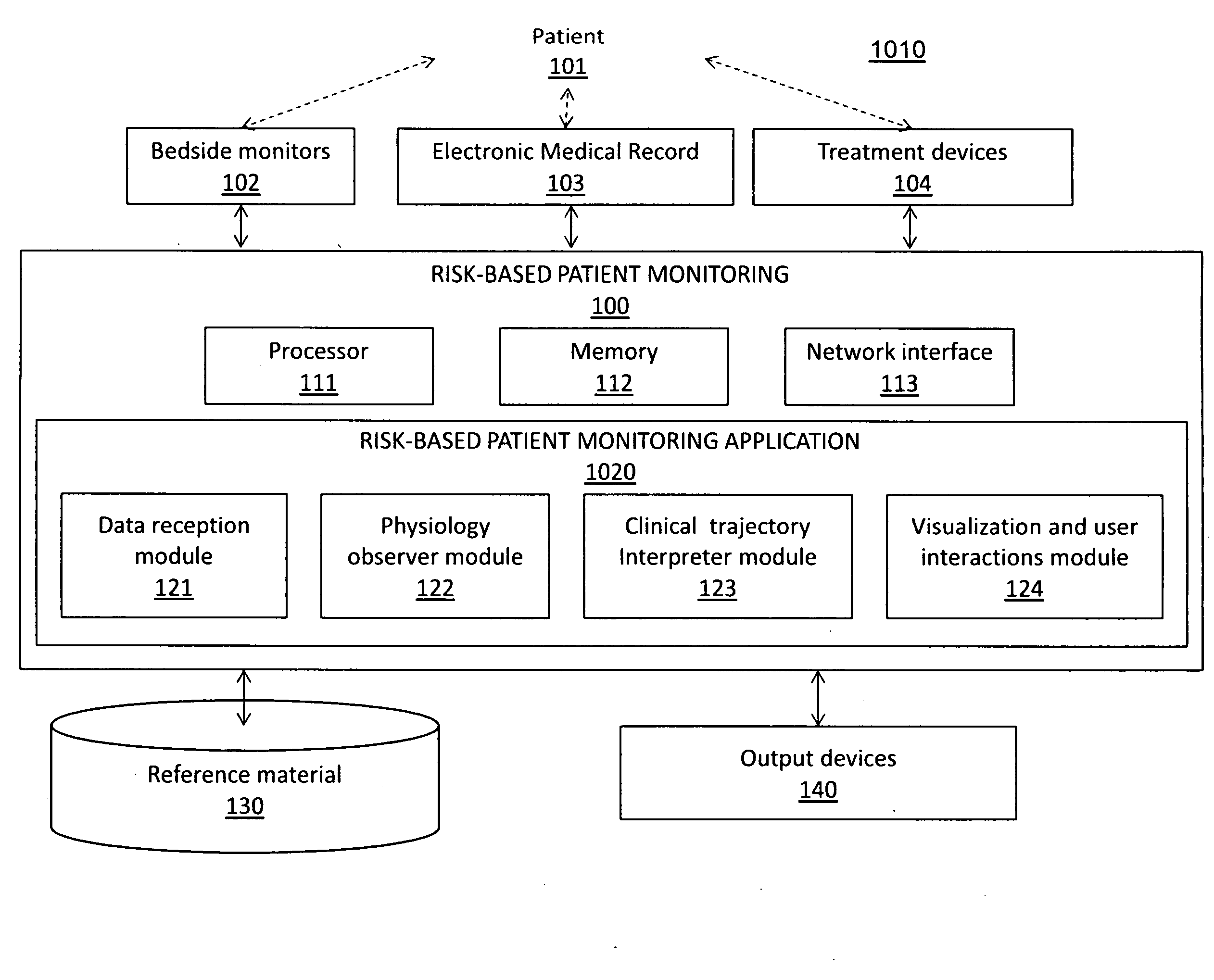
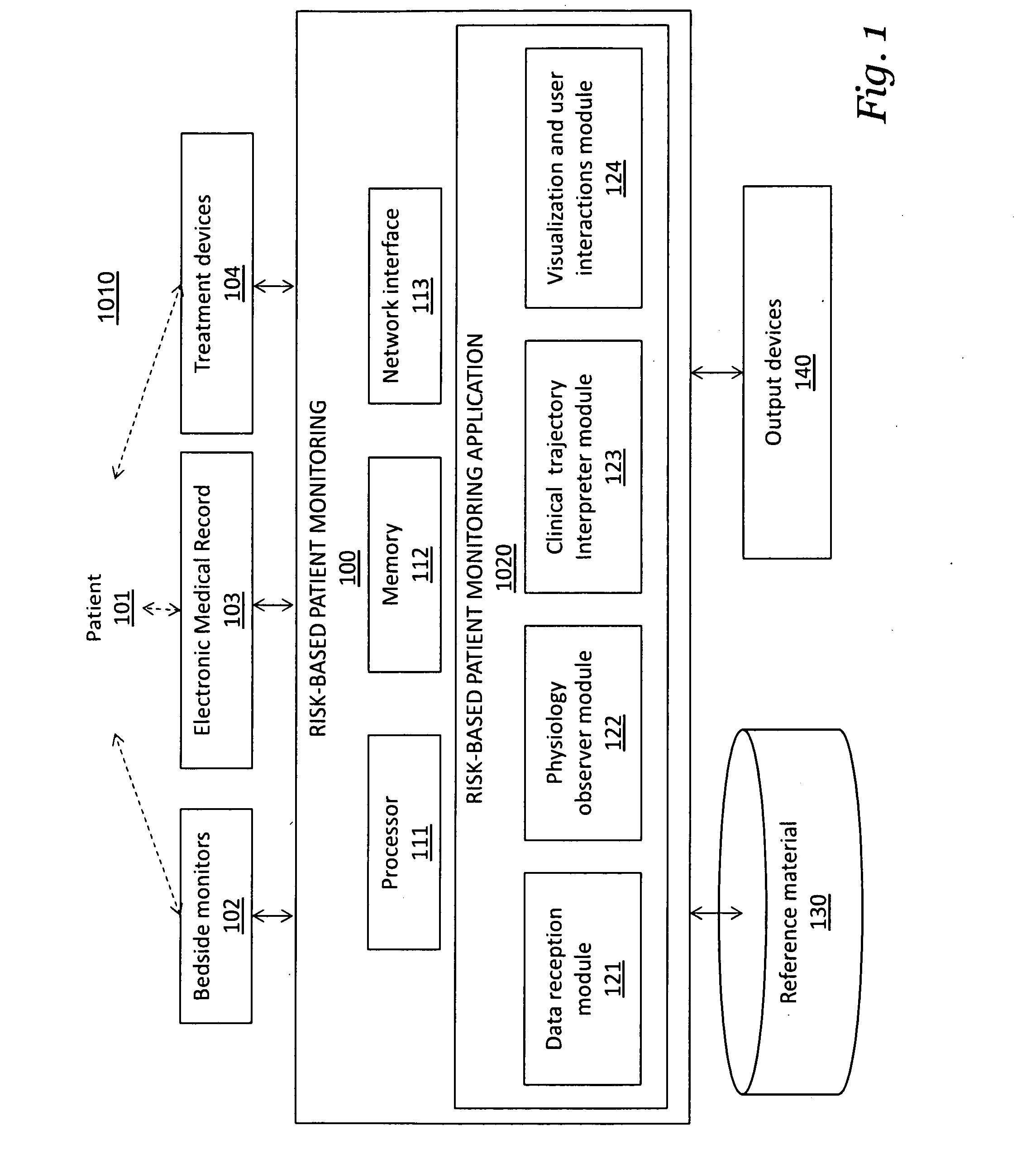
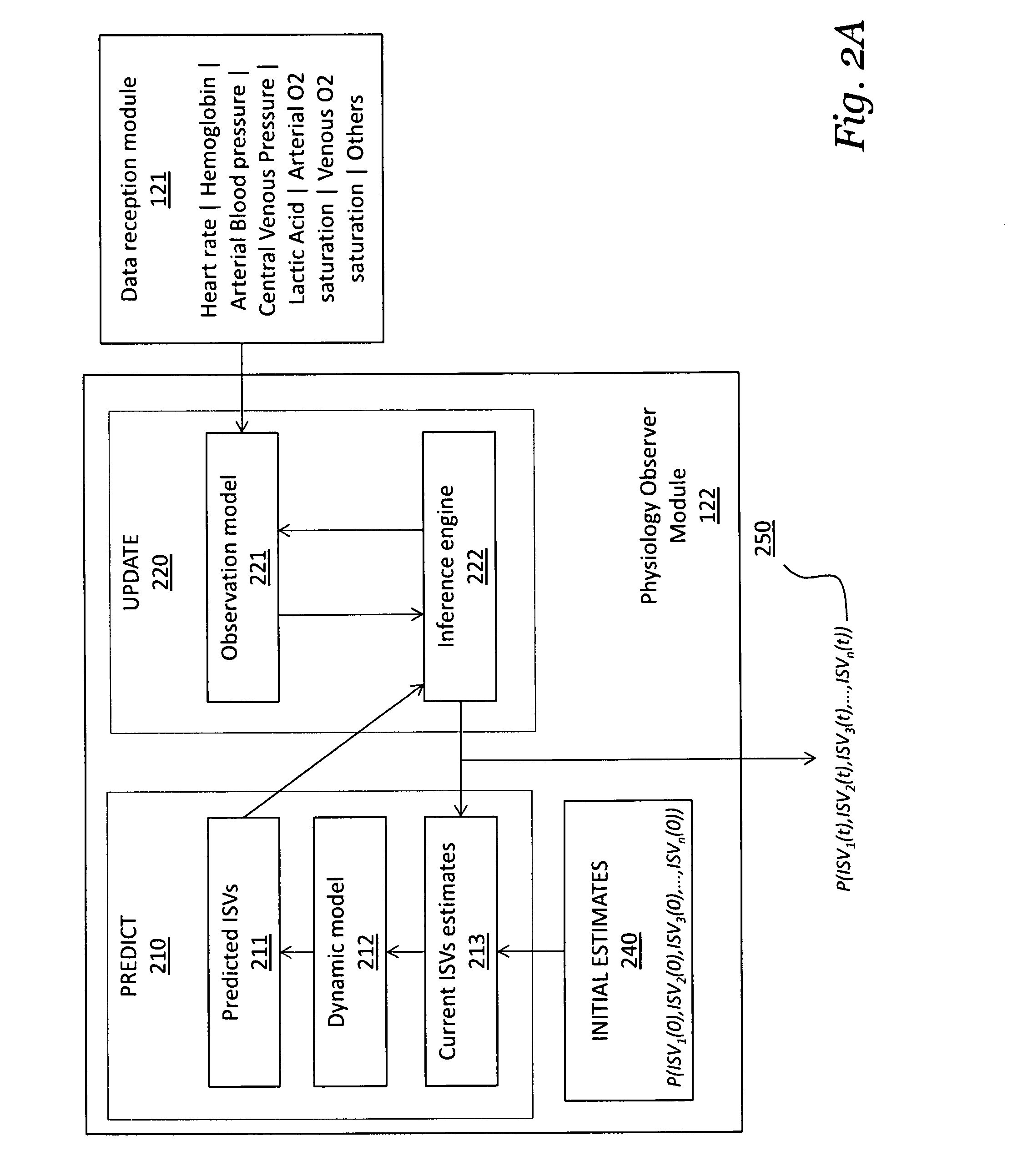
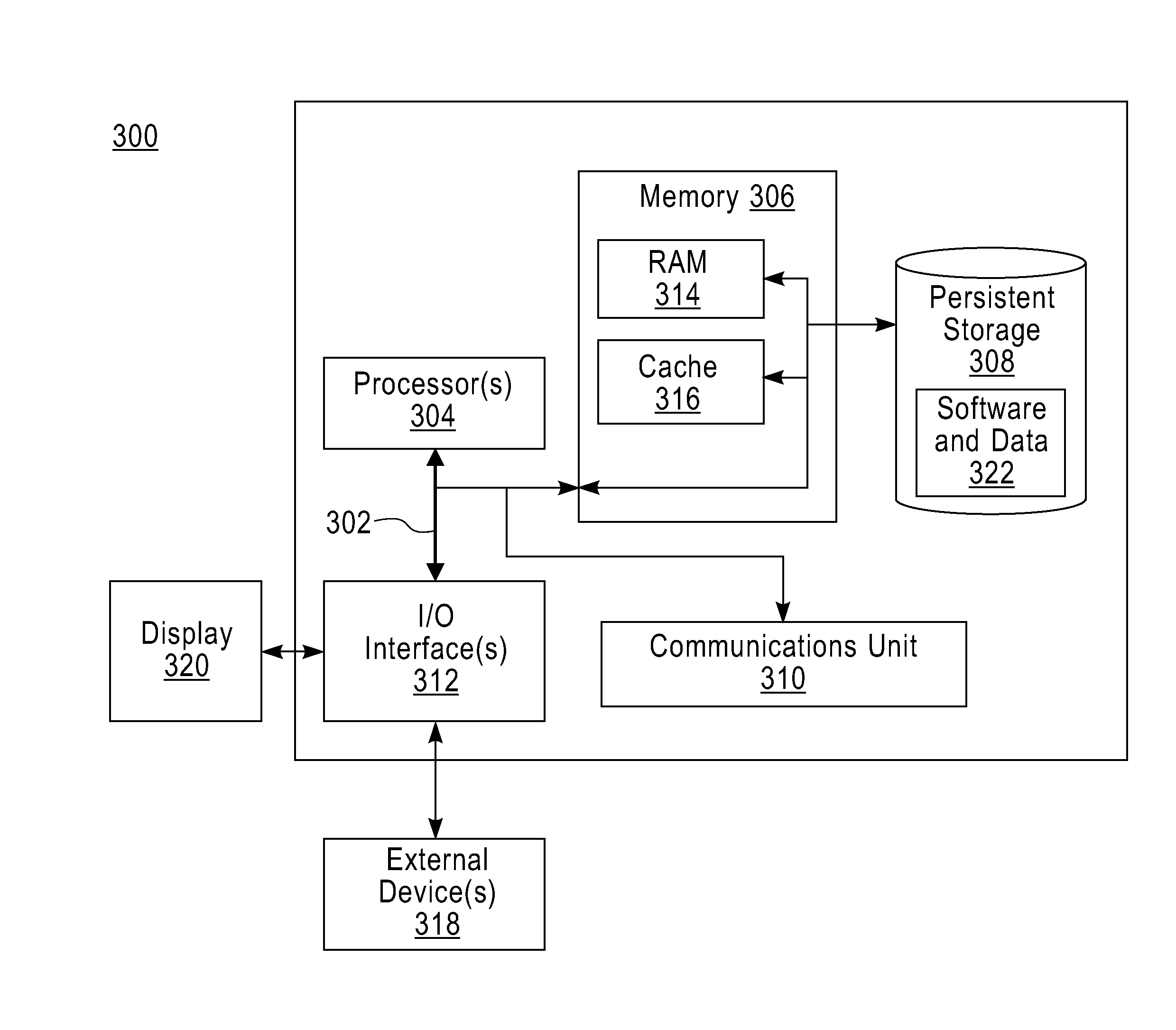
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
95 results about "Patient risk" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
At-Risk Patient: Pressure Ulcers/Injuries. Printer-friendly version. A patient at-risk of developing a pressure ulcer (injury) is an individual who has limitations in daily living activities that could result in chronic problems if exposed to pressure, shear, friction or moisture.
Unified Platform for Monitoring and Control of Blood Glucose Levels in Diabetic Patients
ActiveUS20150018633A1Easy to replaceGuaranteed uptimePeptide/protein ingredientsDrug and medicationsClosed loopSystem call
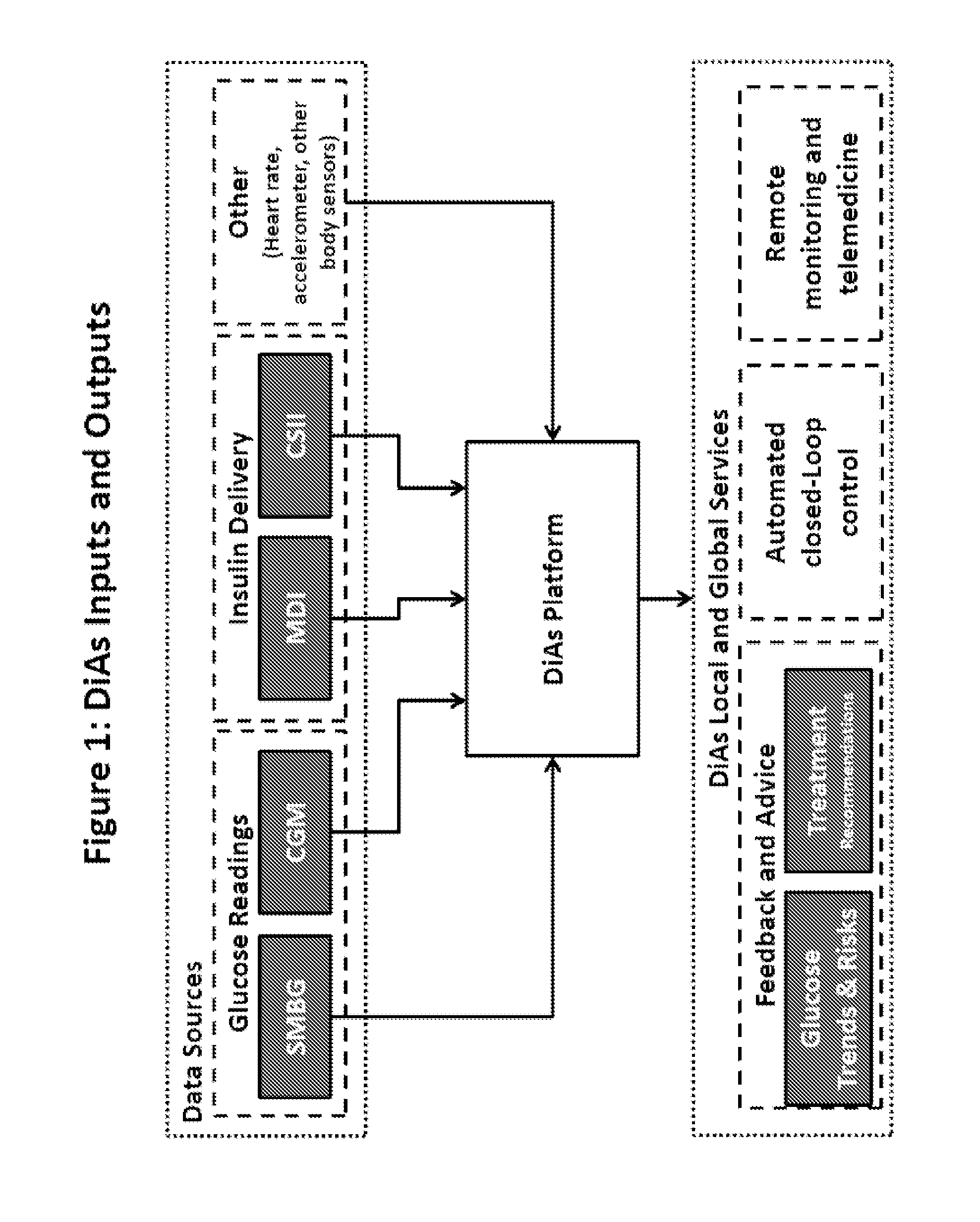
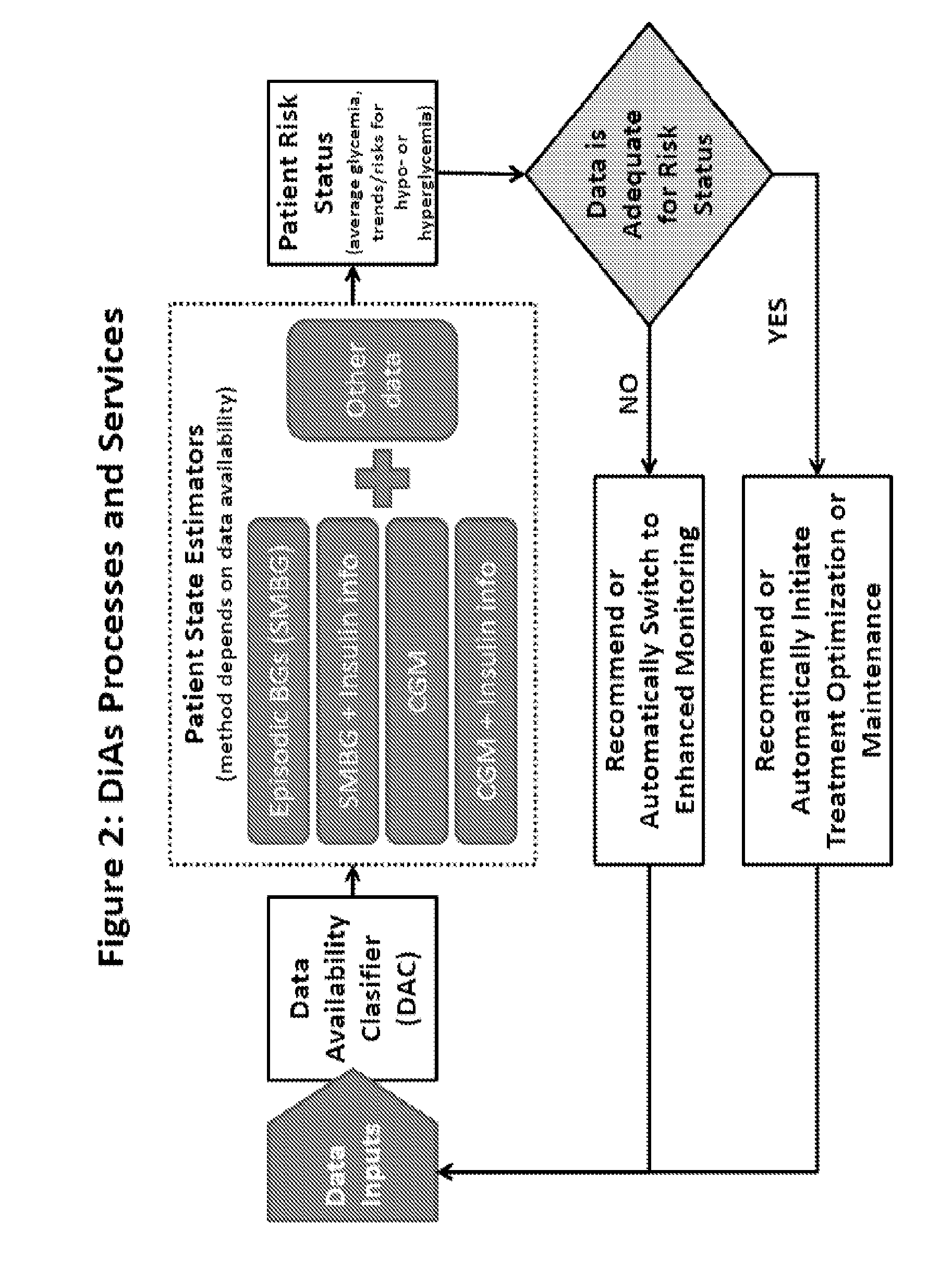
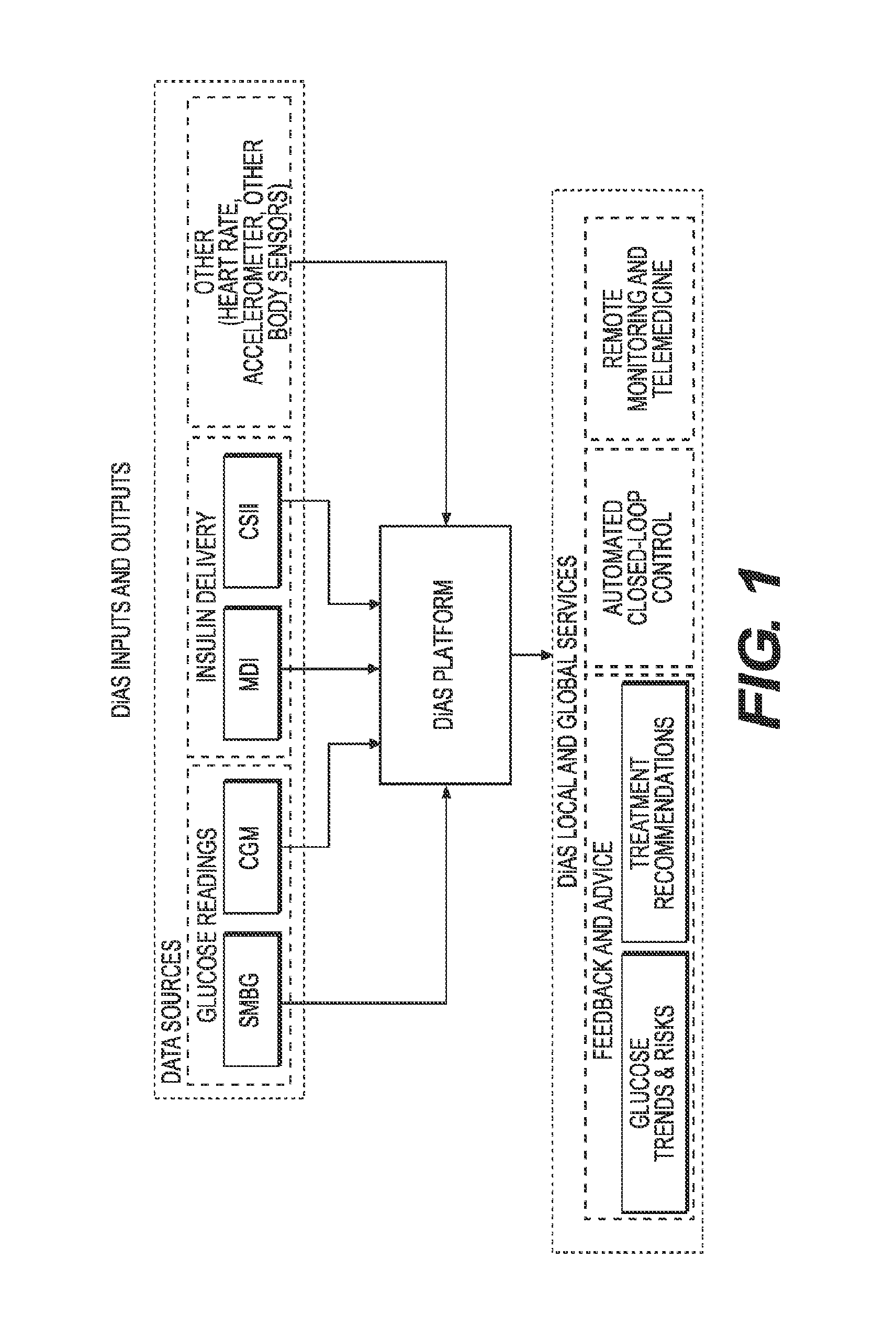
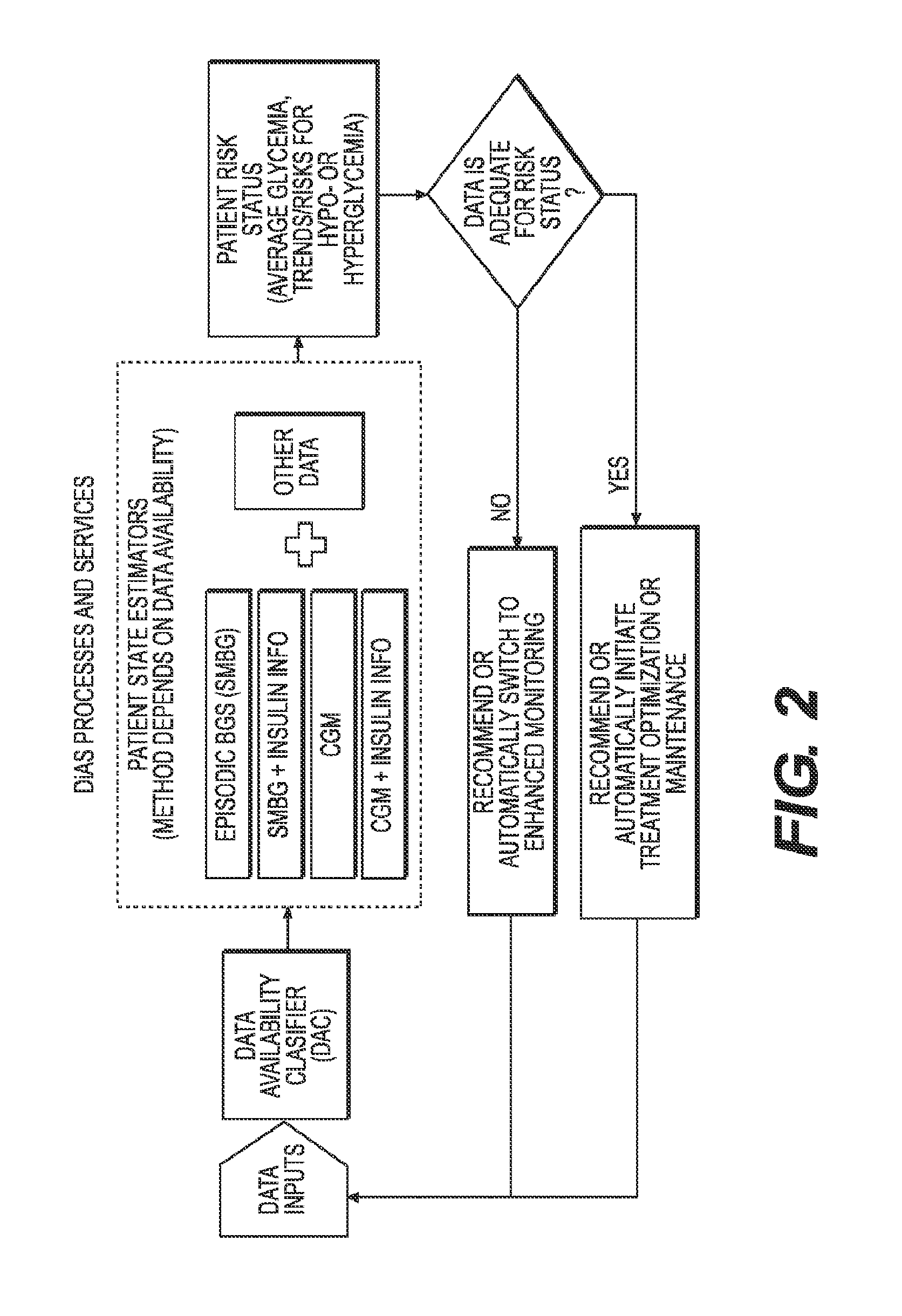
A flexible system capable of utilizing data from different monitoring techniques and capable of providing assistance to patients with diabetes at several scalable levels, ranging from advice about long-term trends and prognosis to real-time automated closed-loop control (artificial pancreas). These scalable monitoring and treatment strategies are delivered by a unified system called the Diabetes Assistant (DiAs) platform. The system provides a foundation for implementation of various monitoring, advisory, and automated diabetes treatment algorithms or methods. The DiAs recommendations are tailored to the specifics of an individual patient, and to the patient risk assessment at any given moment.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
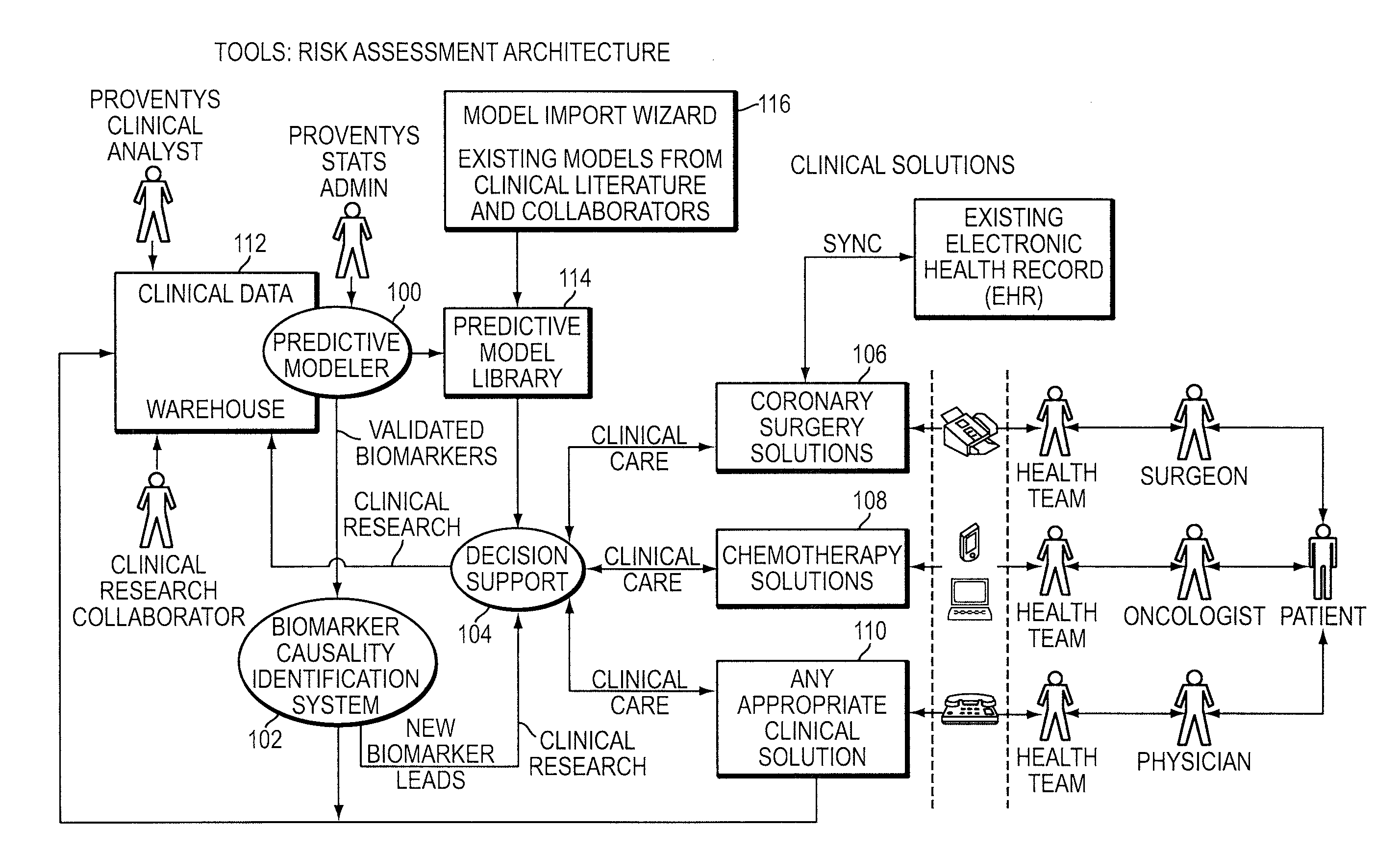
Integrated clinical risk assessment system
InactiveUS20090070138A1Minimize adverse outcomeMaximizing measurable deliveryData processing applicationsHealth-index calculationTherapeutic treatmentTherapy planning
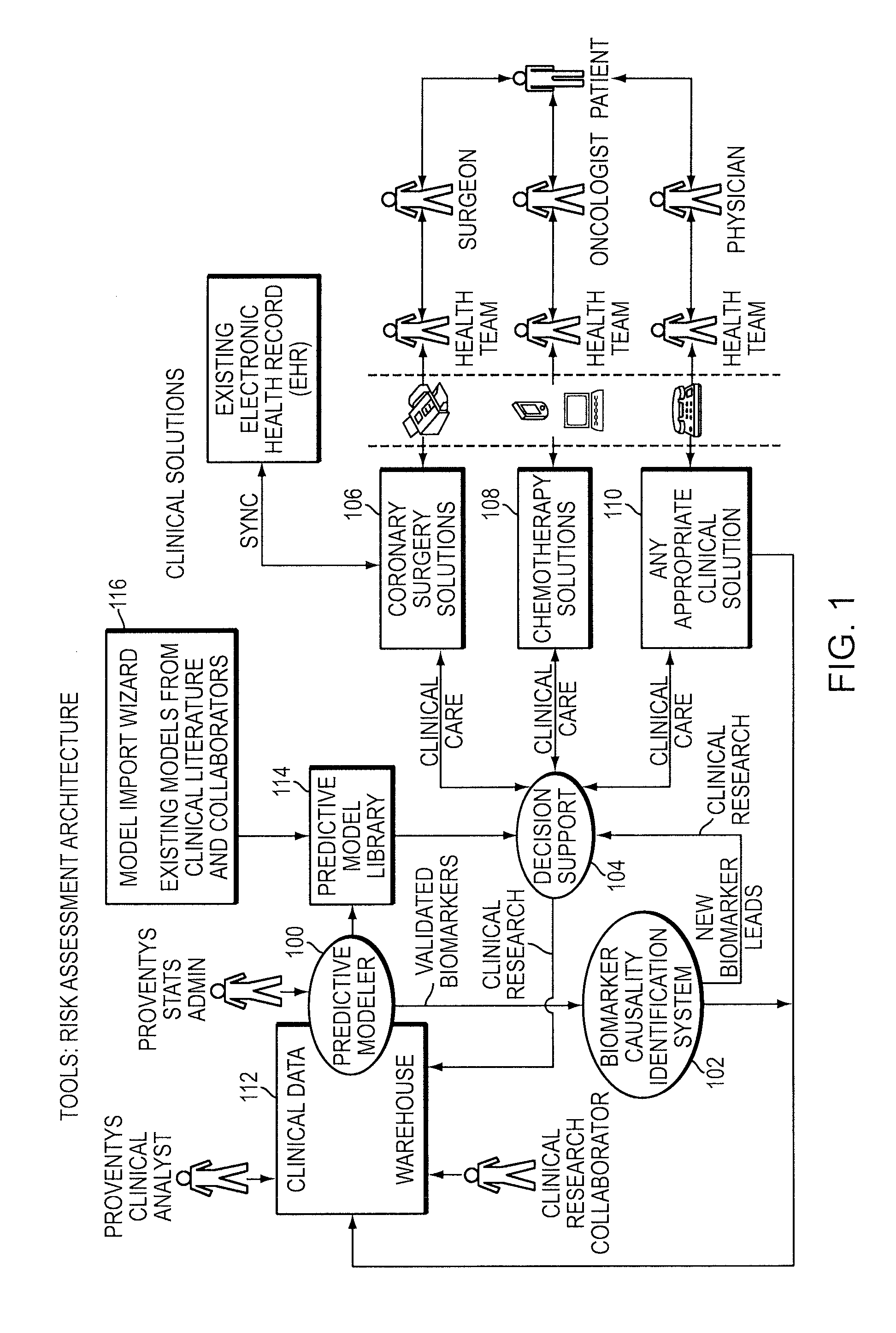
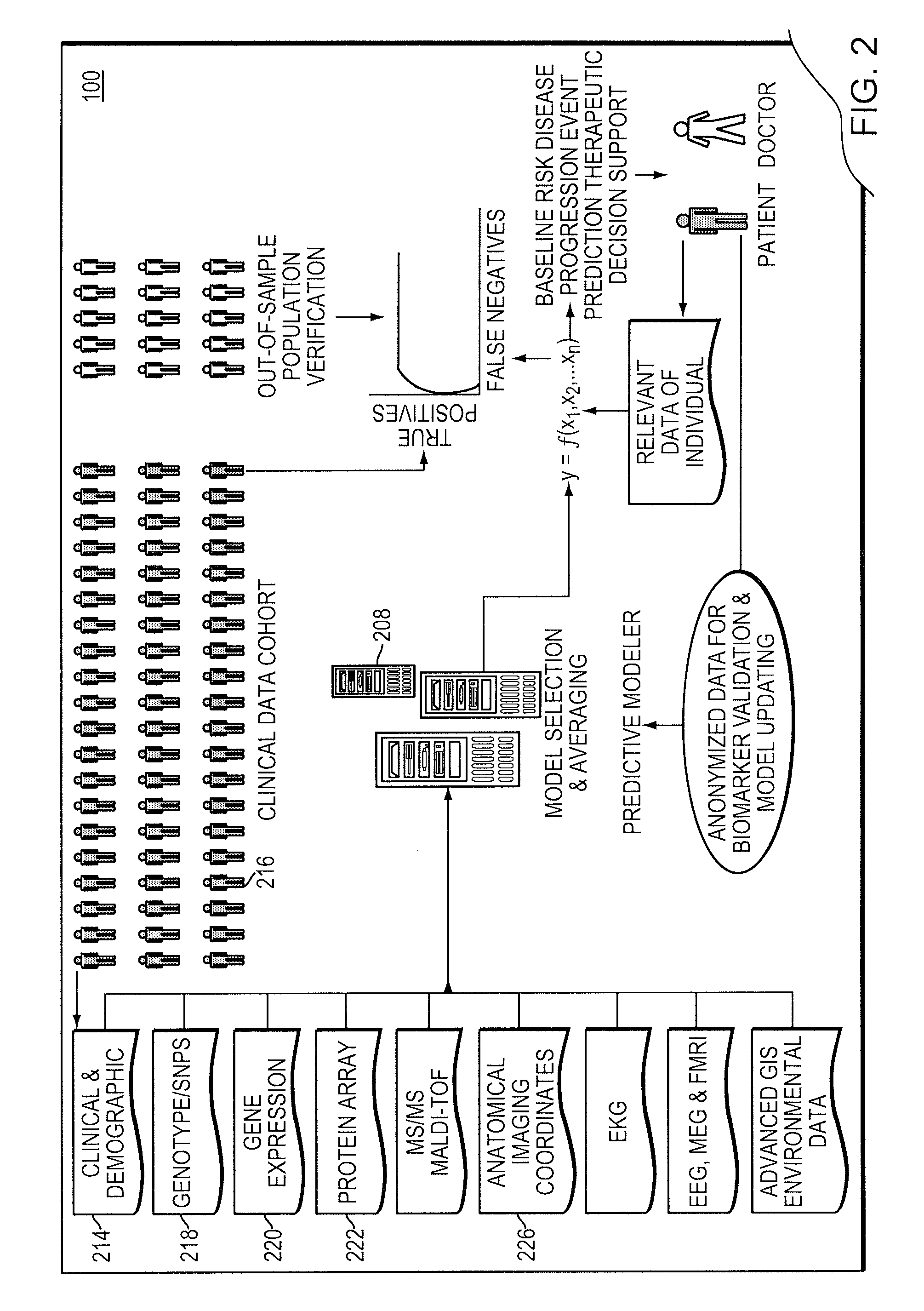
A computer-implemented method is provided for analyzing patient risk and determining an individual therapeutic treatment plan for a cancer patient. The method includes entering patient medical information into a risk assessment tool; identifying any obtaining missing or out-of-date patient information; initializing the risk assessment tool based on the patient's demographics and cancer characteristics and determining a default treatment plan for the patient; modifying the default treatment plan by observing the modification of a risk score for the patient; and confirming a treatment order for the patient based on a balancing of the risk and treatment plan factors.
Owner:PROVENTYS
Method and apparatus for implementing digital video modeling to generate a patient risk assessment model
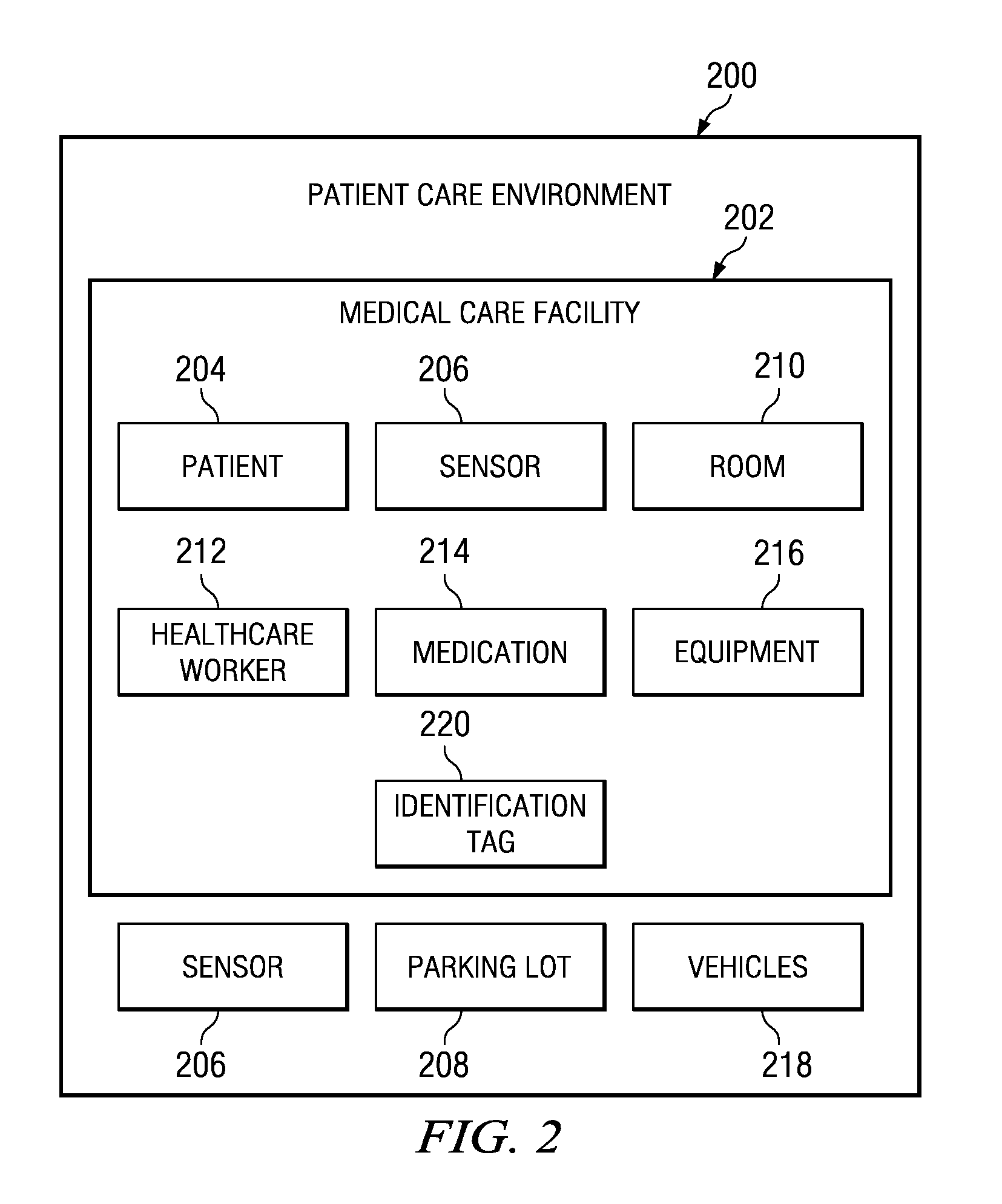
A computer implemented method, apparatus, and computer program product for generating a risk assessment model for an assessment of a patient in a healthcare facility. The process retrieves event data for the patient, wherein the event data is derived from video data, and wherein the event data further comprises metadata describing events affecting the patient in a medical care facility, and parses the event data to form assessment data. The process then generates the risk assessment model using the assessment data.
Owner:IBM CORP
Polynucleotides associated with age-related macular degeneration and methods for evaluating patient risk
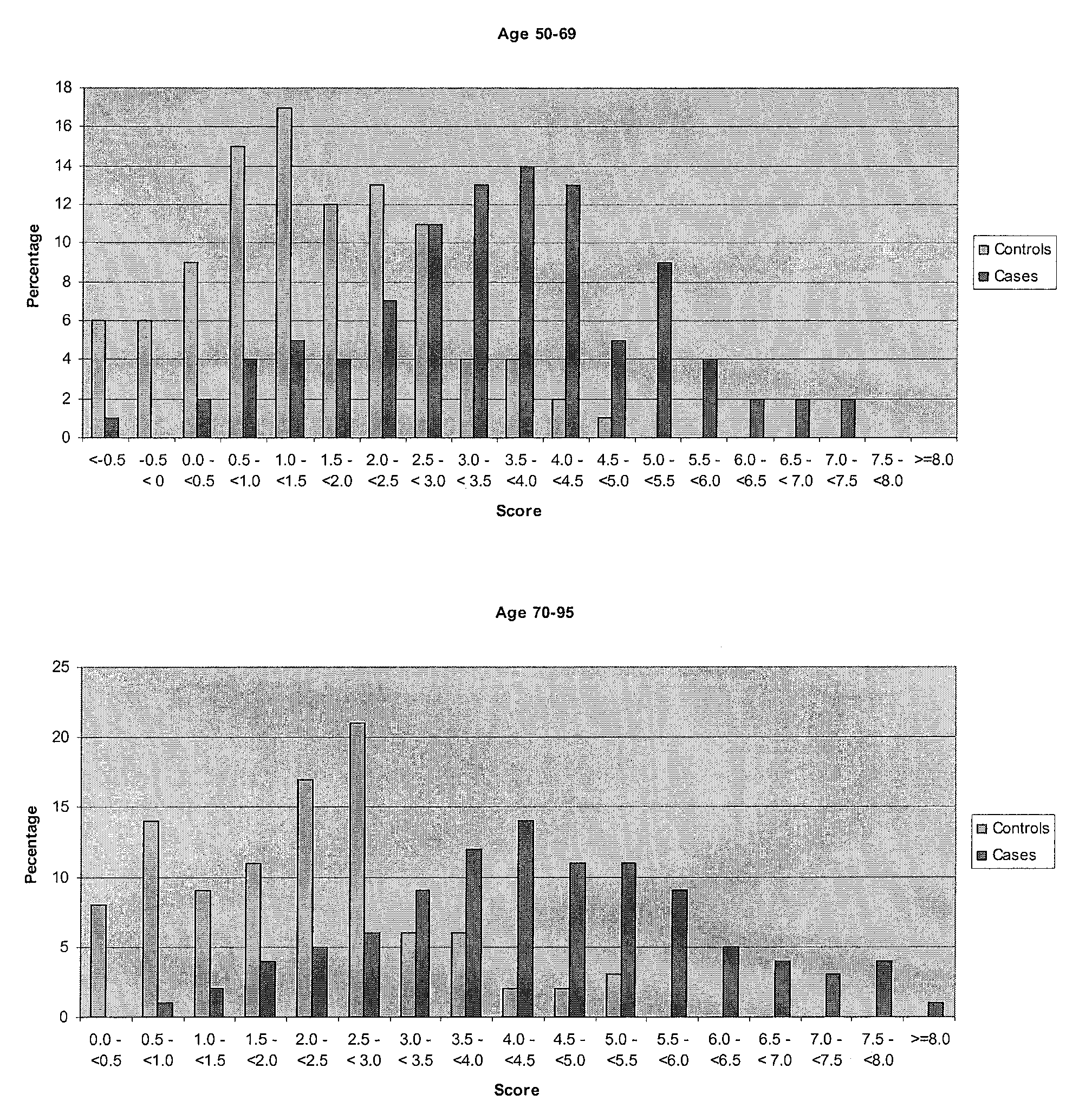
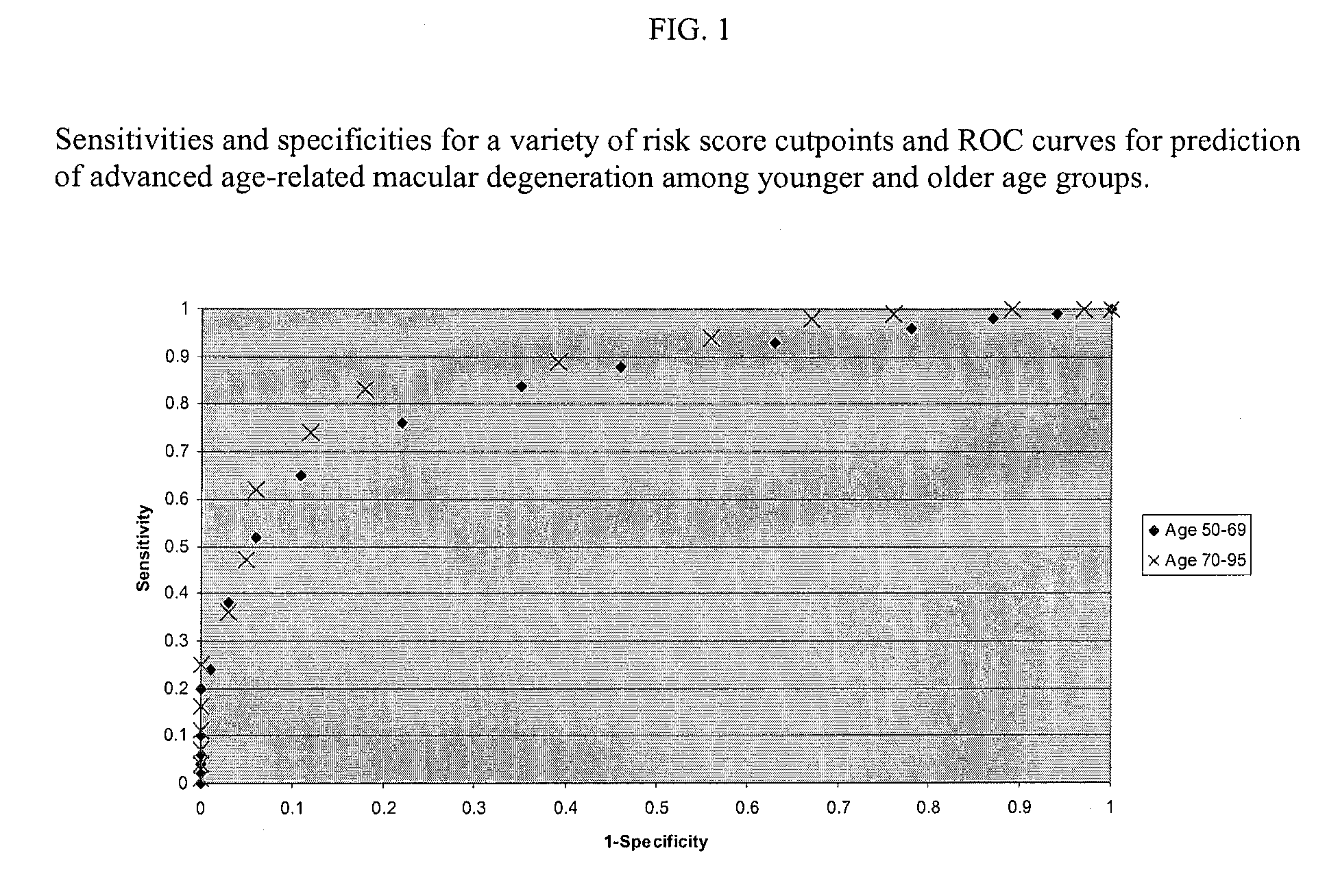
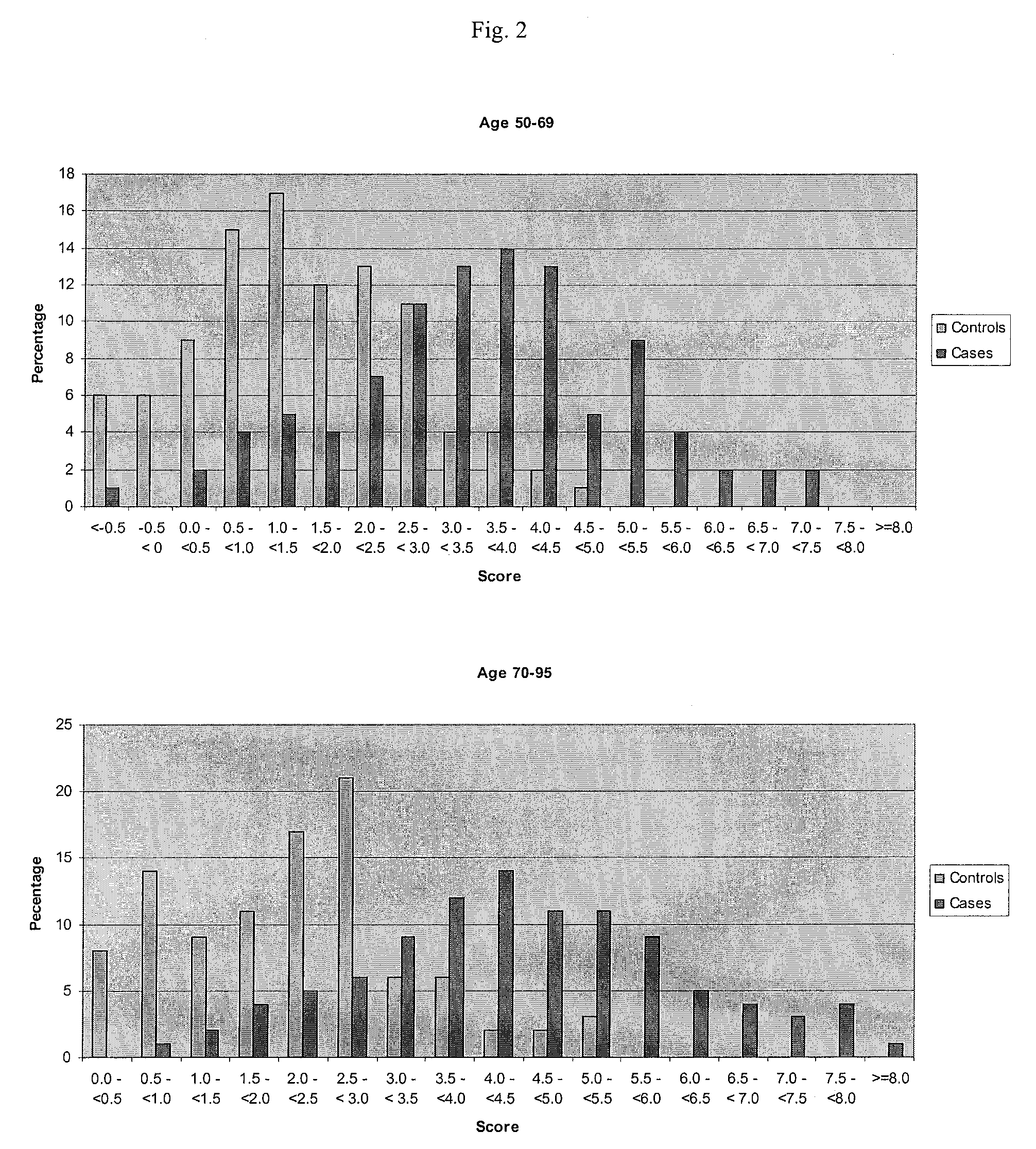
The present invention provides for certain polynucleotide sequences that have been correlated to AMD. These polynucleotides are useful as diagnostics, and are preferably used to fabricate an array, useful for screening patient samples. The array is used as part of a laboratory information management system, to store and process additional patient information in addition to the patient's genomic profile. As described herein, the system provides an assessment of the patient's risk for developing AMD, risk for disease progression, and the likelihood of disease prevention based on patient controllable factors.
Owner:THE GENERAL HOSPITAL CORP A K A MASSACHUSETTS GENERAL HOSPITAL +3
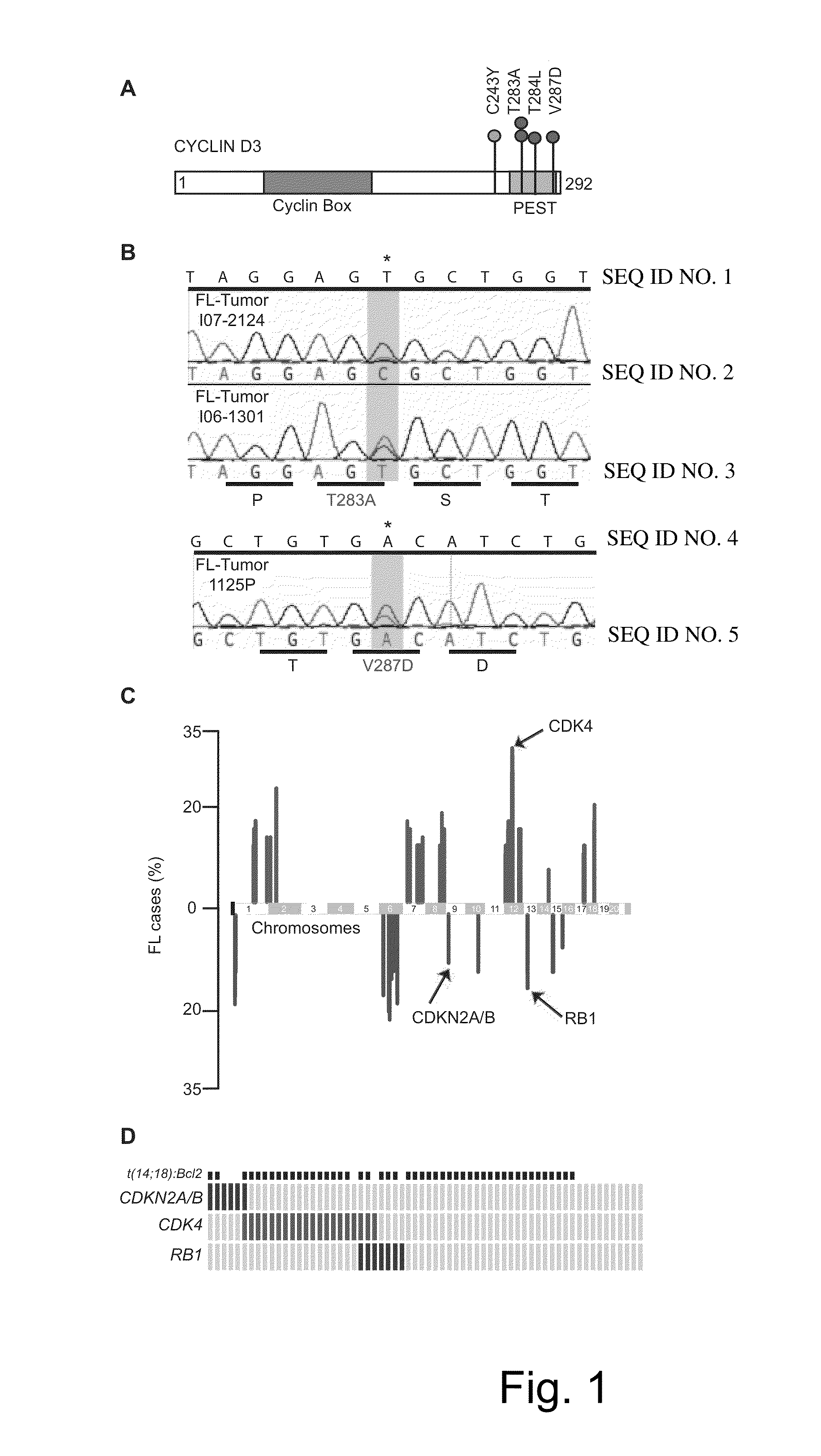
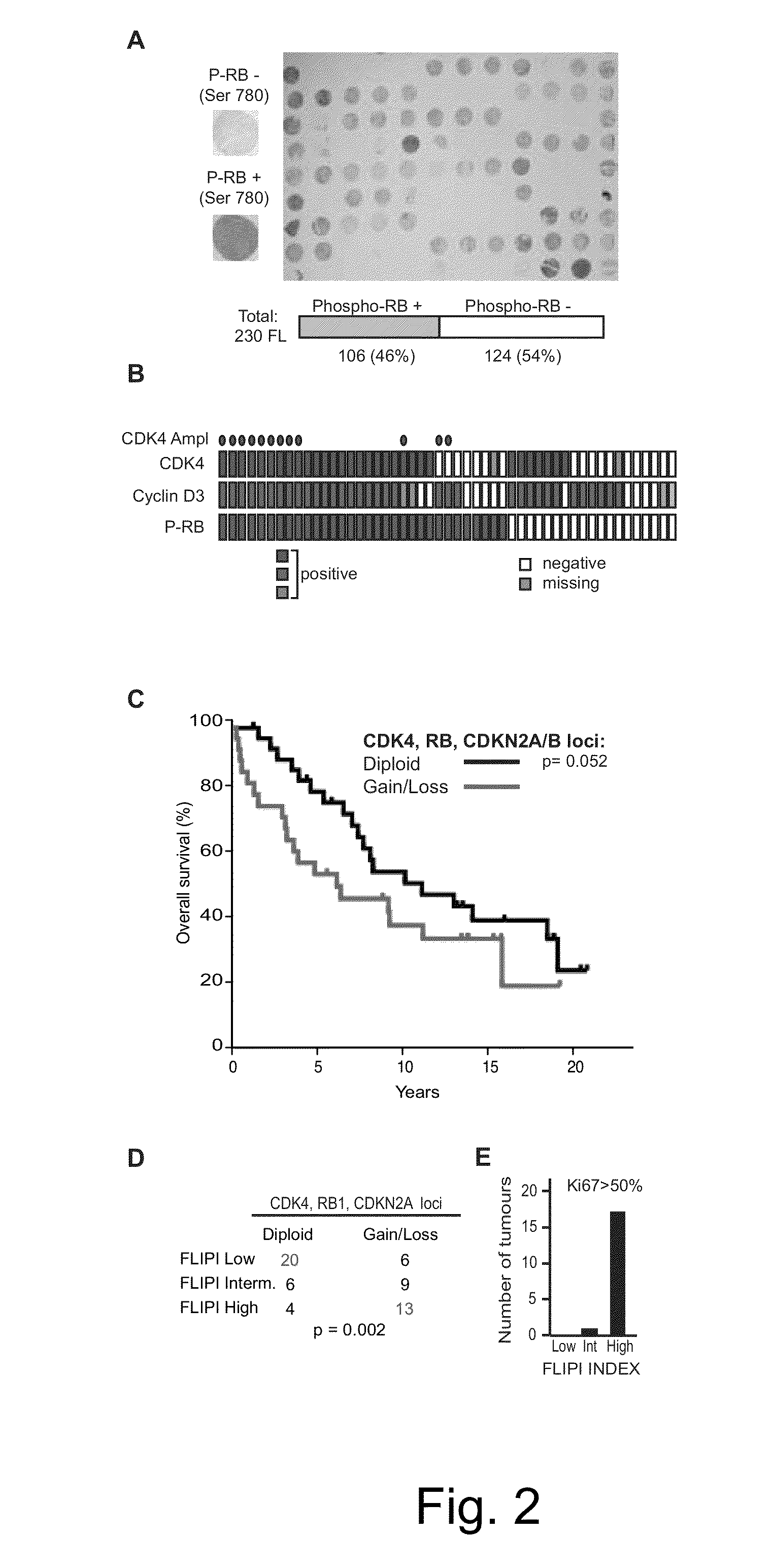
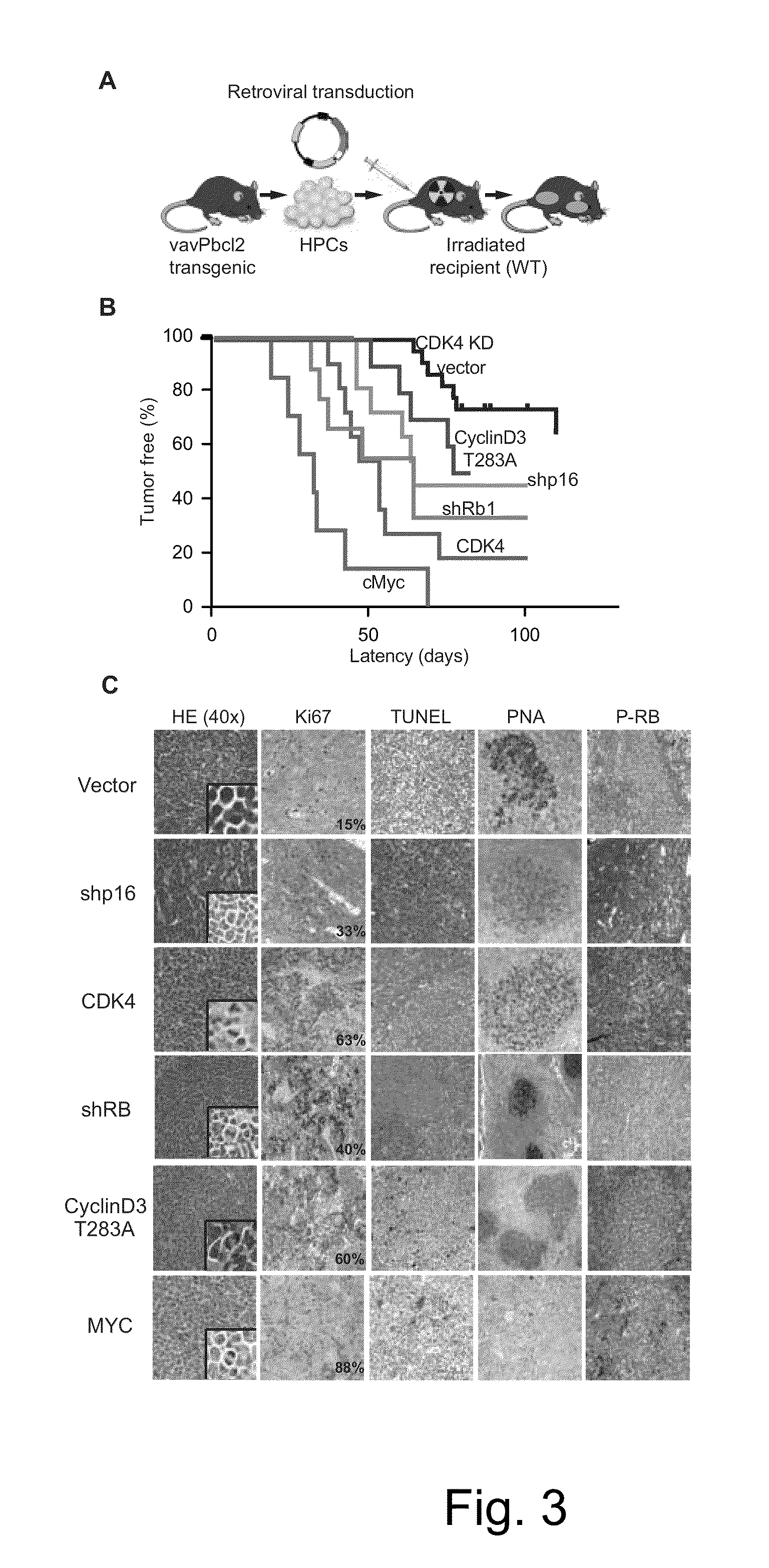
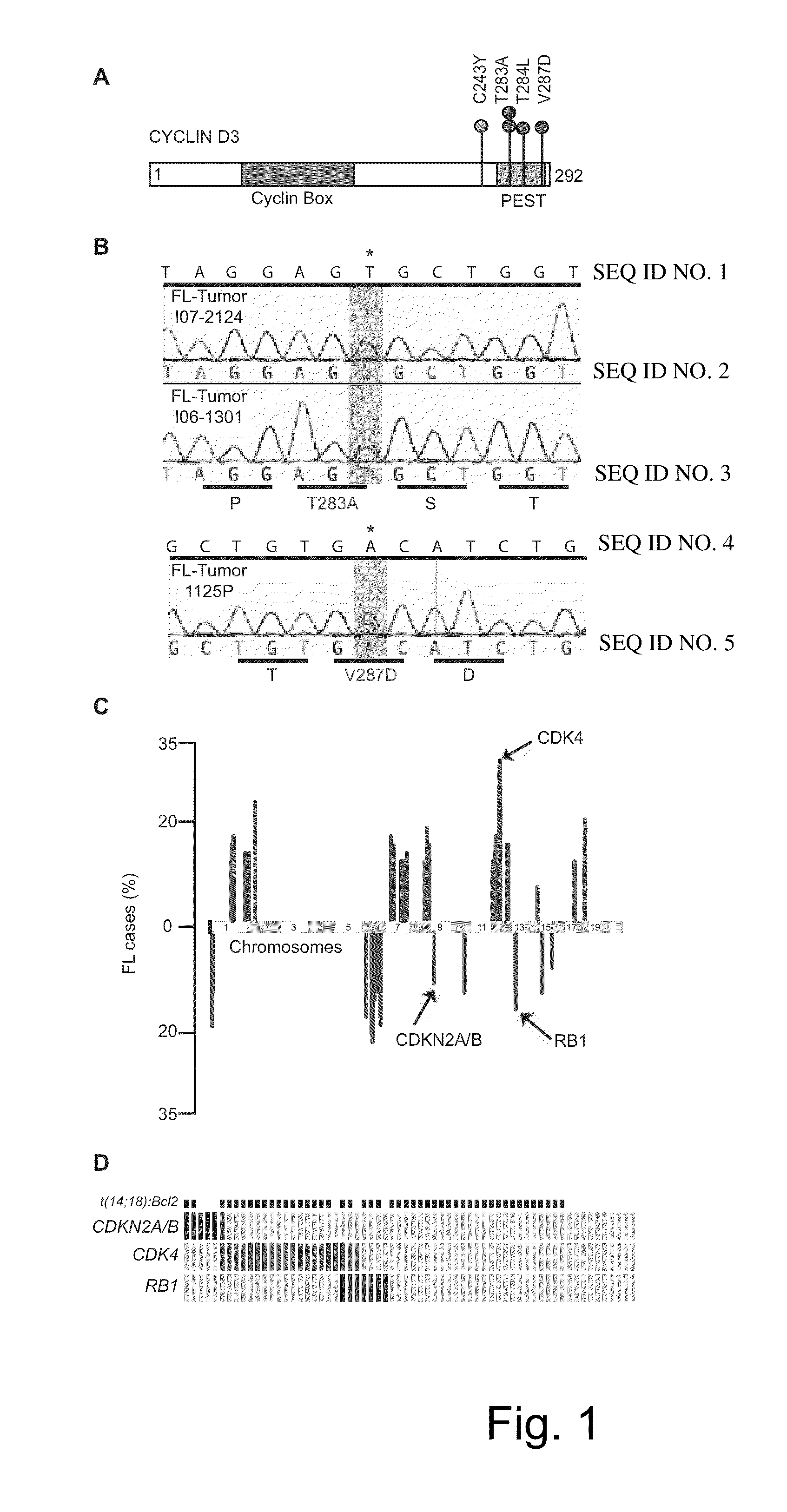
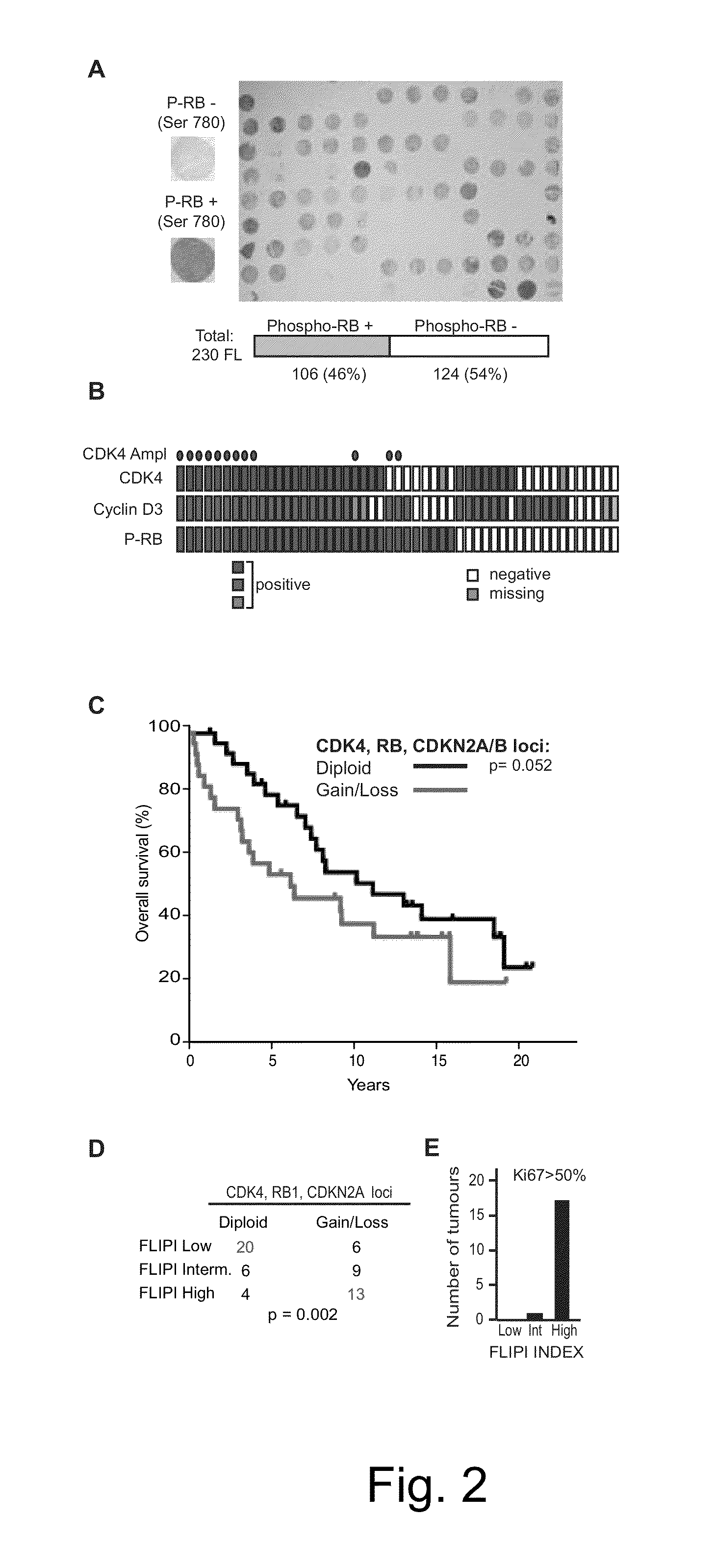
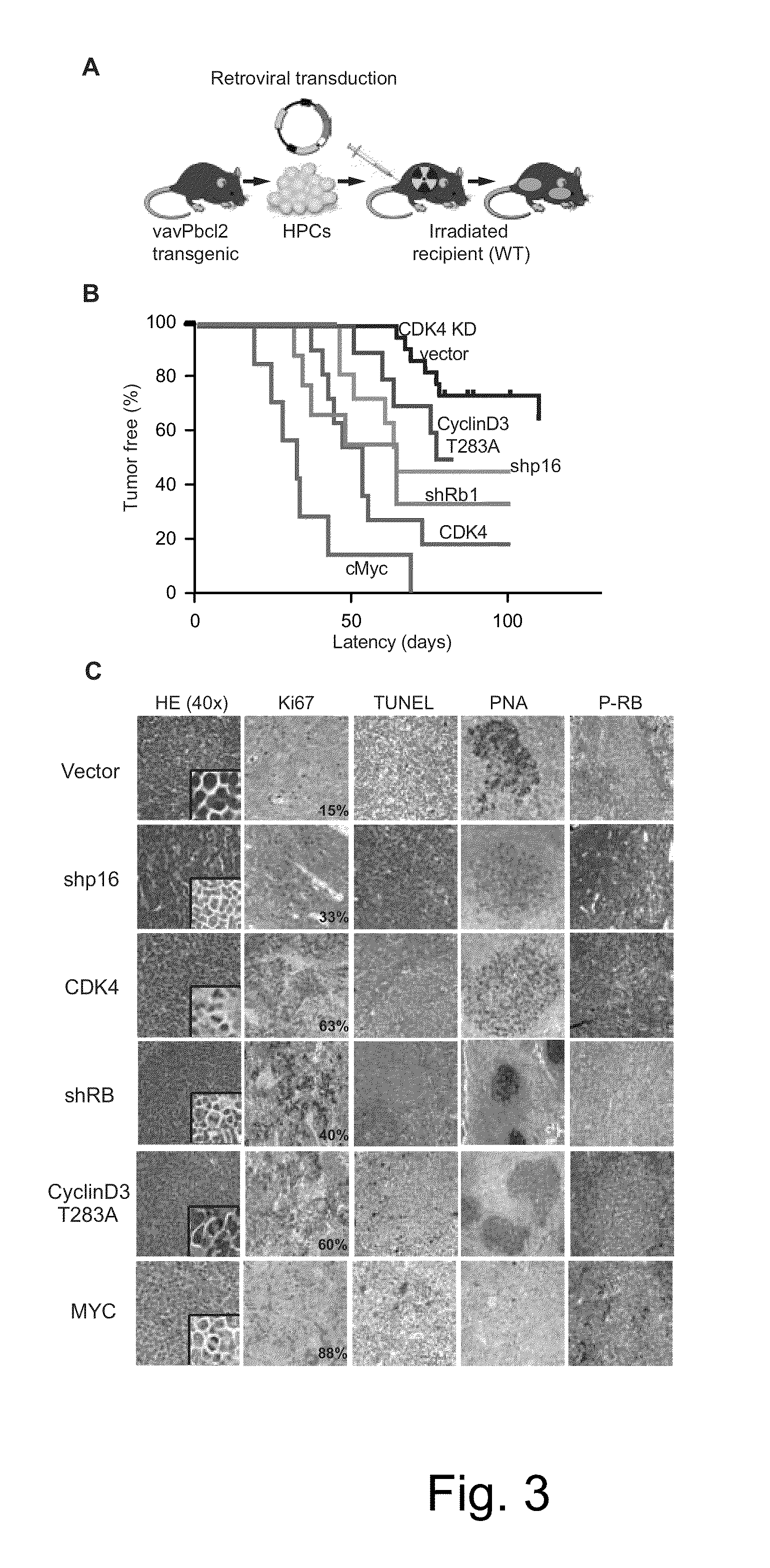
Methods for Treatment of Lymphomas with Mutations in Cell Cycle Genes
InactiveUS20140080838A1Low toxicityEnormous potential to improveOrganic active ingredientsMicrobiological testing/measurementPatient riskCell Cycle Gene
The present disclosure identifies a novel subtype of follicular lymphoma (FL) characterized by dysregulation of the cyclin / CDK / RB proliferative pathway. This subtype of FL is associated with increased malignancy and mortality, relative to FL which is not associated with cell cycle dysregulation. Accordingly, this disclosure presents novel methods to subtype FL and stratify patient risk by detection of biomarkers associated with RB inactivation. This disclosure further presents novel therapies for the treatment of FL subtyped by inactivation of RB.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
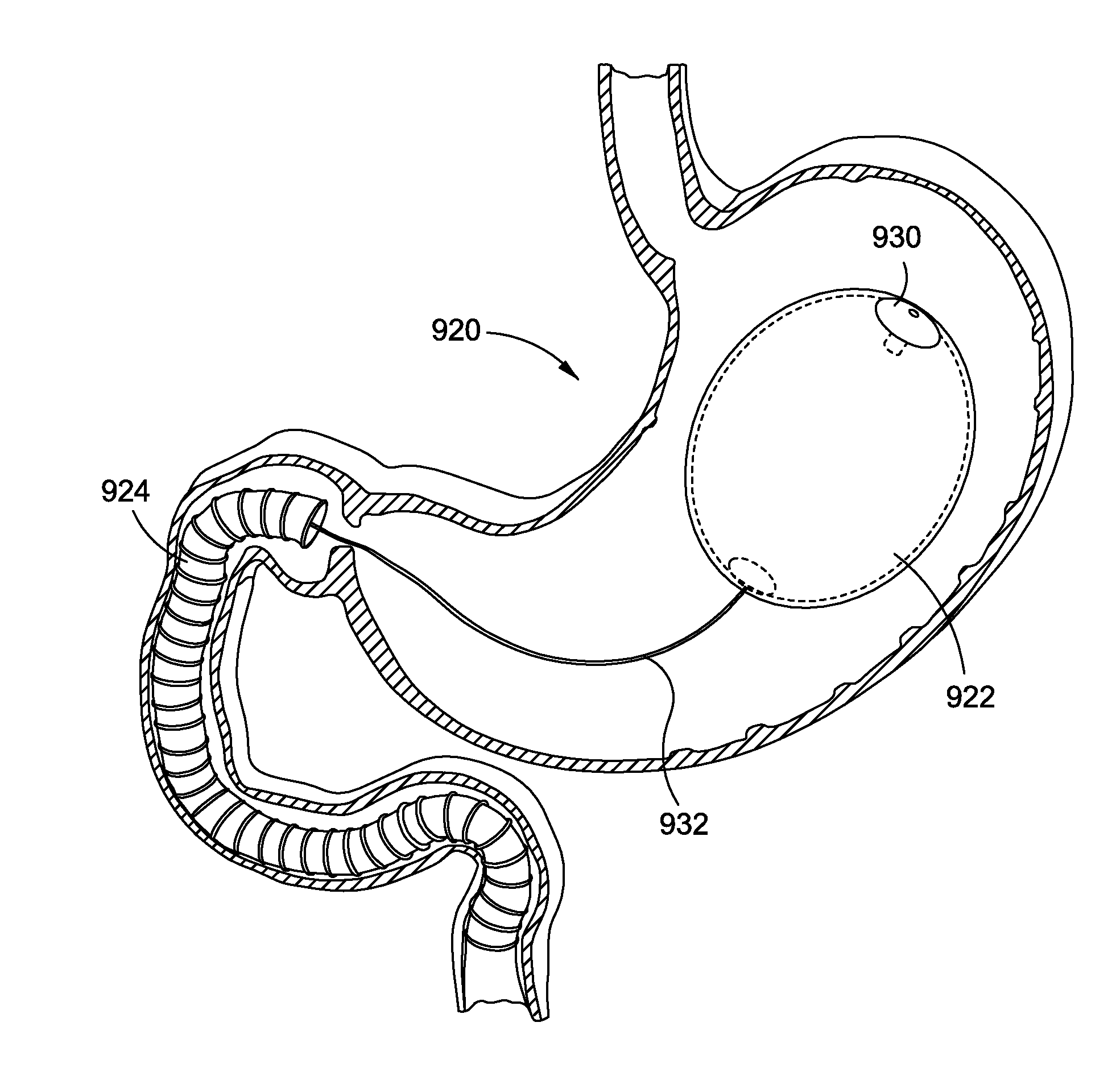
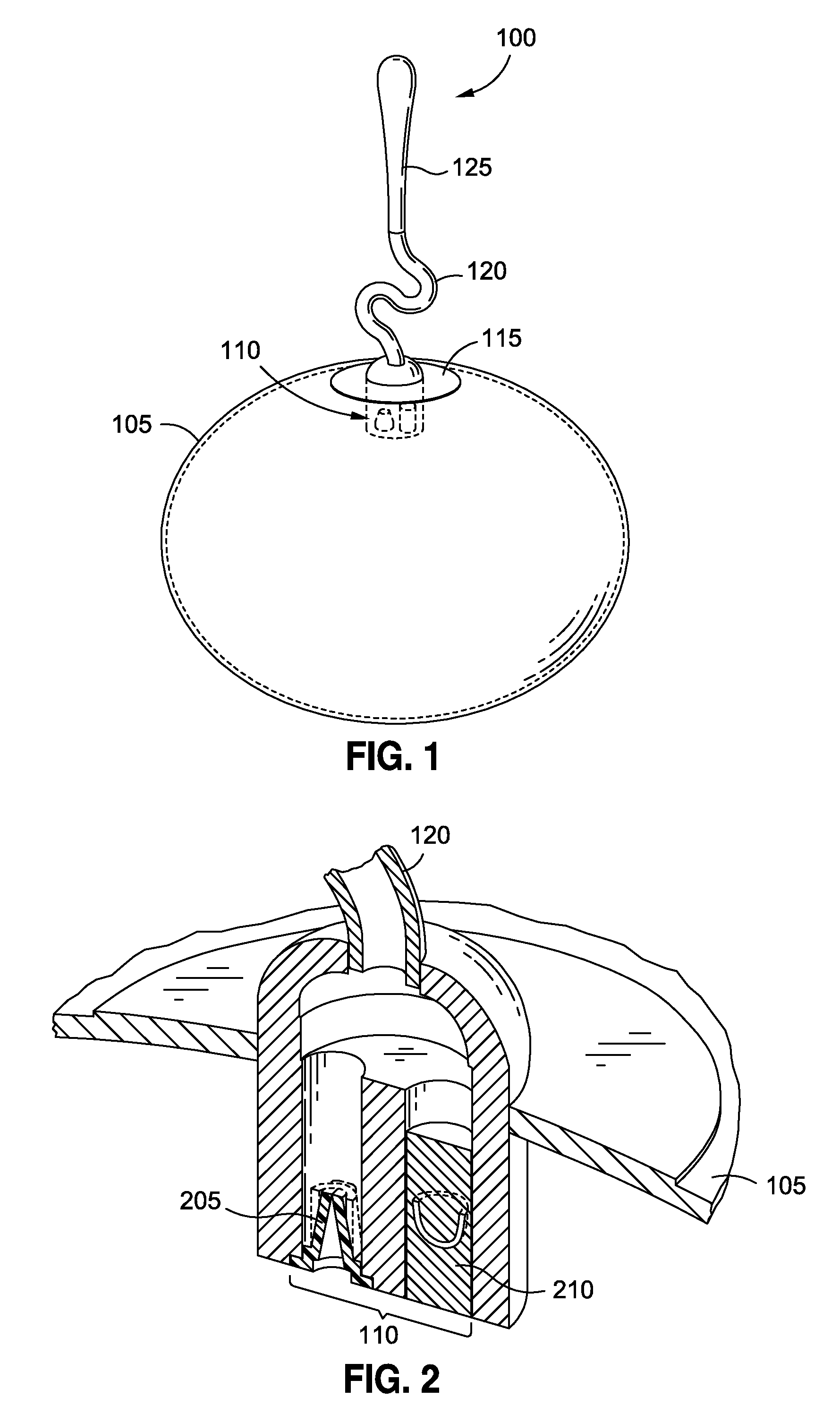
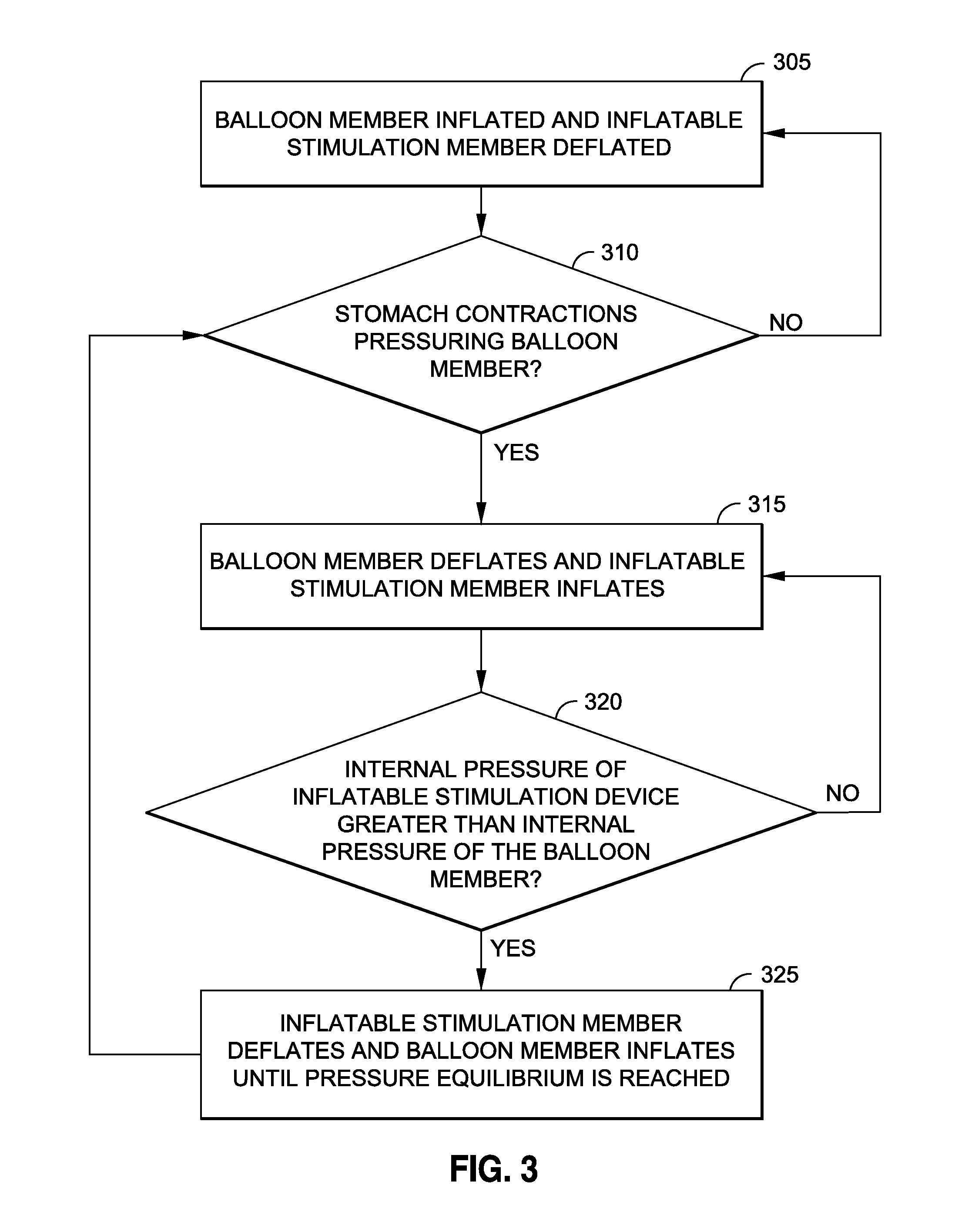
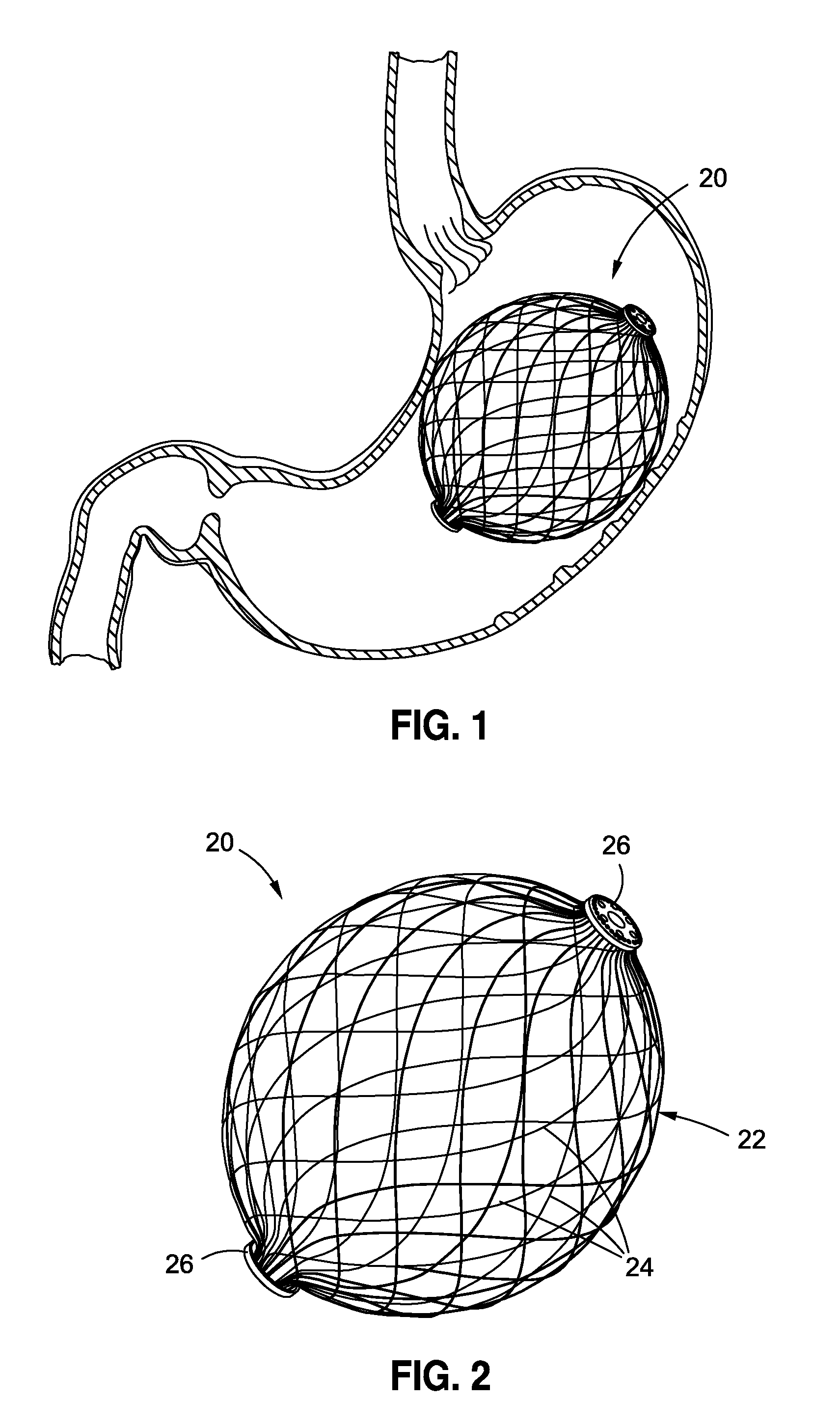
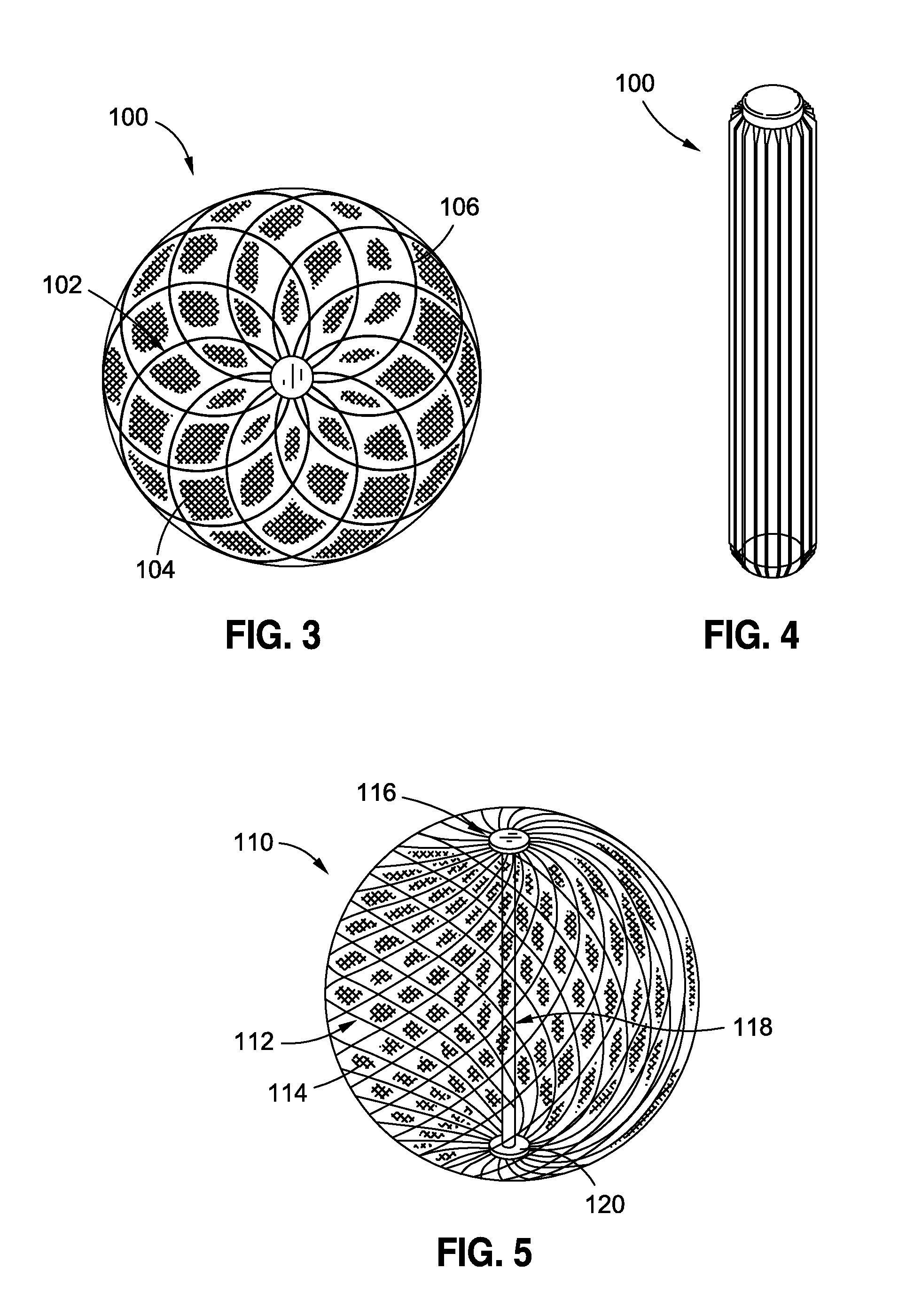
Intragastric implants with duodenal anchors
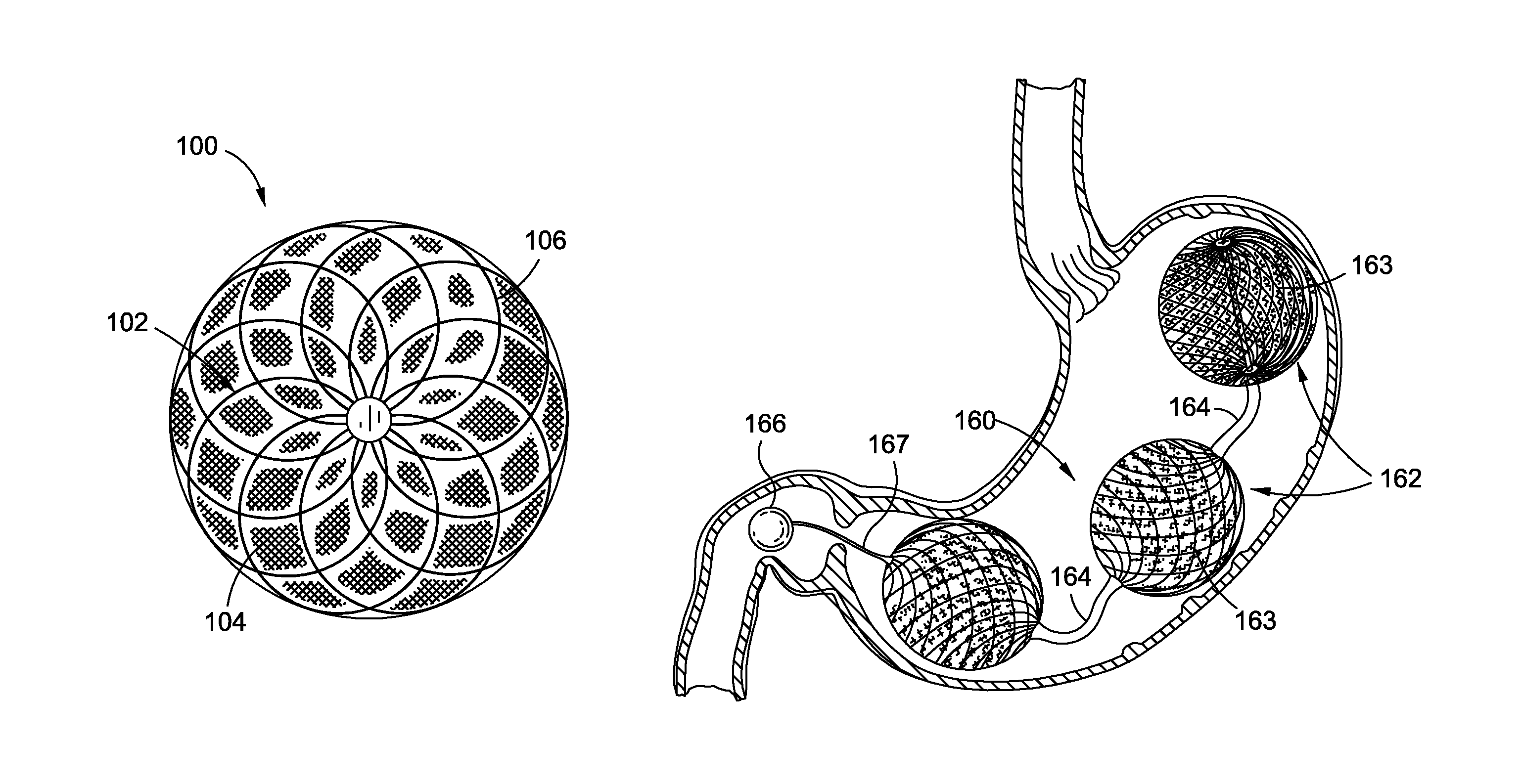
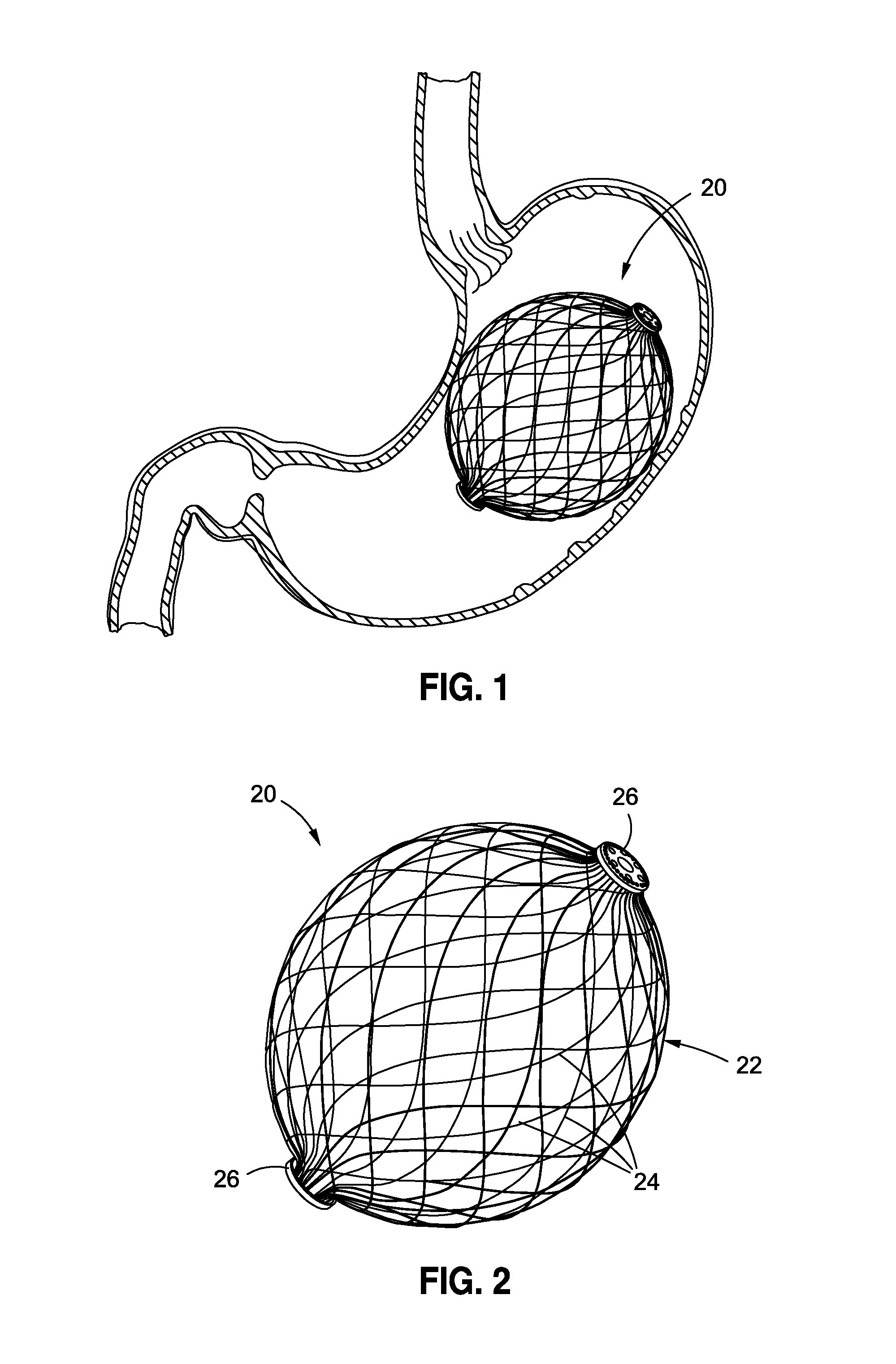
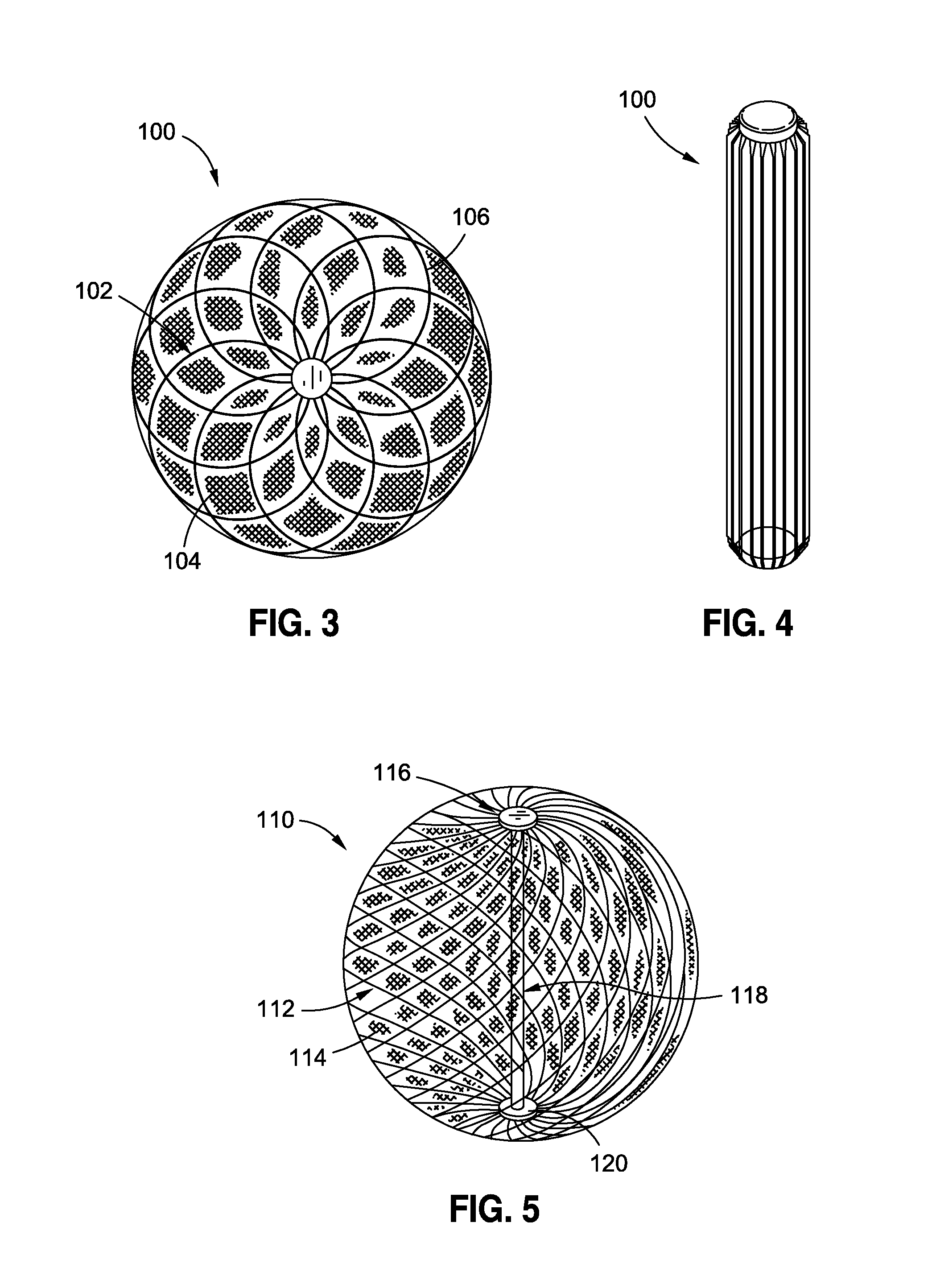
InactiveUS20120095385A1Reducing food ingestedLower the volumeSurgeryDilatorsPatient riskStomach walls
Intragastric fluid transfer devices and related methods for operation thereof are disclosed. The intragastric fluid transfer devices and related methods are intended to assist a patient in maintaining a healthy body weight by stimulating the inner stomach walls and / or the inner duodenum walls. Features of the intragastric fluid transfer device include insertion of the devices transorally and without invasive surgery, without associated patient risks of invasive surgery, and without substantial patient discomfort. The life span of these intragastric fluid transfer devices may be material-dependent upon long-term survivability within an acidic stomach, but is intended to last one year or longer.
Owner:ALLERGAN INC
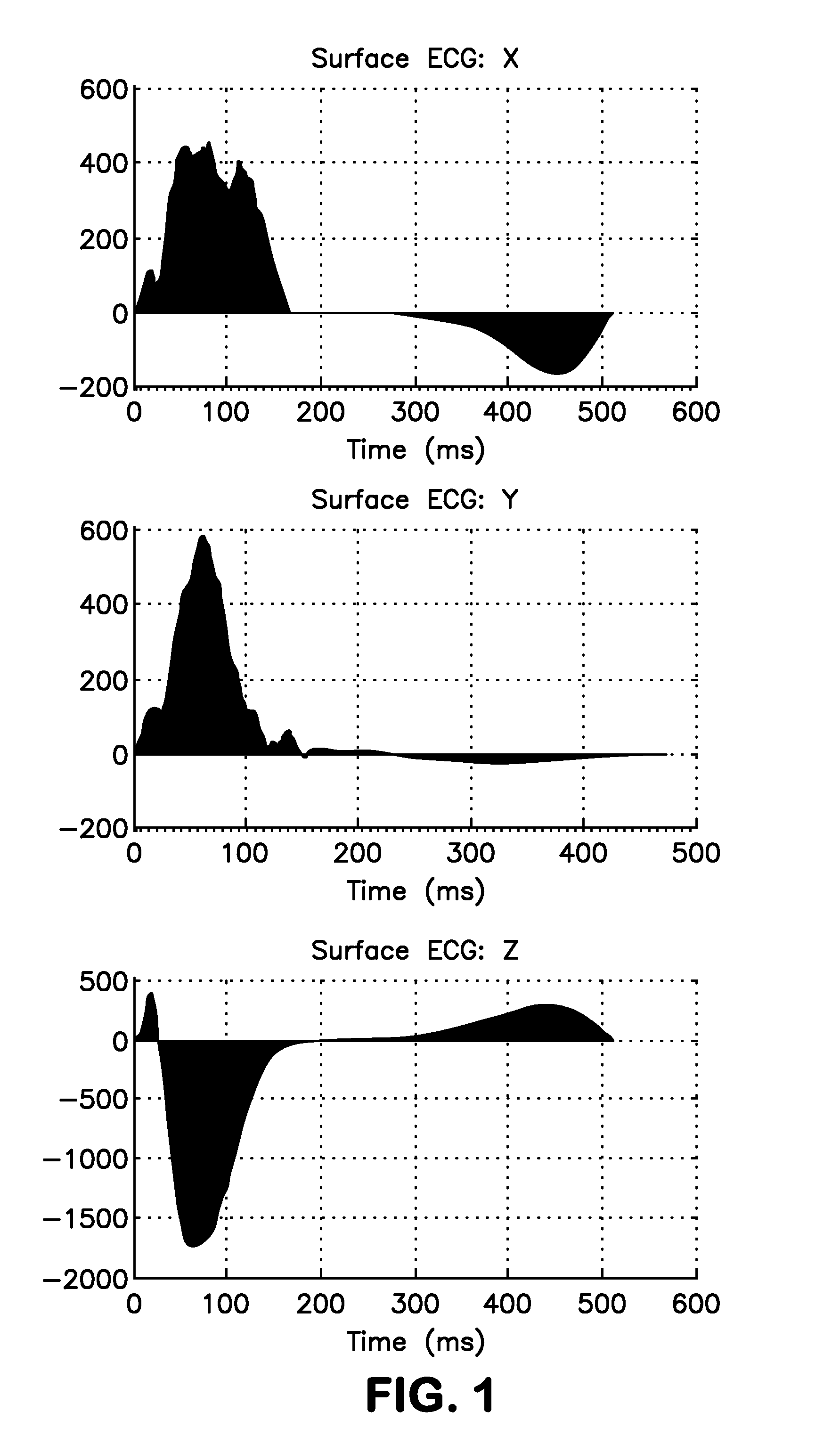
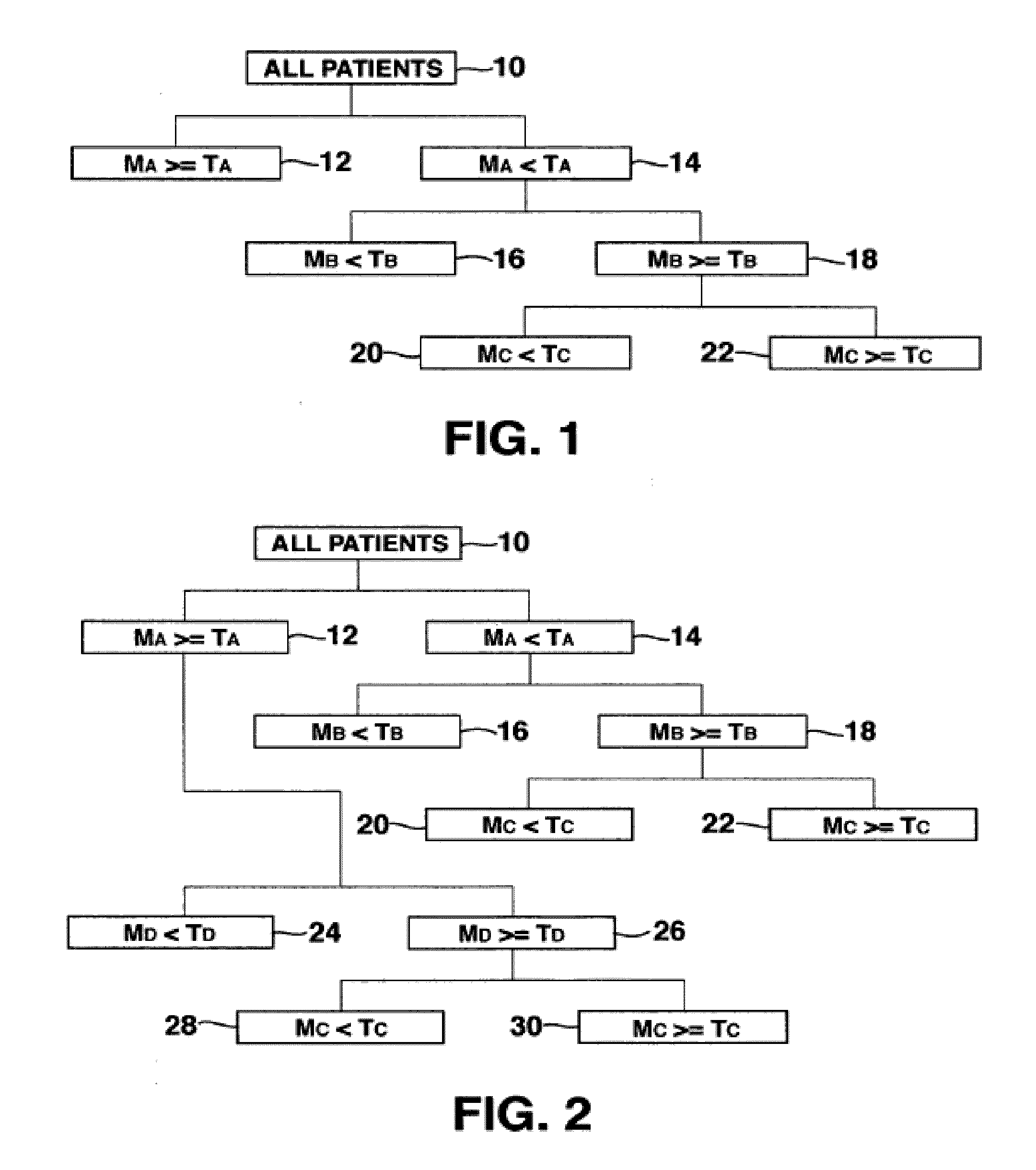
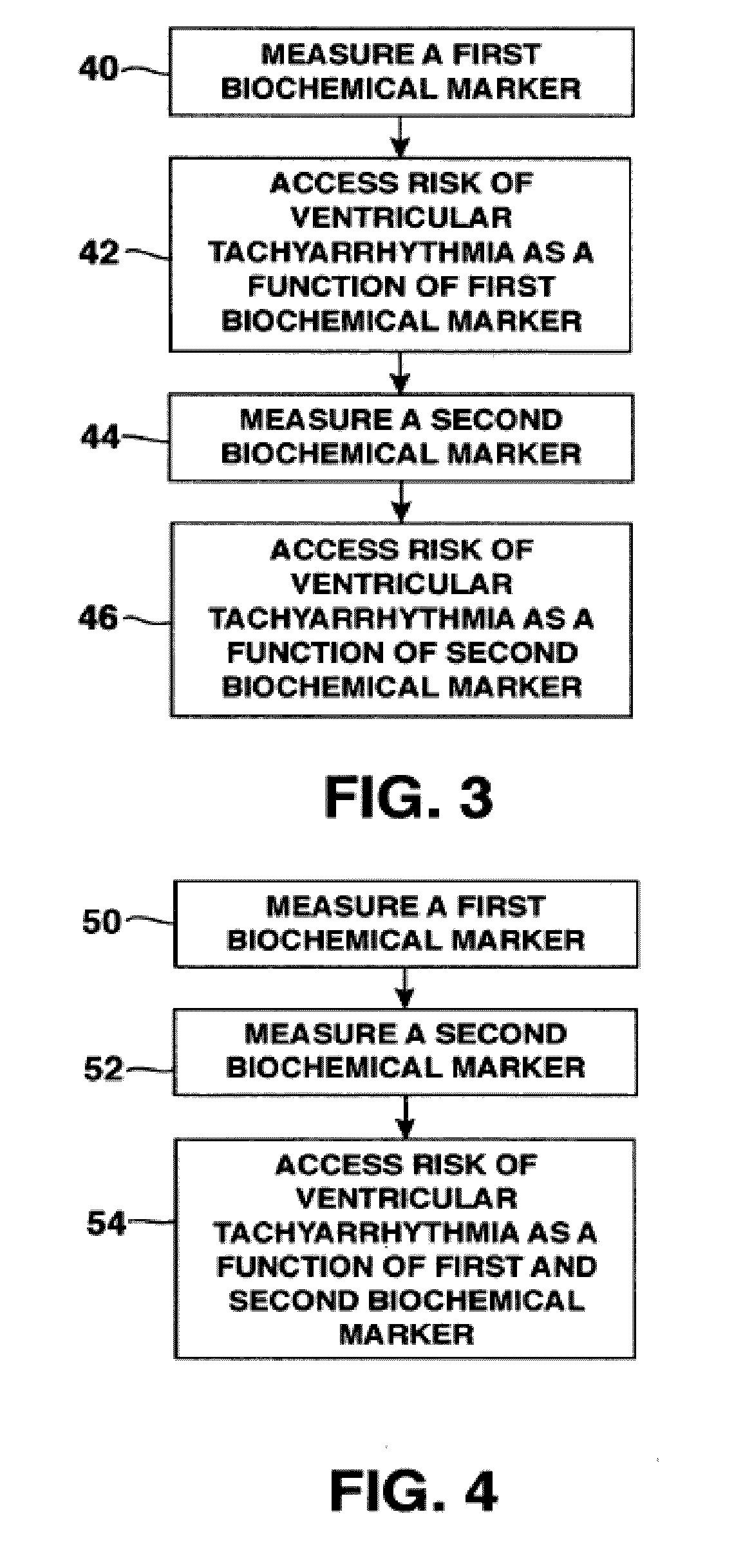
Methods for determining risk of ventricular arrhythmia
ActiveUS20110251504A1Highly determinativeHigh riskElectrocardiographySensorsPatient riskVentricular dysrhythmia
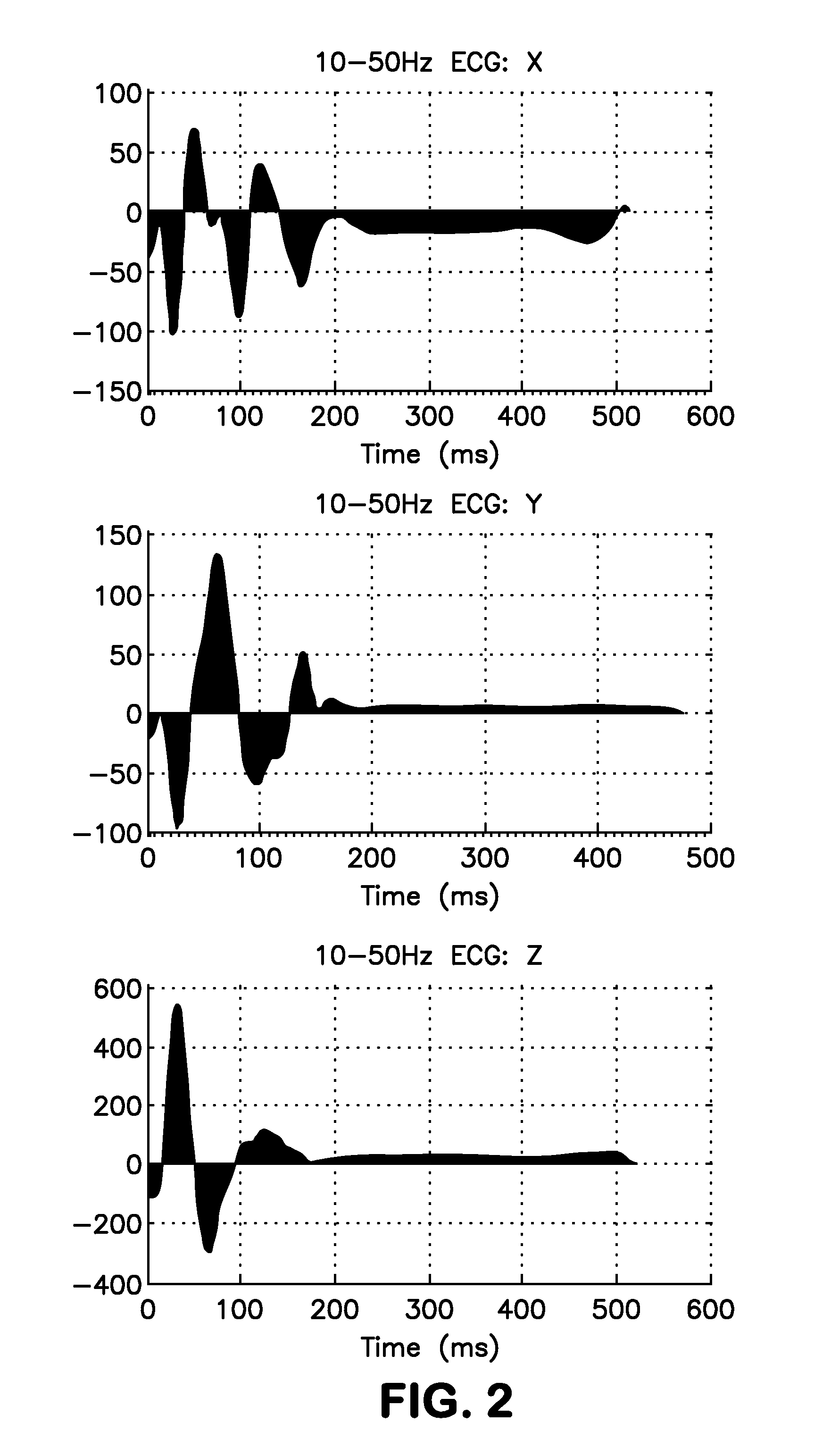
Methods and systems are disclosed for analyzing three dimensional orthogonal ECG measurements to assess patient risk of a subsequent cardiac event based on evaluation of cardiac vector values in view of risk factors defined by the invention.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Central data exchange node for system monitoring and control of blood glucose levels in diabetic patients
ActiveUS20160331310A1Easy to replaceGuaranteed uptimeInertial sensorsMedical devicesNODALClosed loop
A flexible system capable of utilizing data from different monitoring techniques and capable of providing assistance to patients with diabetes at several scalable levels, ranging from advice about long-term trends and prognosis to real-time automated closed-loop control (artificial pancreas). These scalable monitoring and treatment strategies are delivered by a unified system called the Diabetes Assistant (DiAs) platform. The system provides a foundation for implementation of various monitoring, advisory, and automated diabetes treatment algorithms or methods. The DiAs recommendations are tailored to the specifics of an individual patient, and to the patient risk assessment at any given moment. A central data exchange node or server collects patient data from individual DiAs devices and provides safety assurance, monitoring, telemedicine and database building for the DiAs system.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
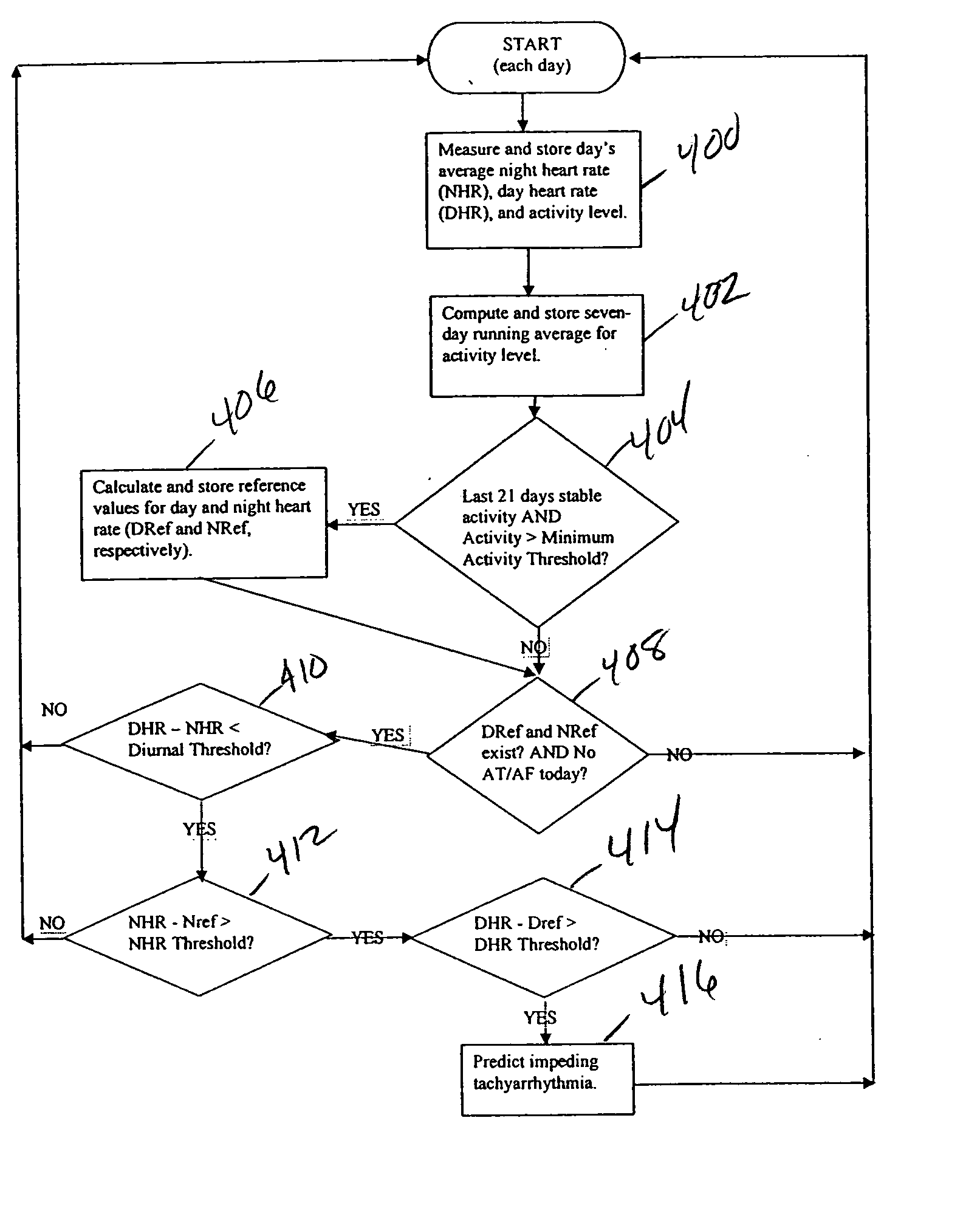
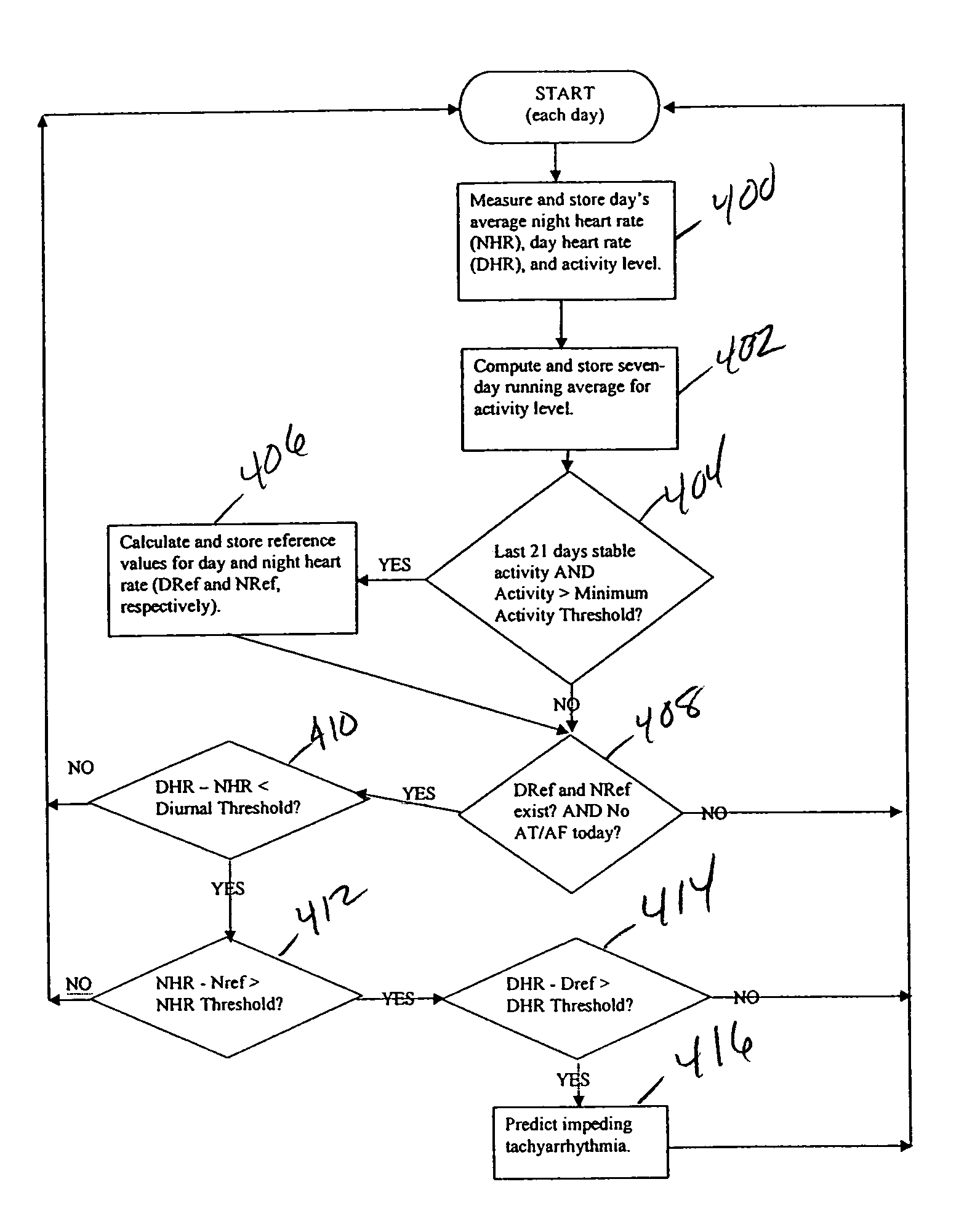
Method and apparatus for predicting arrhythmias using diurnal heart rate
ActiveUS20050137489A1Physical therapies and activitiesElectrocardiographyPatient riskPATIENT PHYSICAL
A method of predicting an arrhythmia, such as ventricular tachycardia, for example, in a medical device using a quantitative measure in order to allow assessment of patient risk and to enable preventative interventions by the device and clinicians. The trending of day and night average heart rates, along with patient physical activity can be analyzed to provide prediction of impending arrhythmia within weeks. By examining day and night average heart rate for crossover points, where the night heart rate equals or exceeds the day rate, and monitoring for a concomitant elevation in the night heart rate from a reference value, specific days heralding an increased risk of arrhythmia can be determined and therapy can be updated accordingly
Owner:MEDTRONIC INC
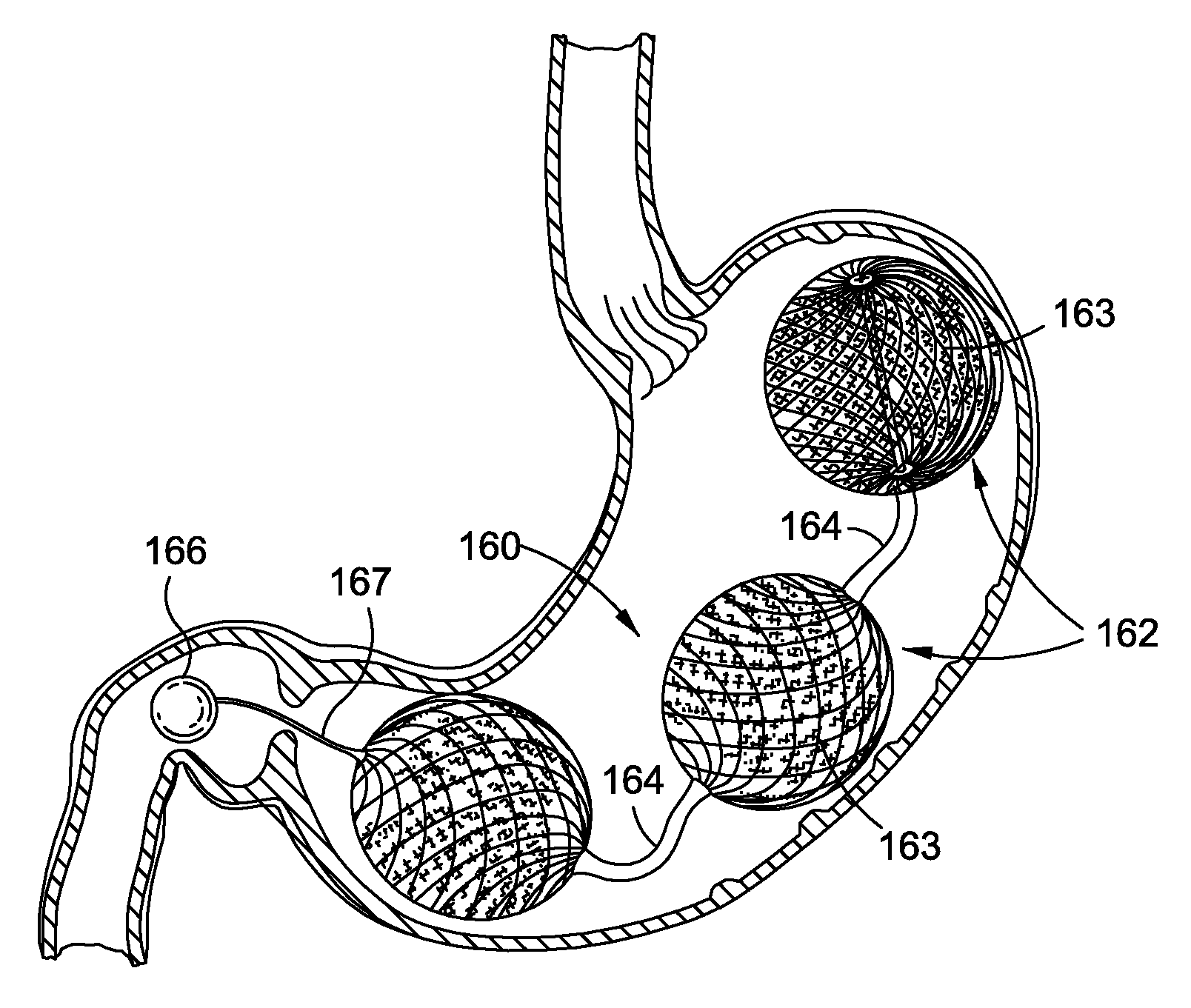
Intragastric implants with collapsible frames
Transoral obesity treatment devices and related methods for operation thereof are described which occupy space within a stomach and / or stimulate the stomach wall. The transoral obesity treatment devices and related methods are intended to assist a patient in maintaining a healthy body weight. Features of the devices include insertion transorally and without invasive surgery, without associated patient risks of invasive surgery, and without substantial patient discomfort. The life span of these devices may be material-dependent upon long-term survivability within an acidic stomach, but is intended to last one year or longer. The devices have the capacity to vary in size and are desirably self-actuating in that they change shape and / or volume using internal motors or actuators. The changing character of the devices helps prevent the person's stomach from compensating for the implant, such as sometimes happens with static intragastric devices.
Owner:APOLLO ENDOSURGERY INC
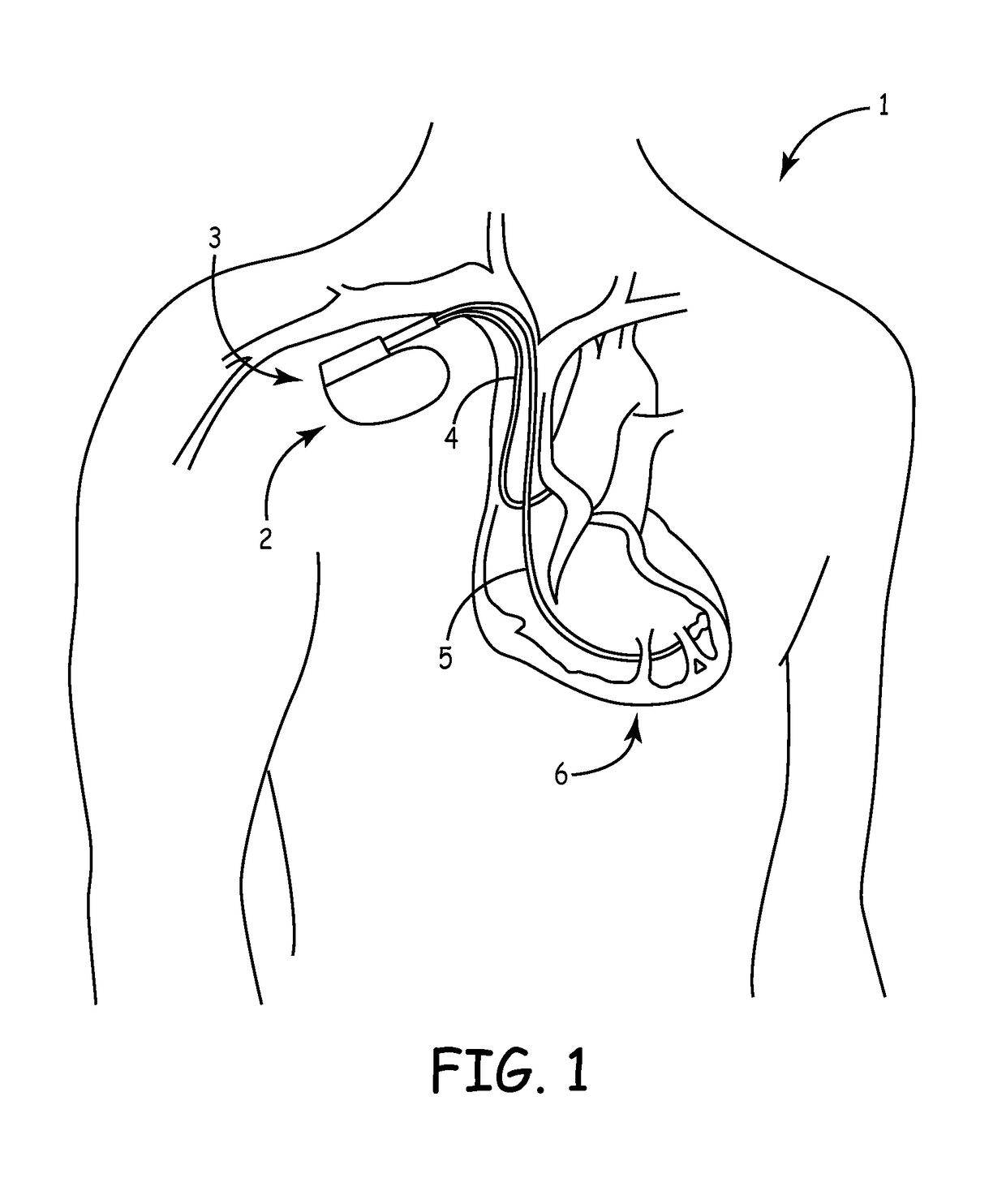
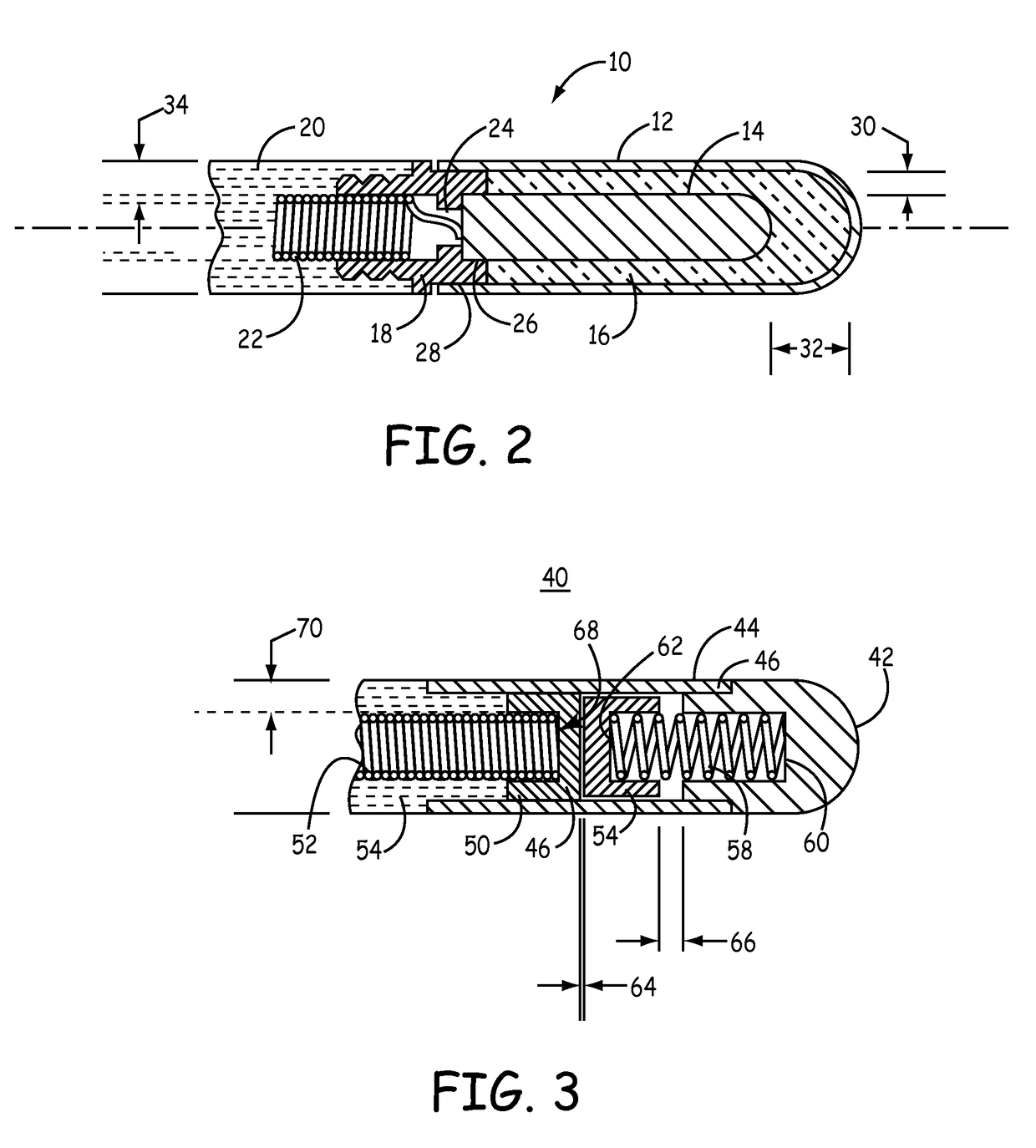
Mri-safe defibrillator electrodes
The present invention reduces patient risks associated with RF-induced thermogenic tissue damage and with pulsed gradient-field-induced arrhythmias by using a defibrillator lead having a self-healing dielectric material that prevents induced voltages from MRI equipment from damaging an ICD or causing unintended defibrillation shocks to a patient. Another aspect of the present invention utilizes a sliding contact arrangement to prevent induced voltages from MRI equipment from being electrically coupled to an ICD thereby reducing patient risks associated with RF-induced thermogenic tissue damage and with pulsed gradient-field-induced arrhythmias.
Owner:MEDTRONIC INC
Method for expanding the domain of imaging software in a diagnostic work-up
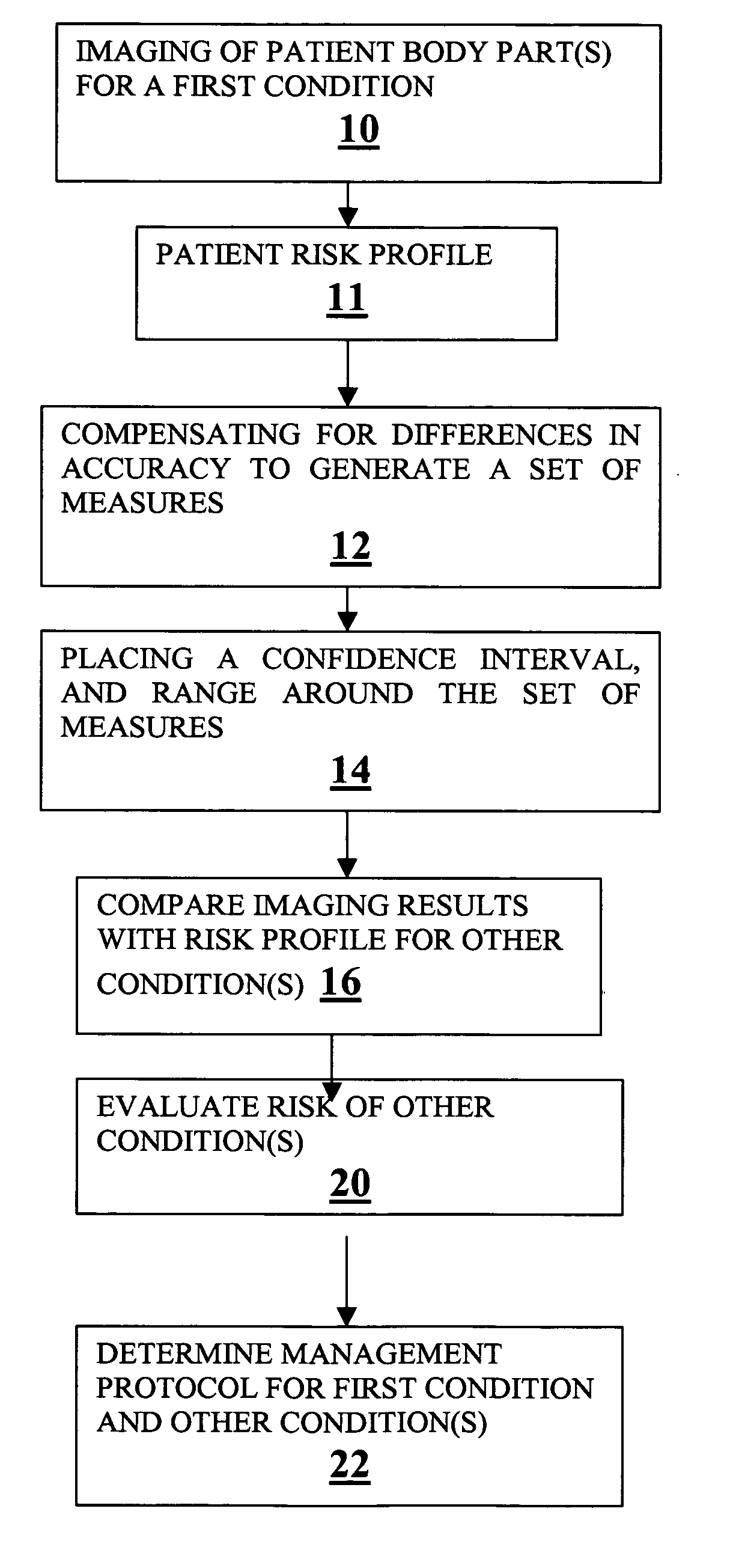
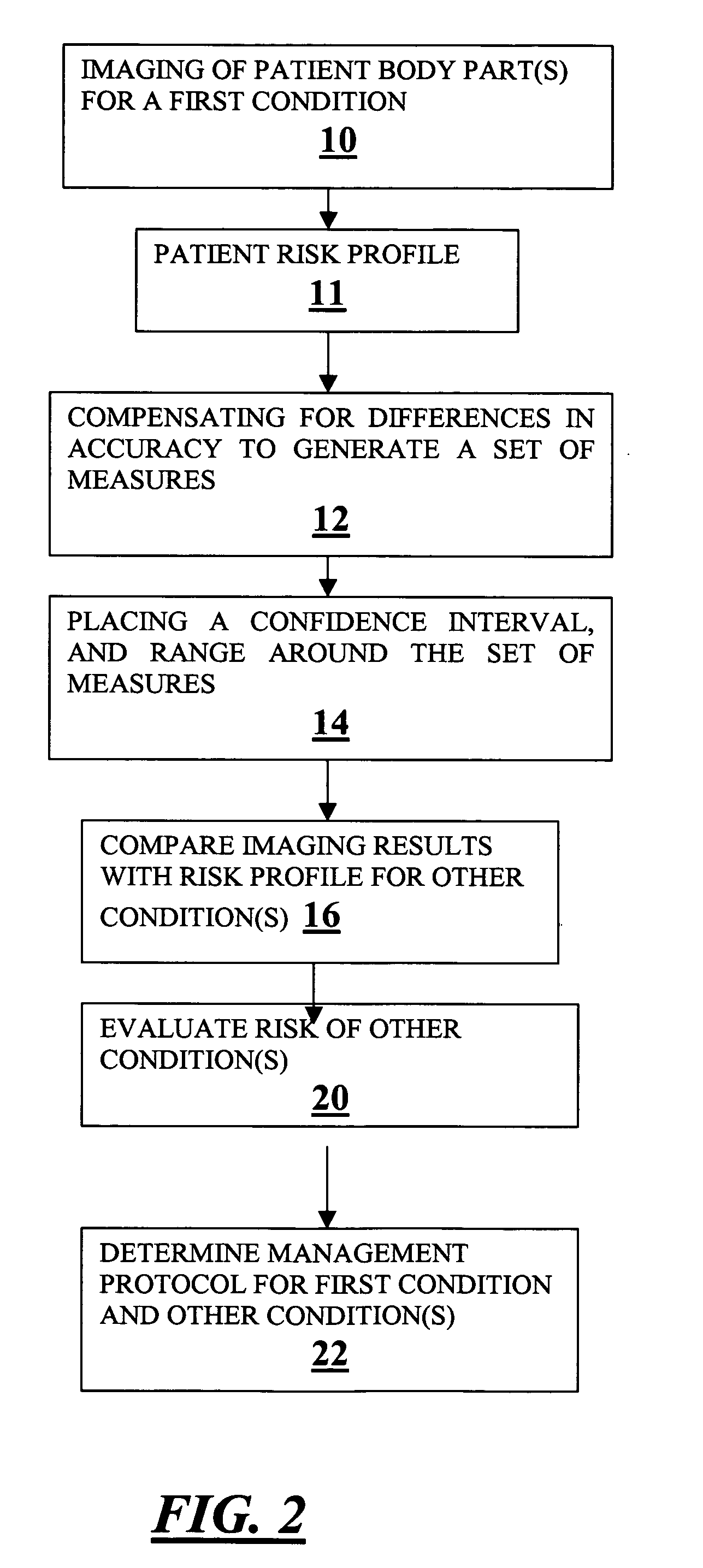
A method for expanding the domain of imaging software in a diagnostic work-up includes the steps of: imaging a patient's body parts for a first condition using a first imaging technique directed to a first condition; acquiring known patient risk factors indicating a second condition; compensating for differences in accuracy between a first imaging technique directed to a first condition, and a second imaging technique directed to testing for the second condition to generate a set of measures; placing a range and confidence interval around the set of measures; and evaluating for a second condition using the set of measures and known patient risk factors.
Owner:YANKELEVITZ DAVID DR +1
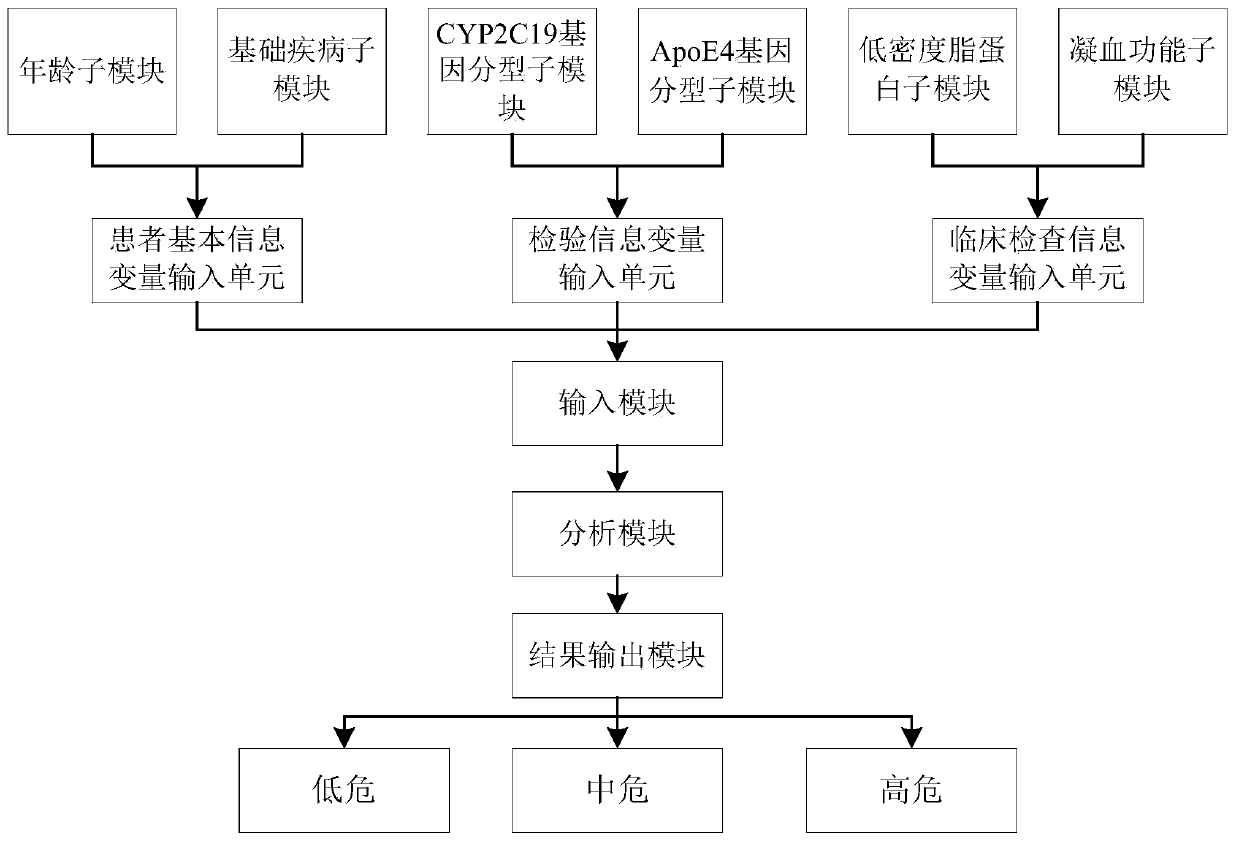
Clinical cerebral infarction patient recurrence risk early warning scoring visual model system and evaluation method thereof
InactiveCN110634573AEasy to operateEasy to handleMedical simulationHealth-index calculationLow risk groupLower risk
The invention discloses a clinical cerebral infarction patient recurrence risk early warning scoring visual model system. The system comprises an input module, an analysis module and a result output module. The invention also discloses an evaluation method of the clinical cerebral infarction patient recurrence risk early warning scoring visual model system. A clinical cerebral infarction patient recurrence risk evaluation nomogram is concise, popular and easy to understand and convenient for clinicians and patients to operate, and a current cerebral infarction recurrence risk of the patients is predicted. And meanwhile, high-risk, medium-risk and low-risk groups can be clearly distinguished according to patient risk scores calculated by a risk scoring formula in the model so that the clinicians are assisted to formulate an efficient treatment scheme. In the invention, a doctor and the patient are guided to evaluate early warning scores of the clinical cerebral infarction patient recurrence risk in combination with a statistical method, and patient data acquisition is verified through a corresponding mathematical statistical method so that the doctor and the patient can quickly grasp the clinical cerebral infarction patient recurrence risk and provide early warning information.
Owner:南昌大学第一附属医院
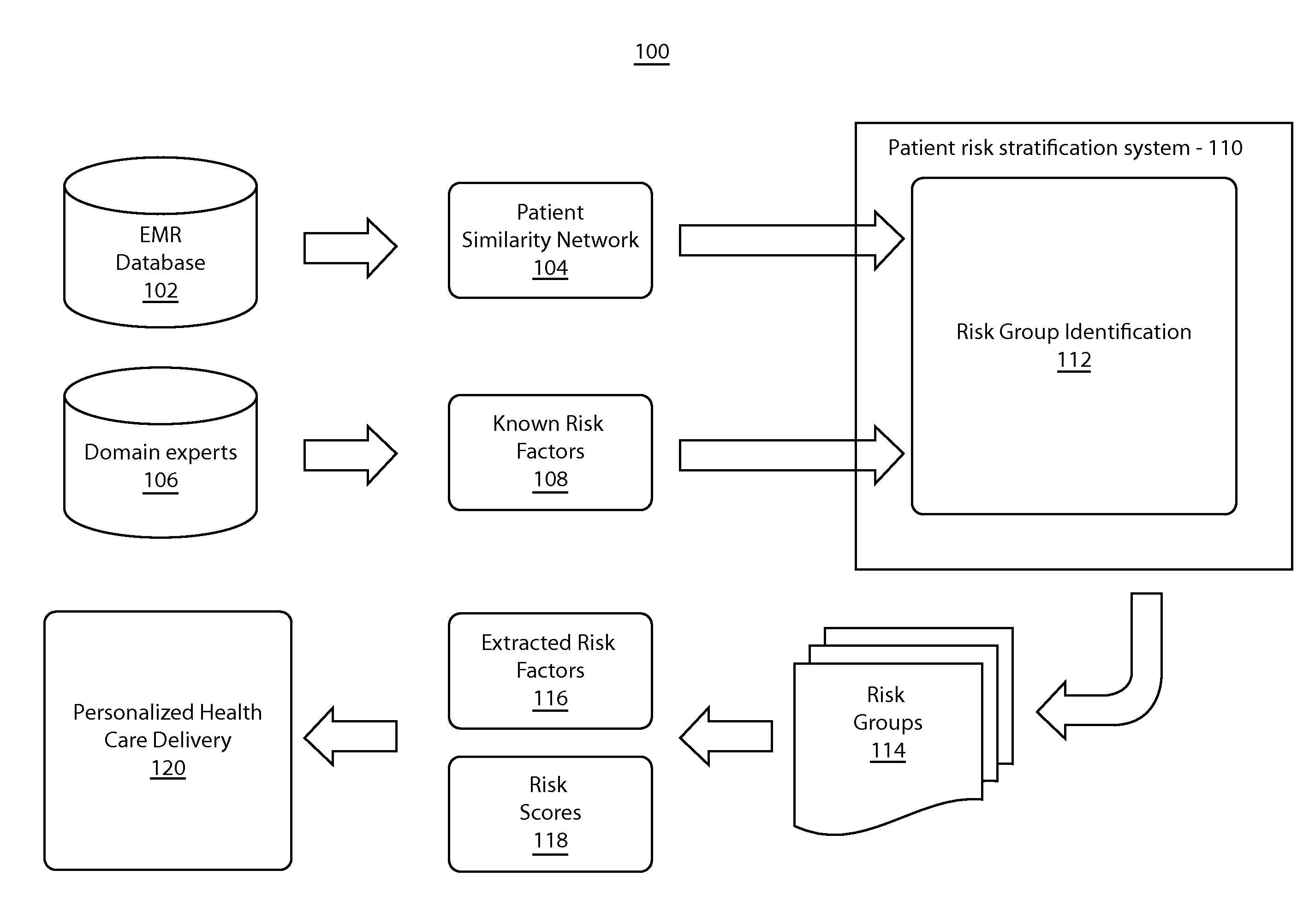
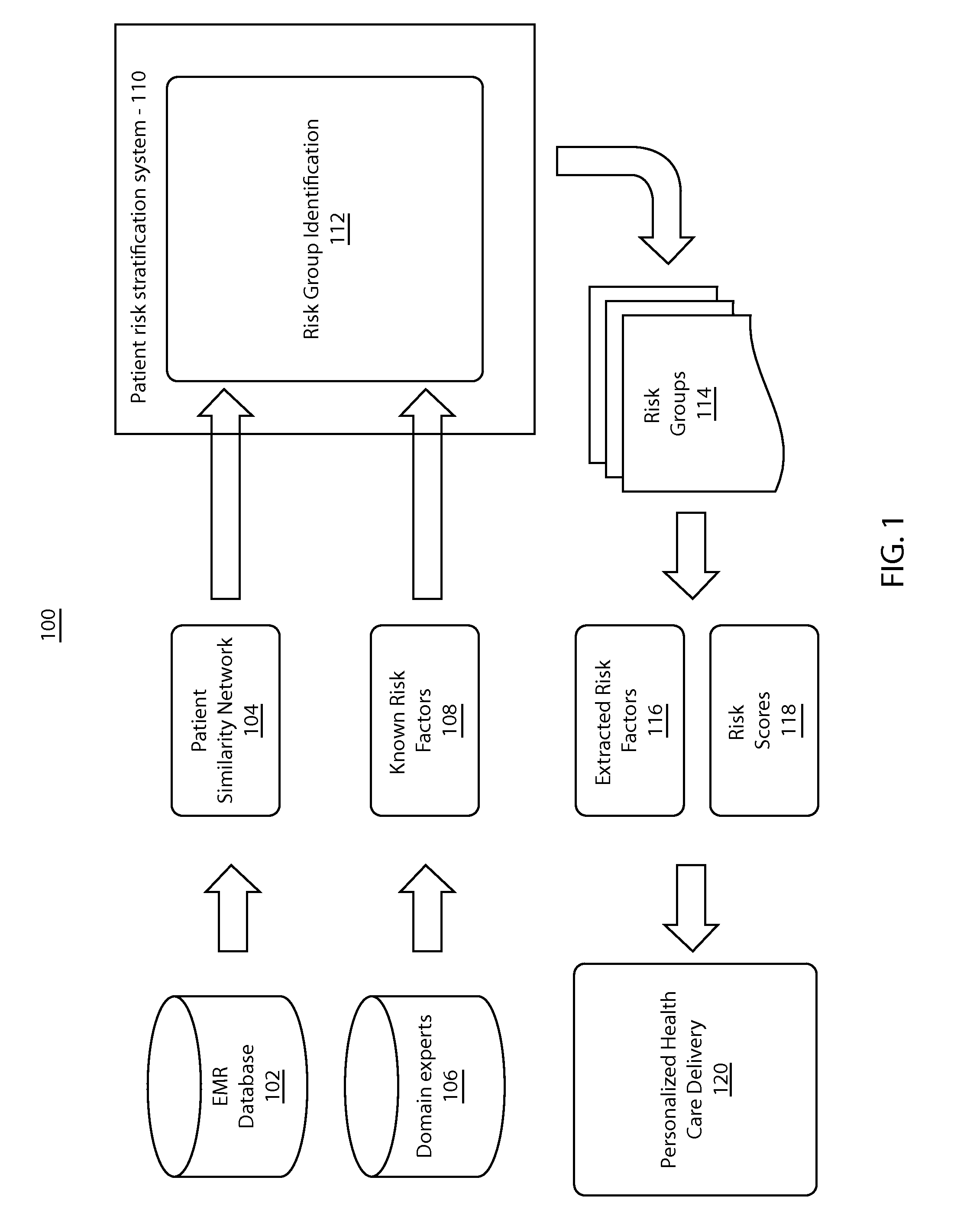
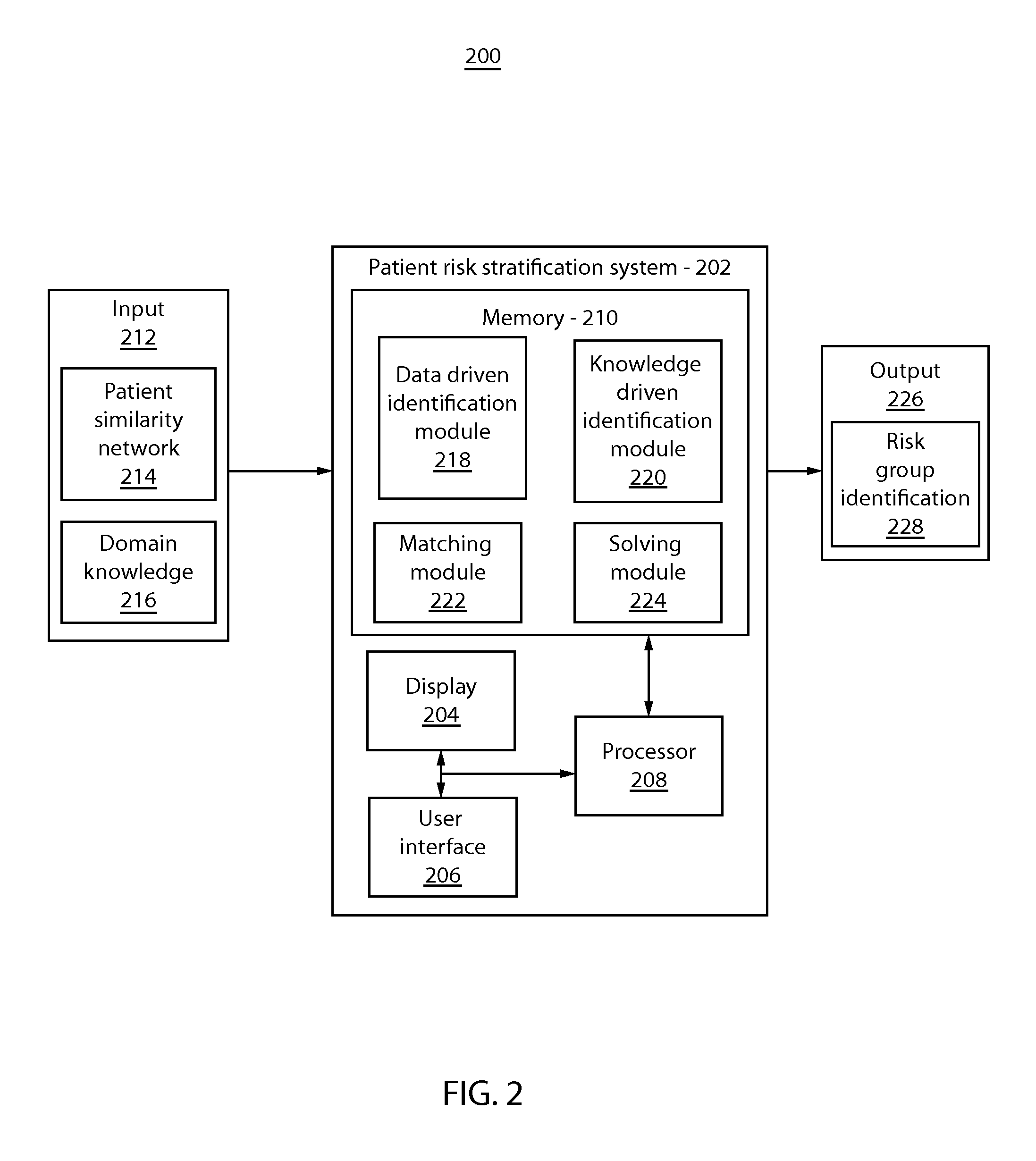
Patient risk stratification by combining knowledge-driven and data-driven insights
A system and method for patient stratification include determining a first set of patient groups from patients in a patient similarity graph based on a similarity structure of the patient similarity graph. A second set of patient groups is identified based on expert domain knowledge associated with the patients. Patients in the first set and the second set are aligned using a processor to stratify patients.
Owner:IBM CORP
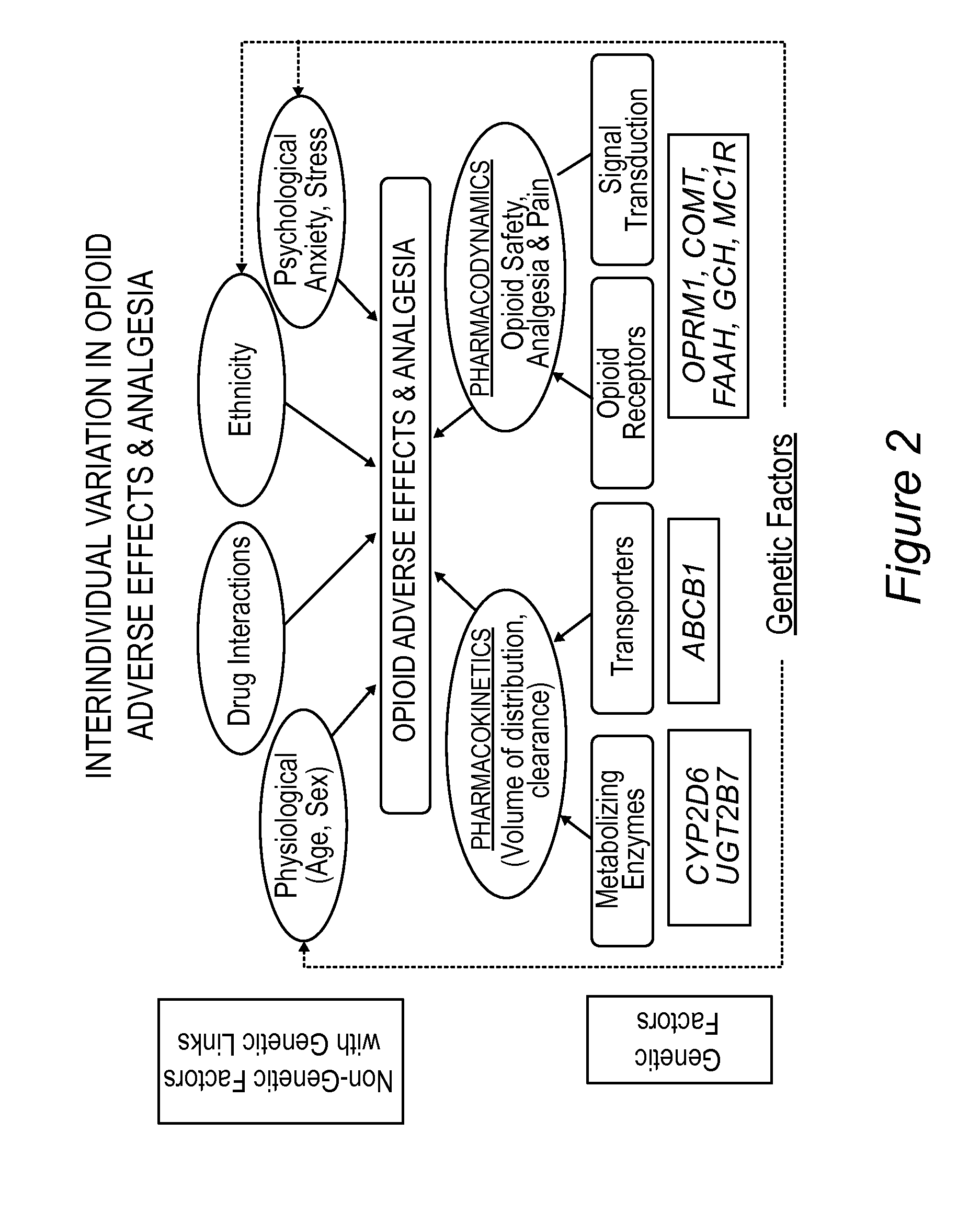
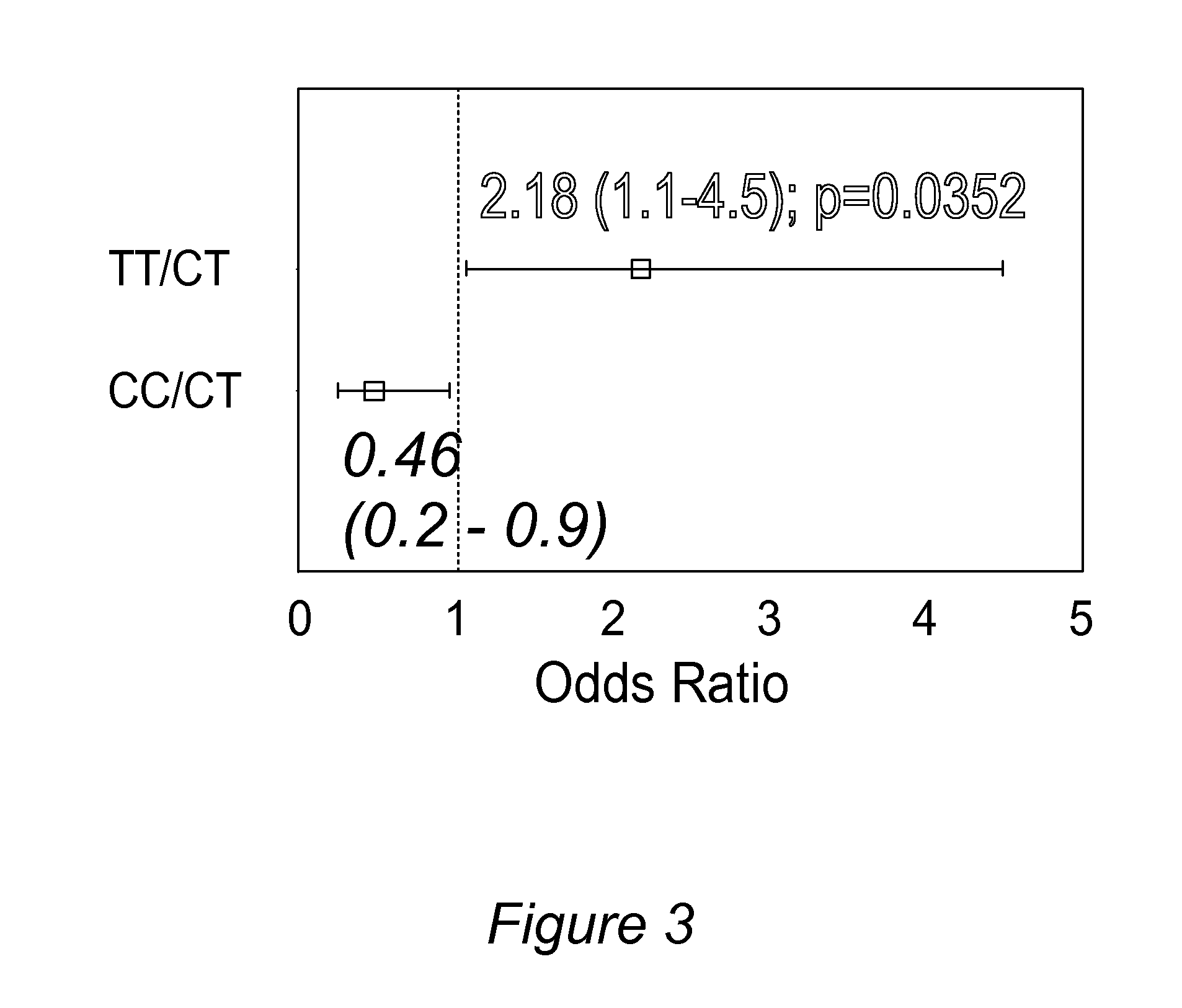
Personalized pain management and anesthesia: preemptive risk identification and therapeutic decision support
ActiveUS20140371256A1Maximize pain reliefAdverse effect in subjectBiocideMicrobiological testing/measurementPersonalizationSide effect
Methods and compositions disclosed herein generally relate to methods of improving clinical and economic outcomes to address adverse effects related to anesthesia, analgesics, opioids, and inadequate pain relief. Embodiments of the invention relate to the association between genes, specific polymorphisms of genes, and non-genetic factors with inadequate pain relief and anesthesia-, analgesic, and / or opioid-related adverse effects. Embodiments of the invention can be used to determine and manage patient risk factors for development of adverse perioperative effects and can allow for personalized anesthesia and pain management for improvement of pain control and reduction of anesthesia-, analgesic-, and opioid-related adverse outcomes. These methods and compositions apply to non-surgical pain management with opioids. Therefore, patients who are genetically predisposed to risk of inadequate pain relief and / or serious side effects from anesthesia, analgesics, and / or opioids can be identified and individualized treatment plans developed for implementation by the clinician to improve clinical and economic outcomes.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI
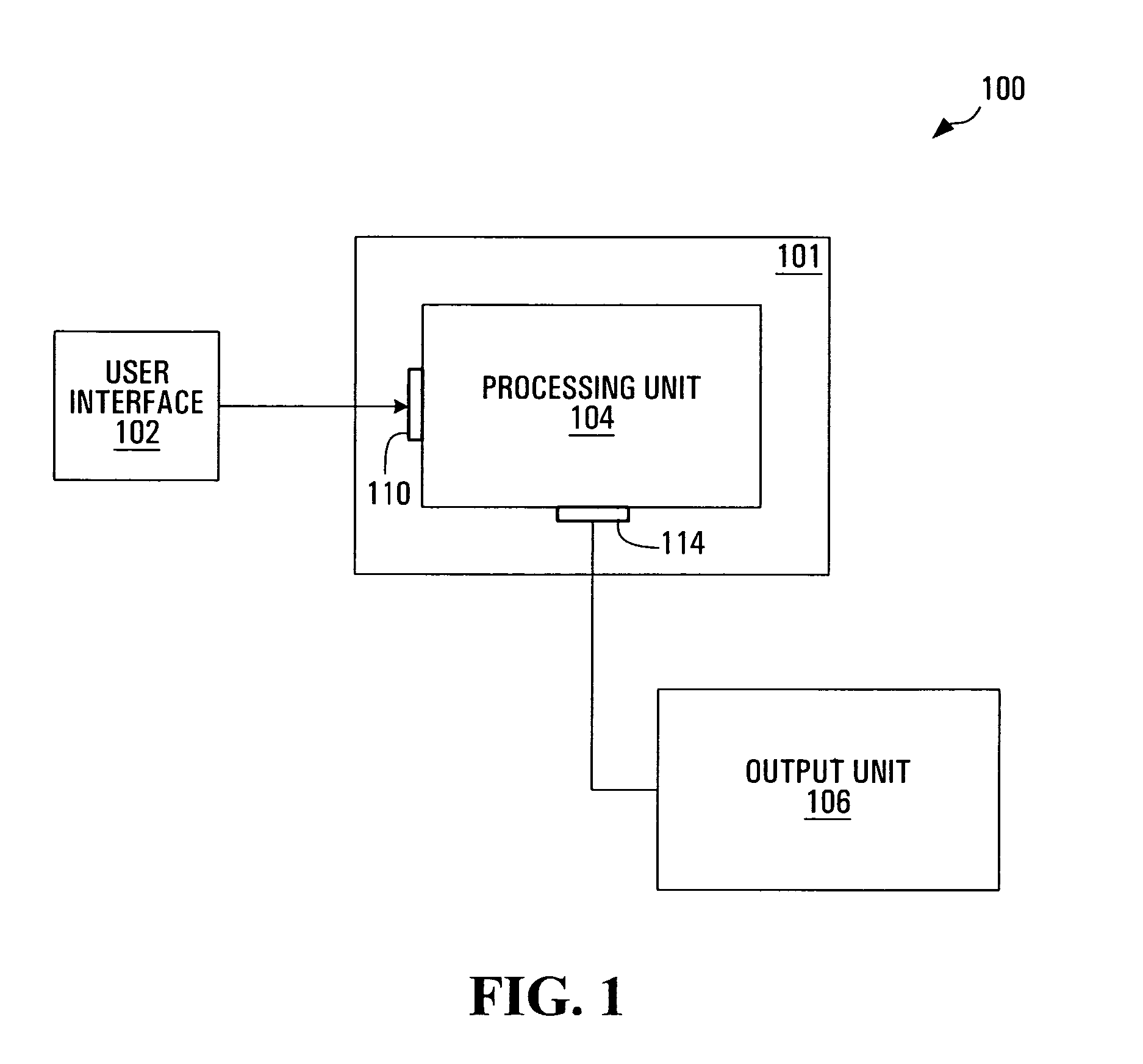
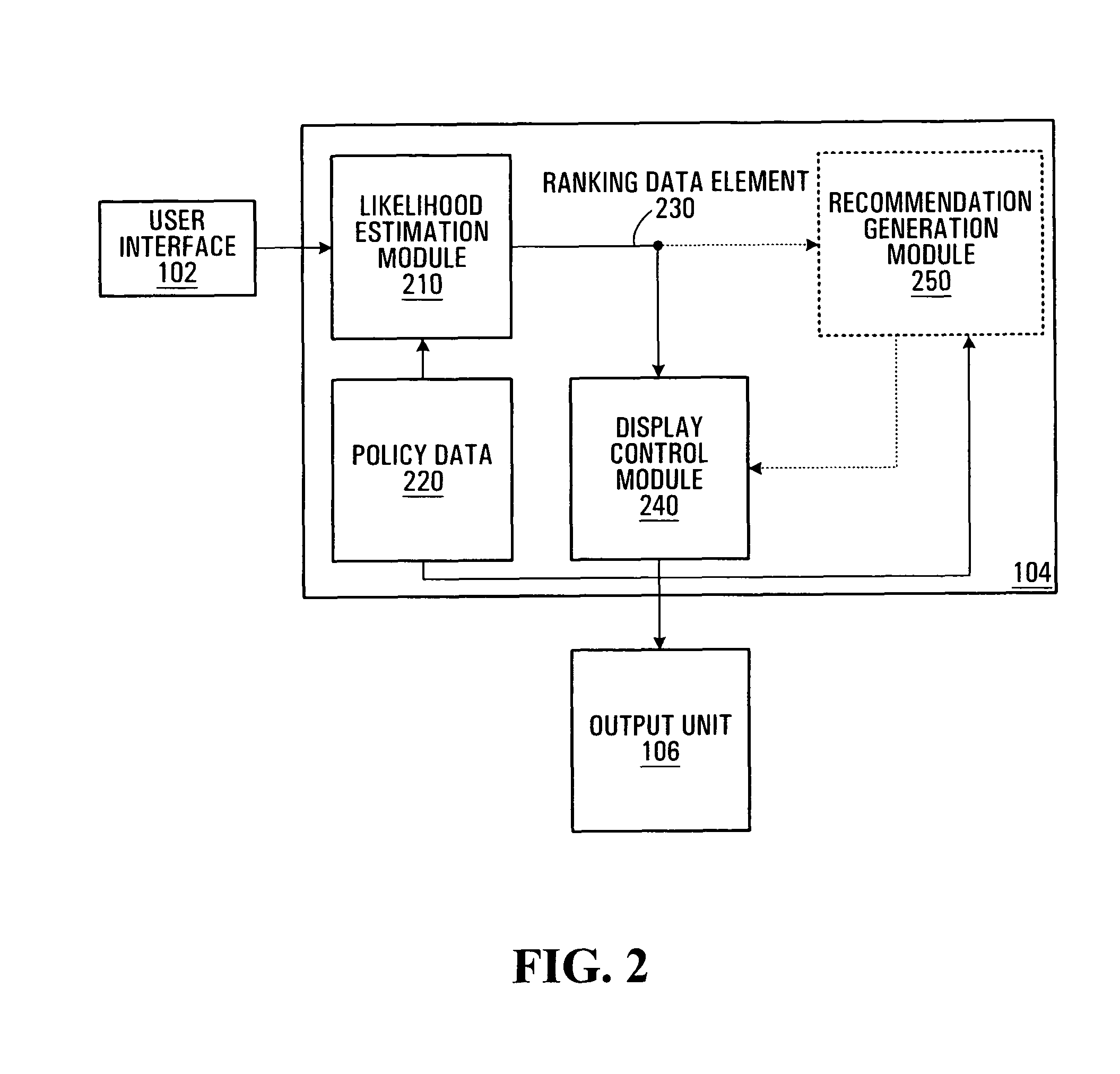
User Interface for Patient Risk Analysis System
A graphical user interface provides a system and method for representing patient risks and other clinical information. The risks correspond to the probabilities that the patient exhibits particular conditions and respective etiologies. In addition the graphical user interface provides a method for representing the utility of different possibly harmful modes of physiologic monitoring in estimating these risks.
Owner:ETIOMETRY
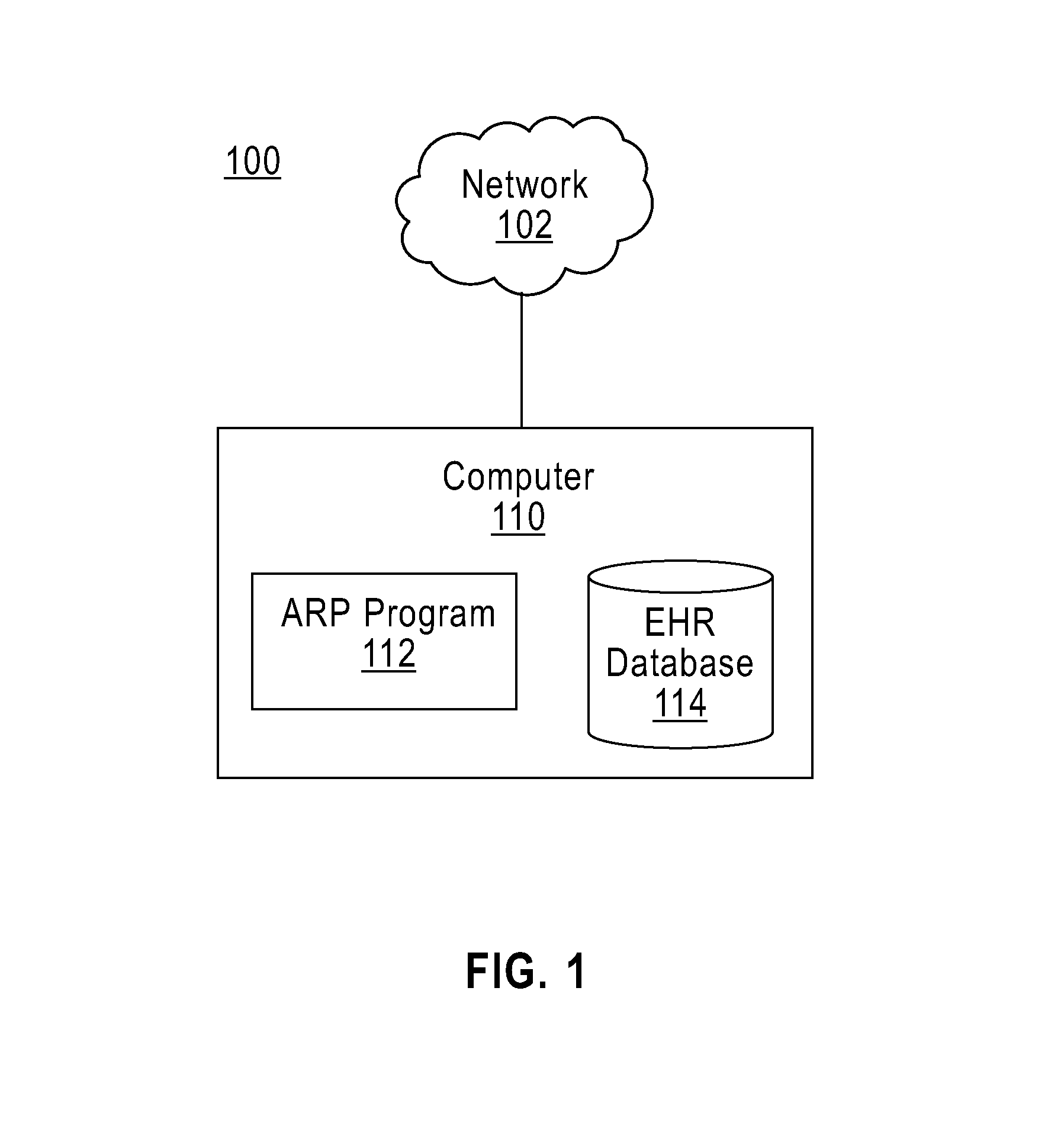
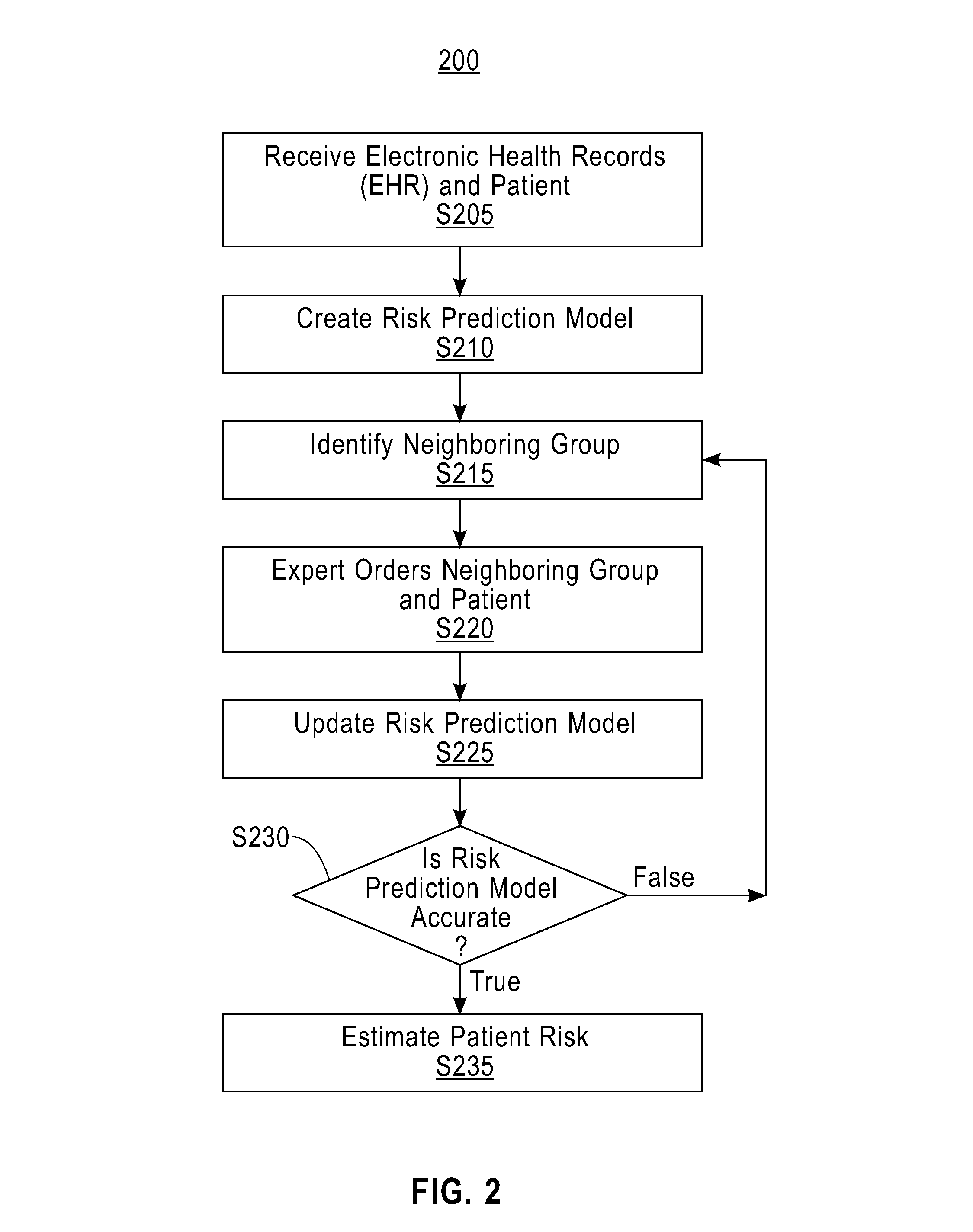
Active patient risk prediction
Electronic health records of a plurality of patients are received. A risk prediction model for a disease based on the electronic health records of the plurality of patients is created. An electronic health record of an original patient is received. A neighboring group of patients of the plurality of patients is identified, wherein the neighboring group of patients is two or more patients similar to the original patient. An ordering of the two or more patients of the neighboring group of patients is received, wherein the ordering of the two or more patients of the neighboring group of patients is based upon how similar each patient of the two or more patients is to the original patient. The risk prediction model is updated based on the ordering of the two or more patients of the neighboring group of patients.
Owner:IBM CORP
Identifying patients at risk for life threatening arrhythmias
The invention is a method for identifying proteins associated with sudden cardiac death (SCD) and for assessing a patient's risk of SCD by determining the amount of one or more SCD-associated proteins in the patient. Typically, the patient submits a sample, such as a blood sample, which is tested for one or more SCD-associated proteins. Based upon the results of the tests, the patient's risk of SCD may be assessed.
Owner:MEDTRONIC INC
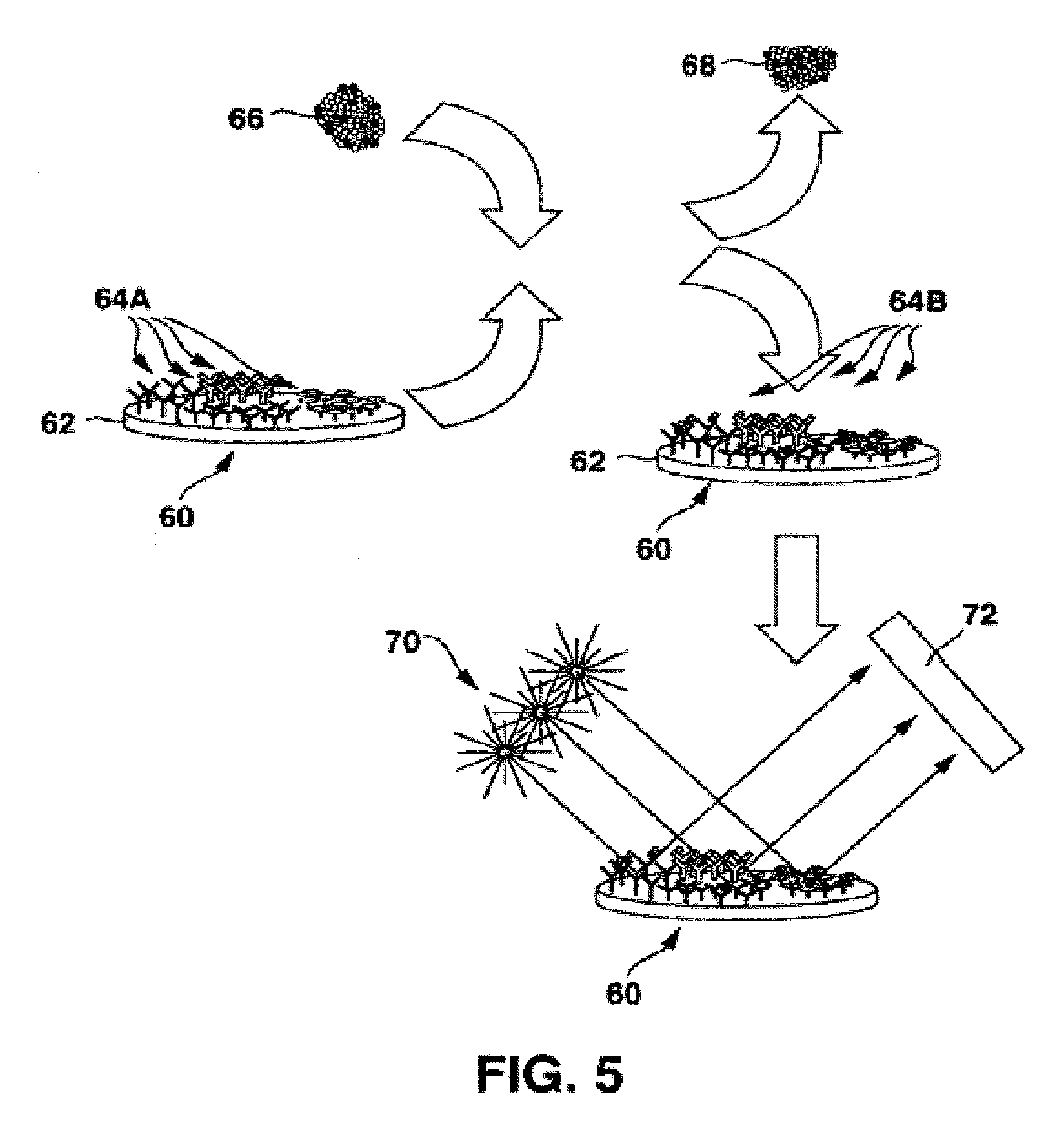
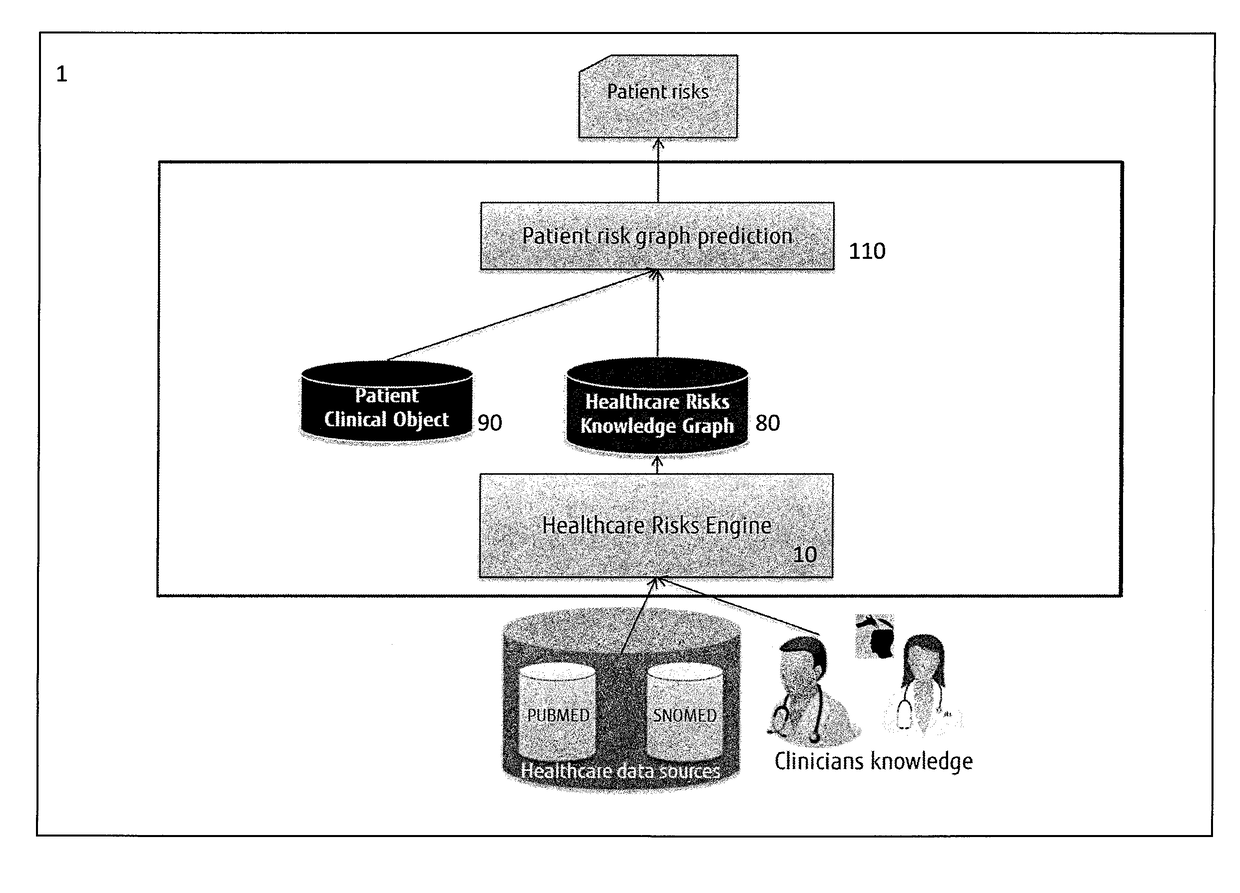
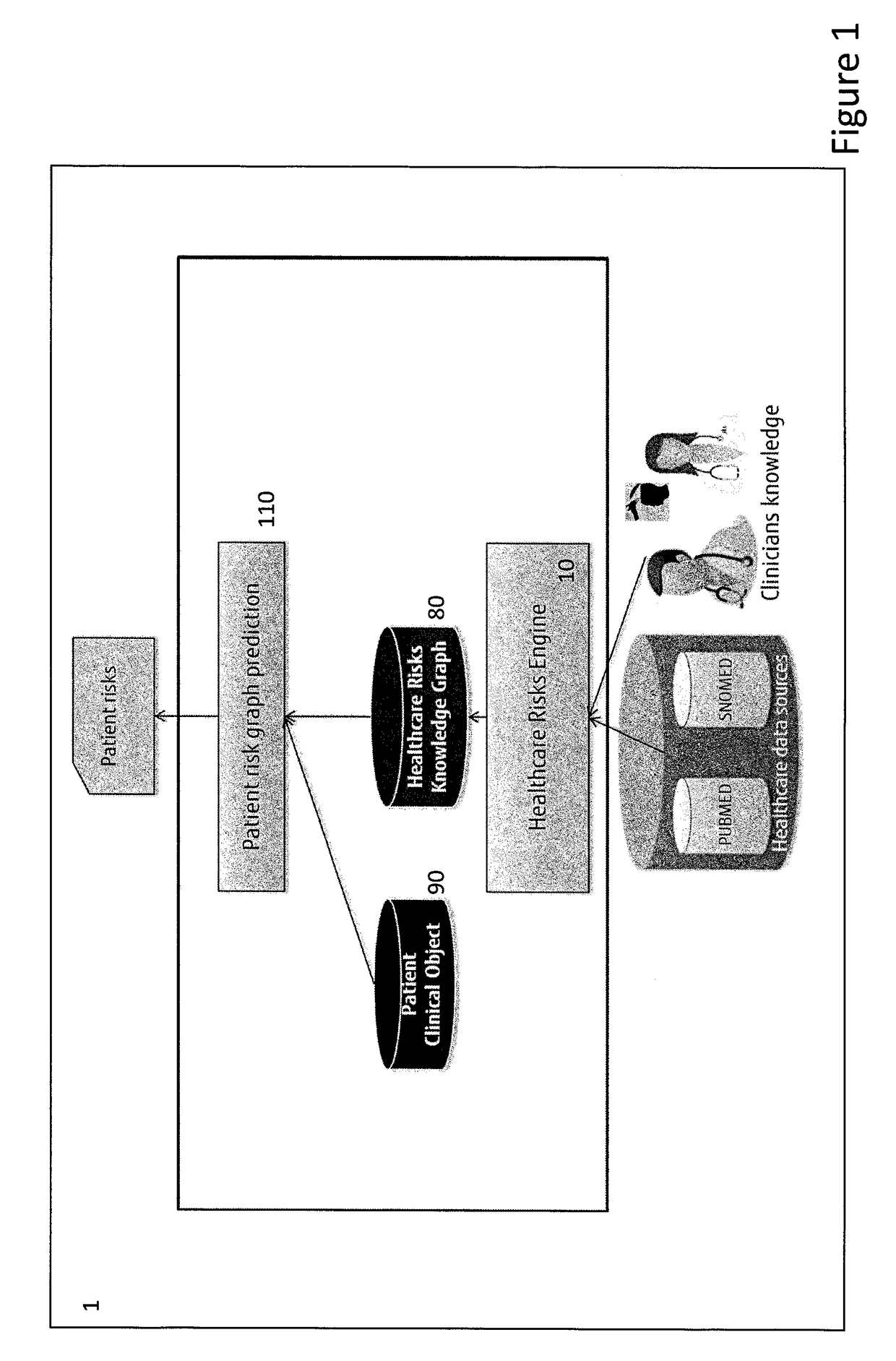
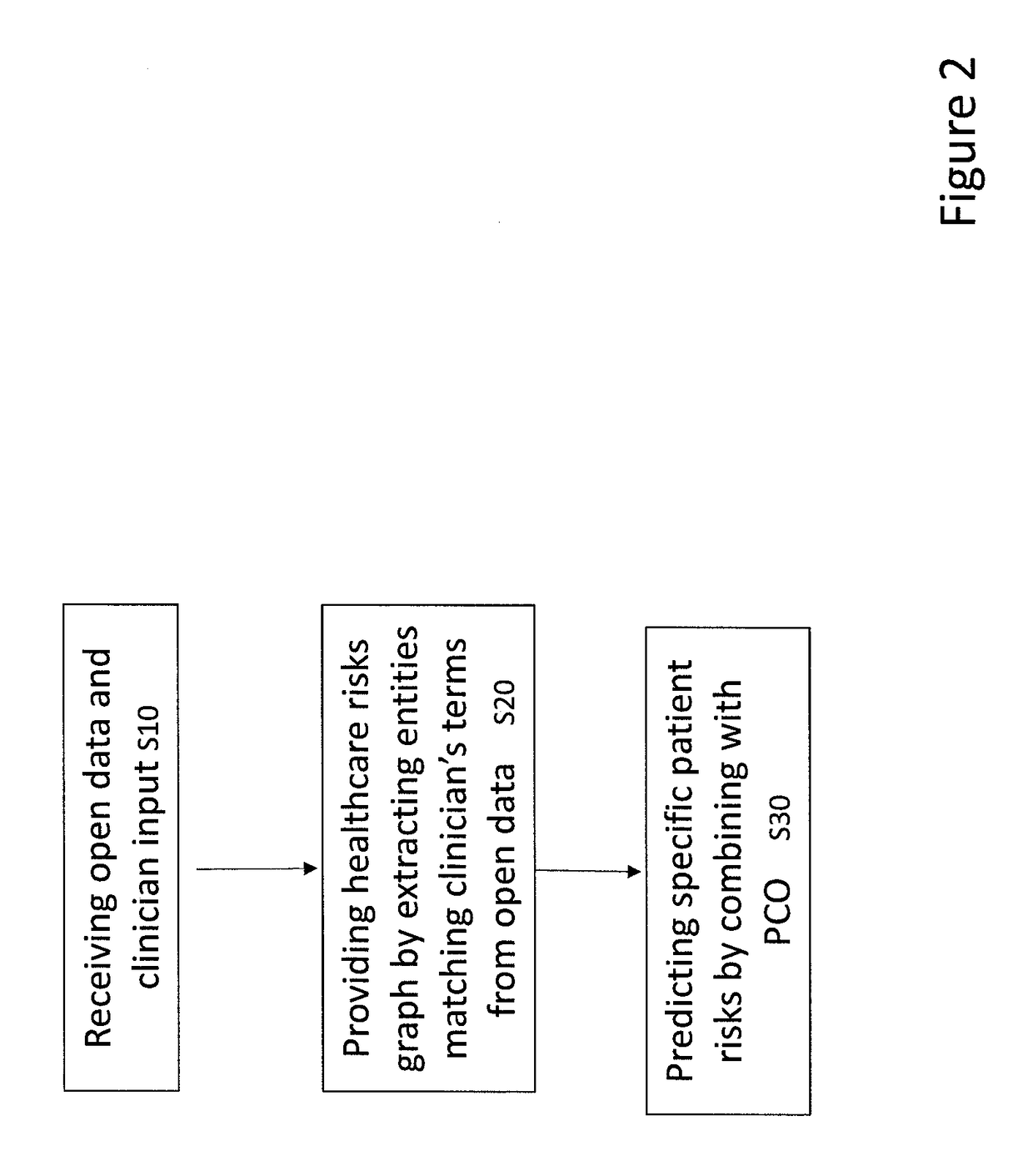
System and a method for assessing patient risk using open data and clinician input
ActiveUS20170277855A1Easy to understandHealth-index calculationMedical referencesPatient riskOpen data
A system for assessing patient risk using open data and input of knowledge data, the system including a healthcare knowledge data input to receive open data and a knowledge input to accept input of knowledge data relating to risk; a healthcare risk engine to provide a healthcare risk knowledge graph from the open data and knowledge data by using input of risk-related terms to retrieve documents from the open data and by extracting the healthcare risk knowledge graph as entities from the documents corresponding to risk-related terms, as well as links between the entities. A patient risk graph prediction module predicts risks for a patient by combining information in a Patient Clinical Object (PCO) with entities in the healthcare risk knowledge graph to produce a patient risk graph.
Owner:FUJITSU LTD
Methods for treatment of lymphomas with mutations in cell cycle genes
InactiveUS9241941B2Enormous potential to improveLow toxicityOrganic active ingredientsMicrobiological testing/measurementPatient riskCell Cycle Gene
The present disclosure identifies a novel subtype of follicular lymphoma (FL) characterized by dysregulation of the cyclin / CDK / RB proliferative pathway. This subtype of FL is associated with increased malignancy and mortality, relative to FL which is not associated with cell cycle dysregulation. Accordingly, this disclosure presents novel methods to subtype FL and stratify patient risk by detection of biomarkers associated with RB inactivation. This disclosure further presents novel therapies for the treatment of FL subtyped by inactivation of RB.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Intragastric implants with collapsible frames
Transoral obesity treatment devices and related methods for operation thereof are described which occupy space within a stomach and / or stimulate the stomach wall. The transoral obesity treatment devices and related methods are intended to assist a patient in maintaining a healthy body weight. Features of the devices include insertion transorally and without invasive surgery, without associated patient risks of invasive surgery, and without substantial patient discomfort. The life span of these devices may be material-dependent upon long-term survivability within an acidic stomach, but is intended to last one year or longer. The devices have the capacity to vary in size and are desirably self-actuating in that they change shape and / or volume using internal motors or actuators. The changing character of the devices helps prevent the person's stomach from compensating for the implant, such as sometimes happens with static intragastric devices.
Owner:APOLLO ENDOSURGERY INC
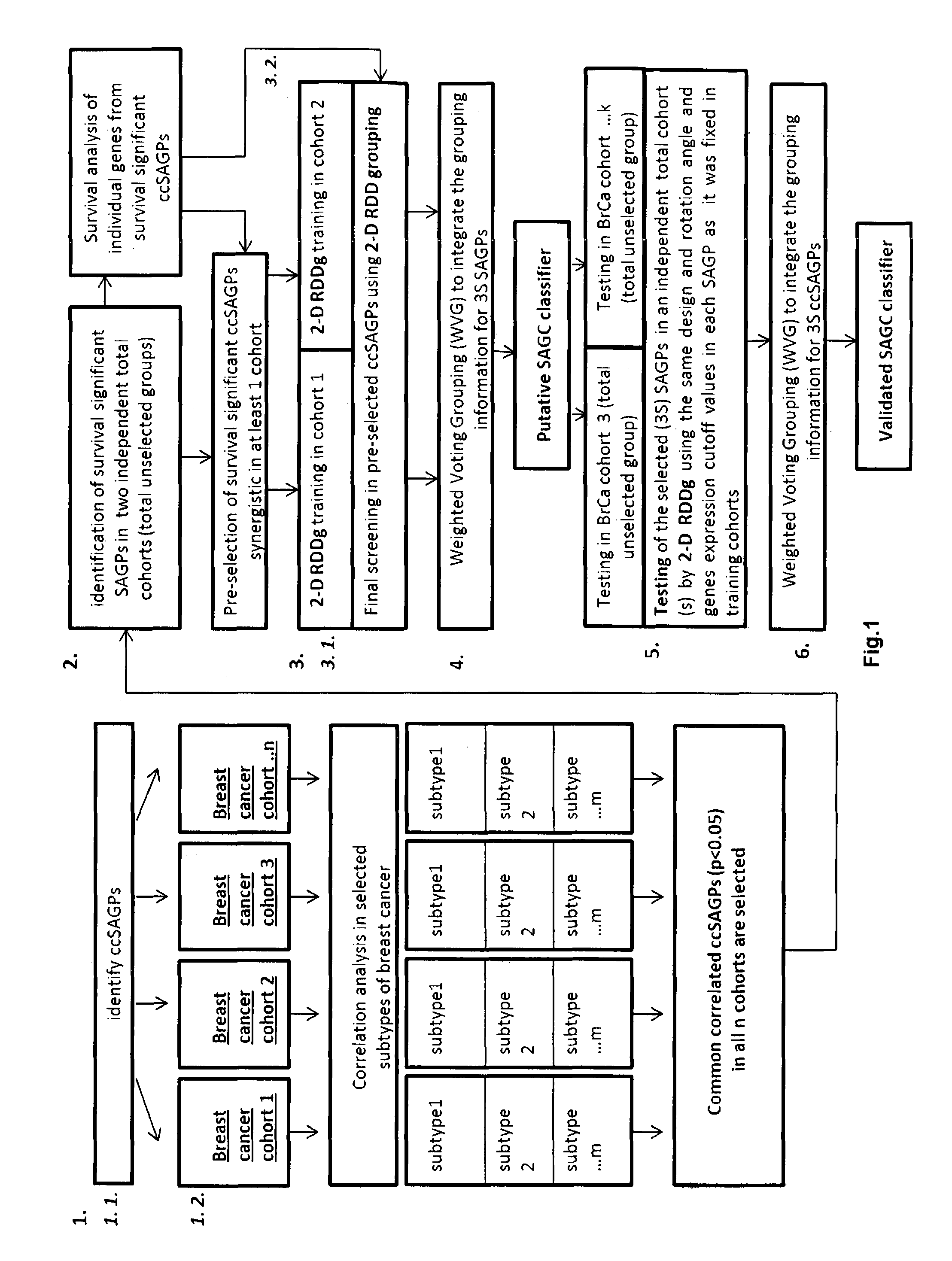
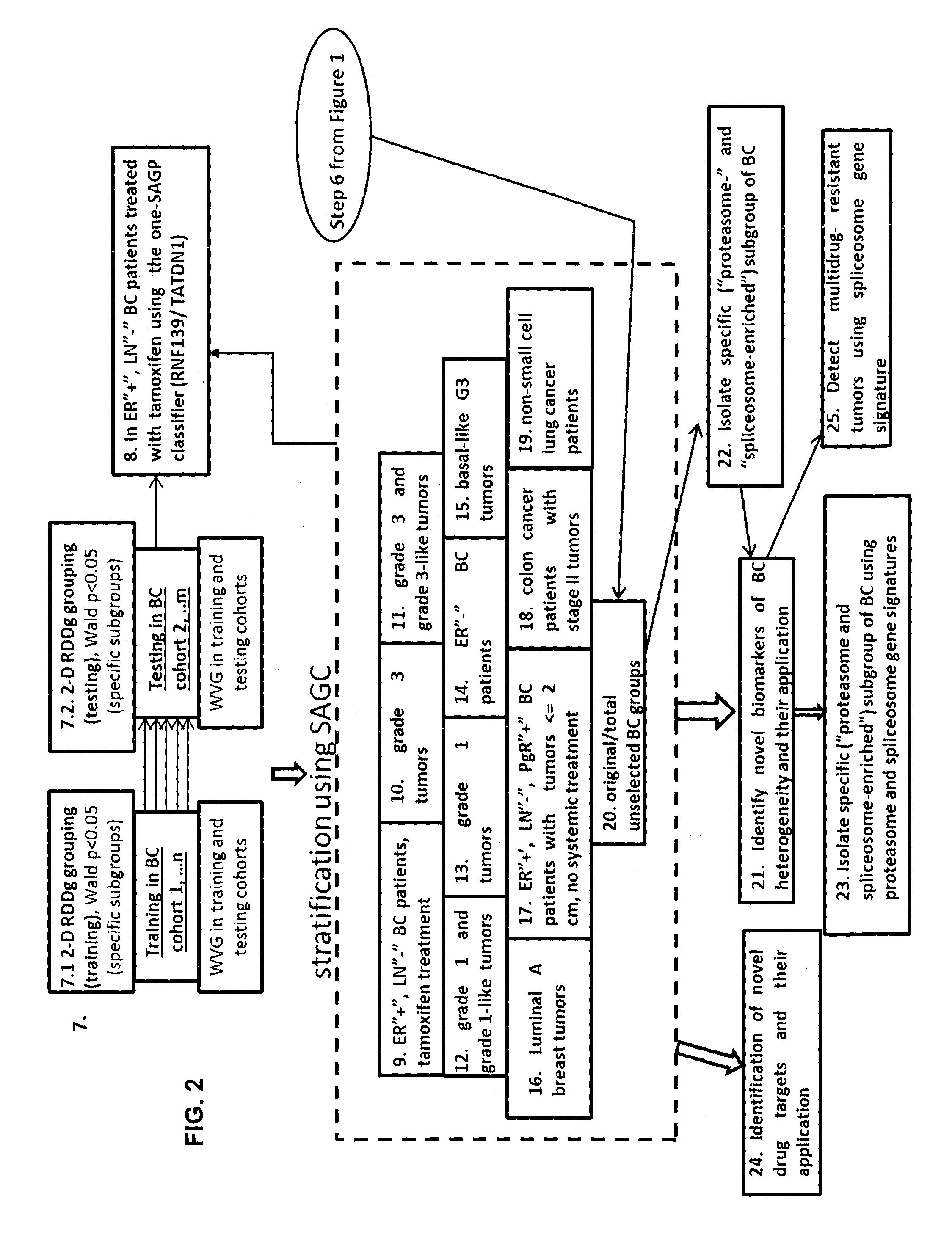
Sense-antisense gene pairs for patient stratification, prognosis, and therapeutic biomarkers identification
InactiveUS20160259883A1Quality improvementHighly prognostically significantMicrobiological testing/measurementLibrary screeningPrognostic signaturePatient stratification
The present invention relates to a method of identification of clinically and genetically distinct sub-groups of patients subject to a medical condition, particularly breast, lung, and colon cancer patients using a composition of respective gene expression values for certain gene pairs. Sense-antisense gene pairs (SAGPs) which are relevant for a medical condition and the disease prognosis are used by the method to generate statistical models based on the expression values of the SAGPs. SAGPs for which the statistical models are found to have high value in prognosis of the variation of medical condition and the diseases are selected and integrated in the prognostic signature including specified parameters (e.g. cut-off values) of the prognostic model. It further relates to using respective gene expression values for these genes to predict patient′ risk groups (in context of patient's survival or / and disease progression) and to using the predicted groups for identification of patient risk, and specific and robust prognostic biomarkers with mechanistic interpretations of biological changes (associated with the gene signatures) appropriating for an implementation of therapeutic targeting.
Owner:AGENCY FOR SCI TECH & RES
Oral cancer point of care diagnostics
ActiveUS20130295580A1Component stabilityImprove stabilityBioreactor/fermenter combinationsBiological substance pretreatmentsPatient riskPoint of care
A point of care diagnostic test, device and disposables for determining a patient risk for oral cancer in the same visit that a sample is collected.
Owner:RICE UNIV +2
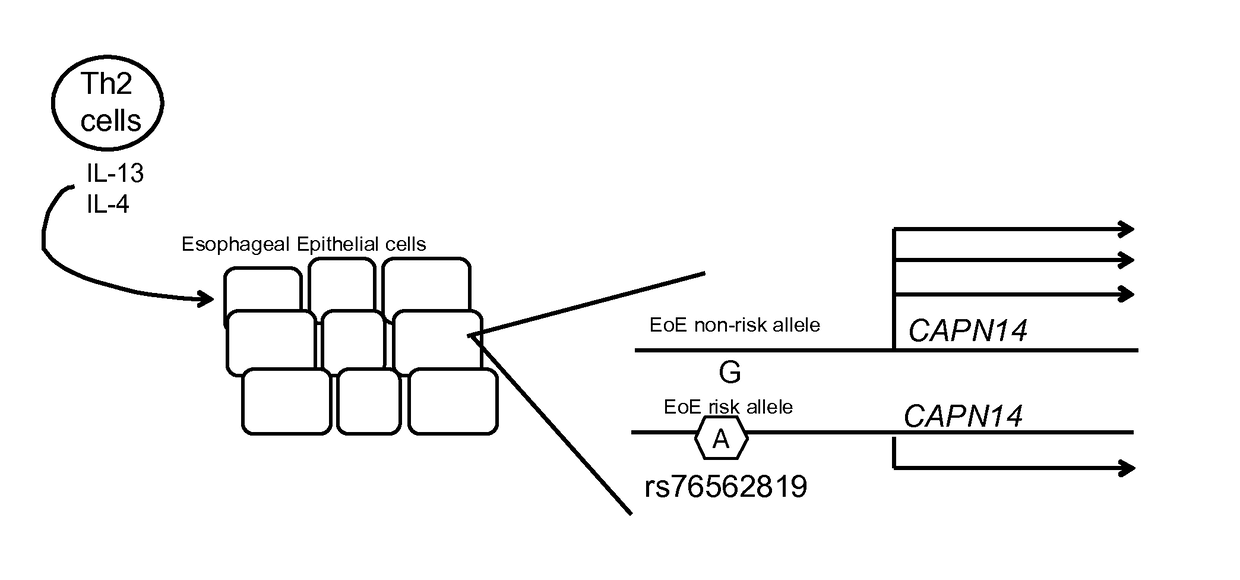
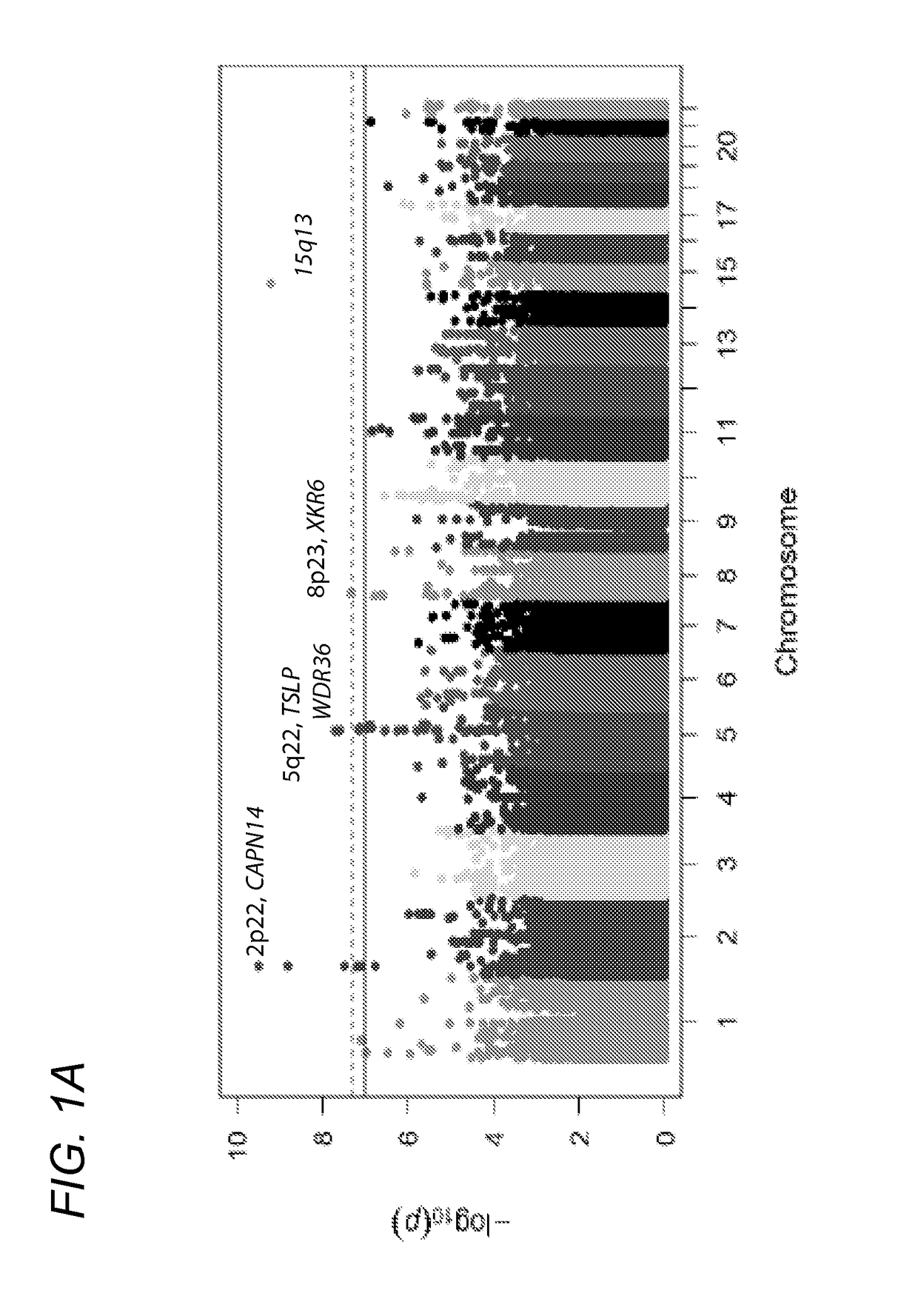
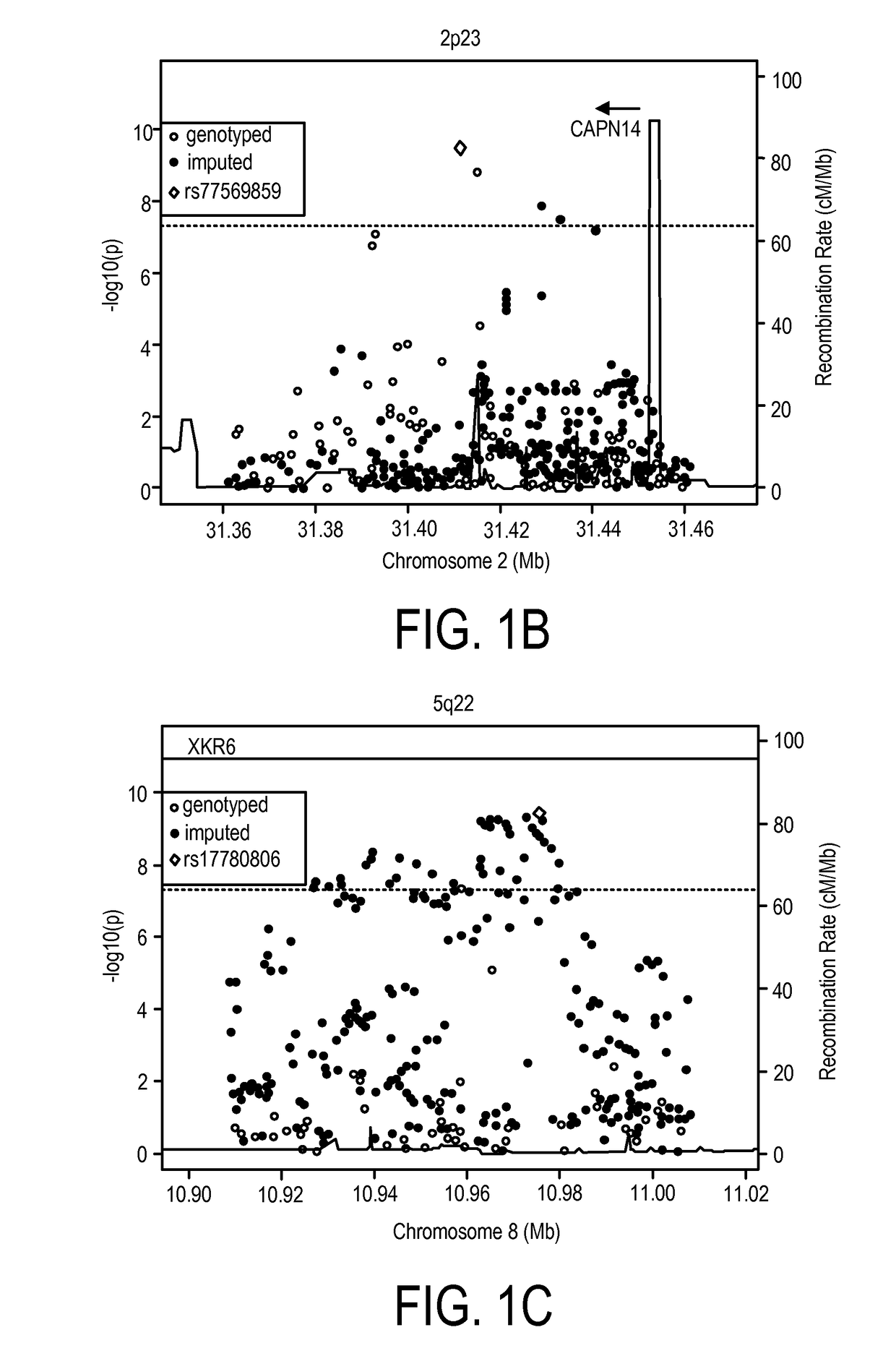
Genetic Test for Determining Susceptibility for Eosinophilic Esophagitis
Methods and compositions disclosed herein generally relate to determination of susceptibility to eosinophilic esophagitis, asthma, and / or allergic diseases, disorders, and / or pulmonary and / or upper gastrointestinal conditions arising therefrom and / or related thereto and the diagnosis, treatment, and / or management of eosinophilic esophagitis, asthma, and / or allergic diseases, disorders, and / or pulmonary and / or upper gastrointestinal conditions arising therefrom and / or related thereto. Embodiments of the invention relate to the association between genes and specific polymorphisms of genes with eosinophilic esophagitis. Embodiments of the invention can be used to determine and manage patient risk factors for development of eosinophilic esophagitis; this determination can then be used to diagnose eosinophilic esophagitis and to treat a patient diagnosed with eosinophilic esophagitis.
Owner:CHILDRENS HOSPITAL MEDICAL CENT CINCINNATI +1
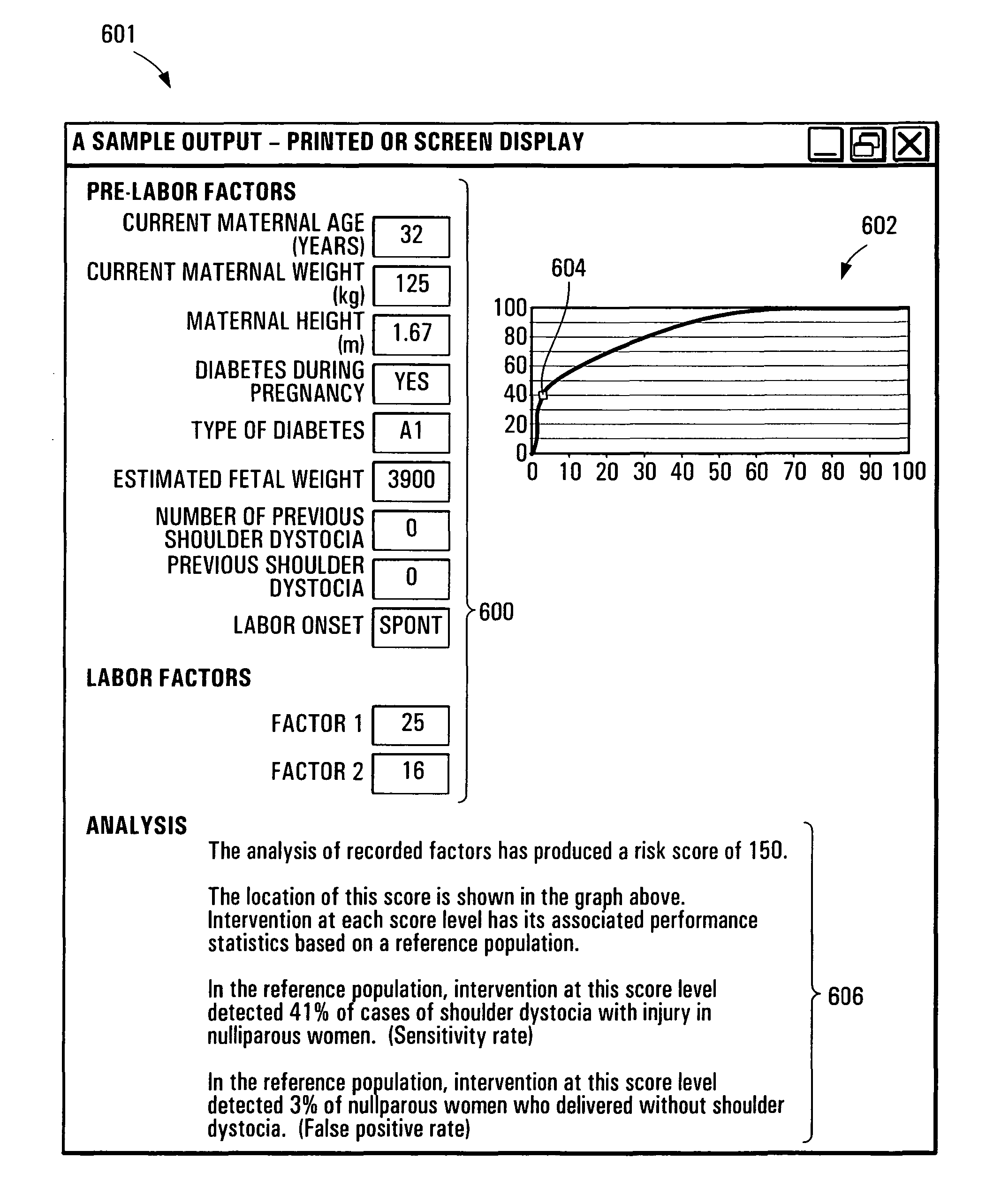
Method and apparatus for estimating a likelihood of shoulder dystocia
A system and apparatus implementing a graphical user interface module for displaying information conveying a level of risk of delivery with shoulder dystocia associated to an obstetrics patient. The graphical user interface module displays a set of user modifiable information fields for allowing a user to enter a set of patient information data elements associated to the obstetrics patient. The graphical user interface module also displays a control allowing a user to cause the set of information data elements to be transmitted to a processing unit adapted to derive patient risk data at least in part on the basis of the set of information data elements, the patient risk data conveying a level of risk of delivery with shoulder dystocia. The graphical user interface module then displays the patient risk data with reference to risk assessment information.
Owner:PERIGEN
MRI-safe defibrillator electrodes
The present invention reduces patient risks associated with RF-induced thermogenic tissue damage and with pulsed gradient-field-induced arrhythmias by using a defibrillator lead having a self-healing dielectric material that prevents induced voltages from MRI equipment from damaging an ICD or causing unintended defibrillation shocks to a patient. Another aspect of the present invention utilizes a sliding contact arrangement to prevent induced voltages from MRI equipment from being electrically coupled to an ICD thereby reducing patient risks associated with RF-induced thermogenic tissue damage and with pulsed gradient-field-induced arrhythmias.
Owner:MEDTRONIC INC
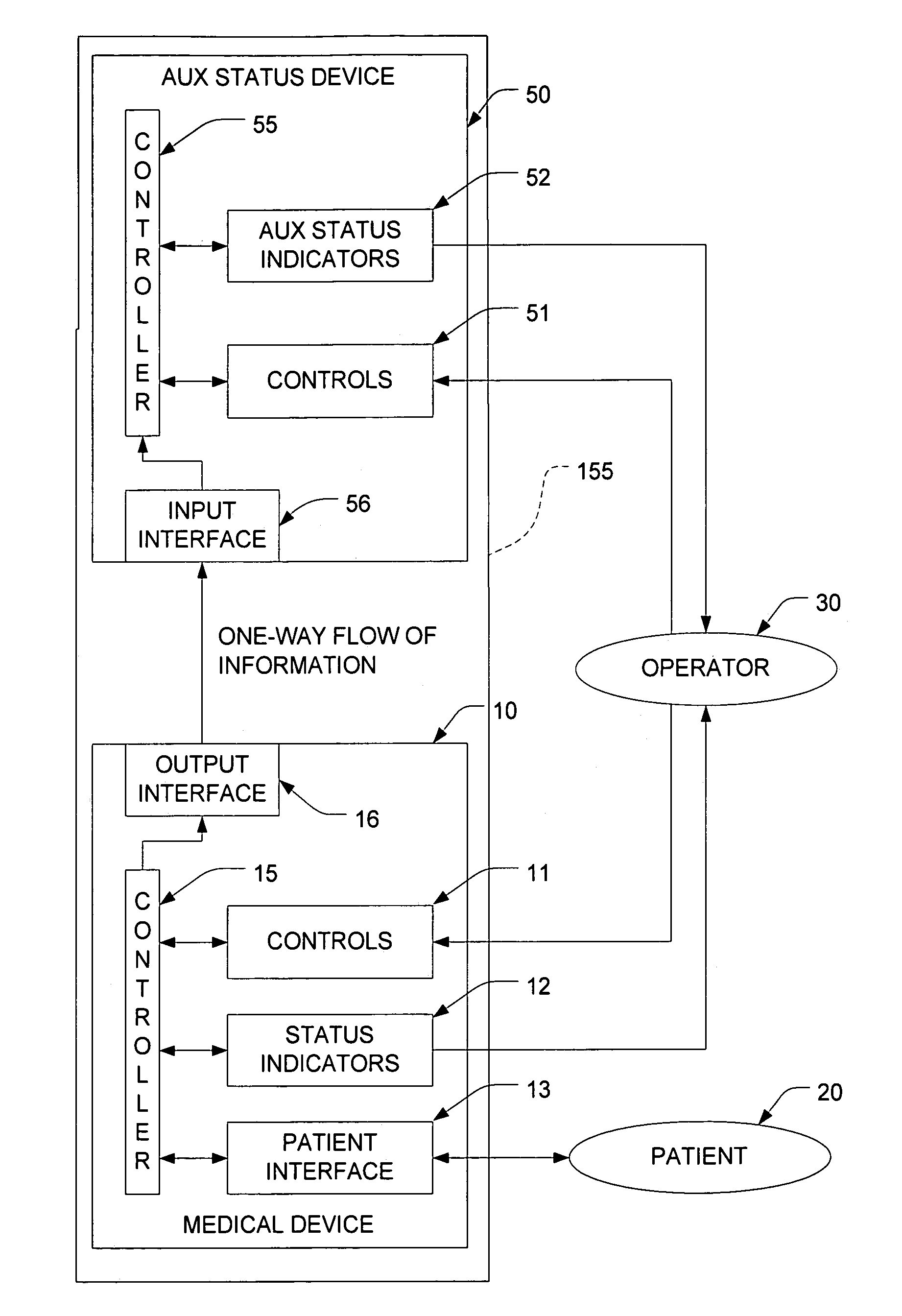
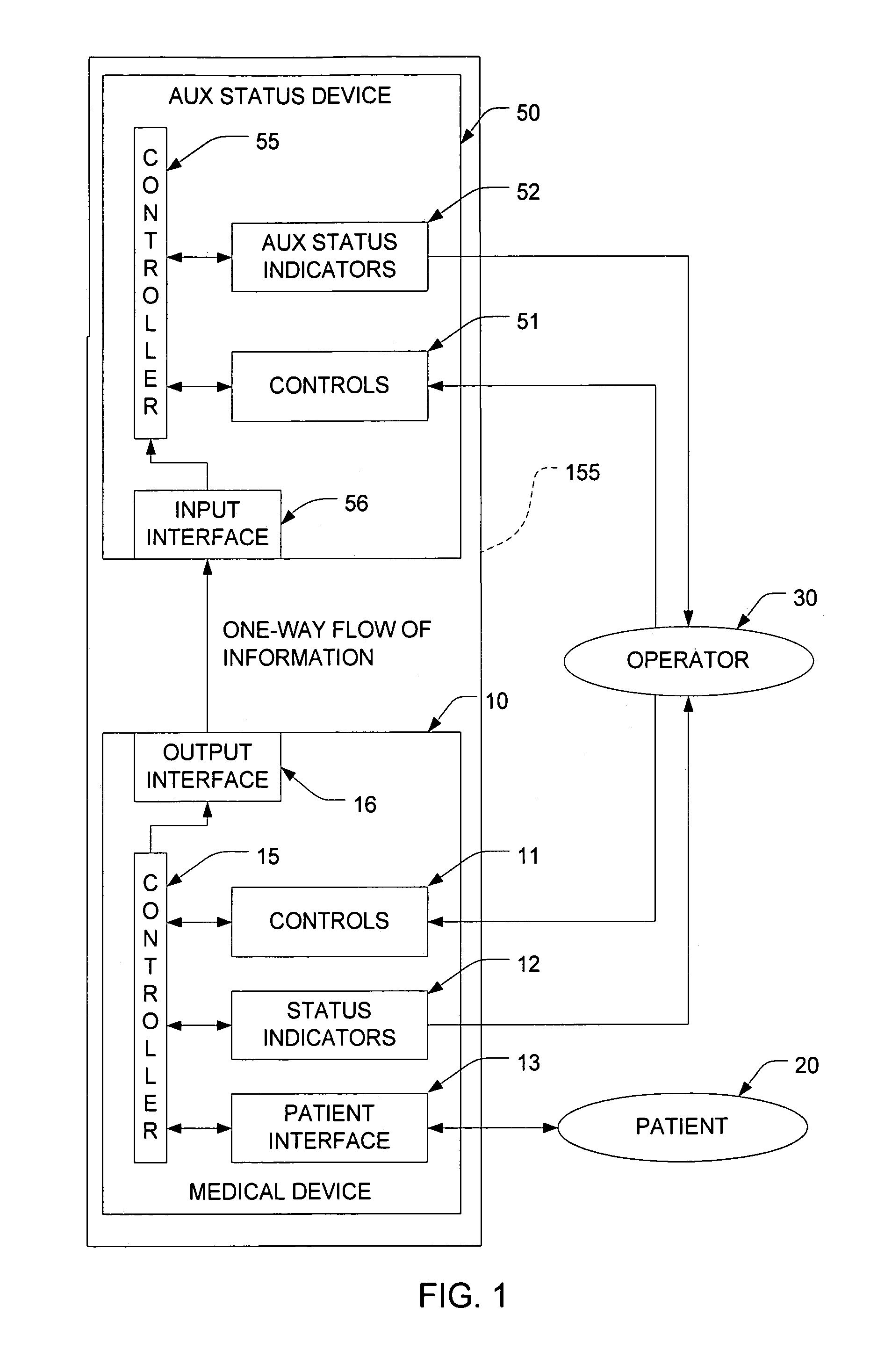
Functional isolation of upgradeable components to reduce risk in medical treatment devices
InactiveUS7771379B2Without of adversely affectingCritical errorRespiratorsLocal control/monitoringPatient riskMedicine
A medical treatment system isolates more risk-sensitive equipment from less risk-sensitive components of a treatment device to allow upgrades to be made to the latter more easily without an concomitant increase in risk to a patient caused by upgrades. For example, the latter may serve a pure monitoring function while the former encapsulates the treatment functions thereby preventing errors from the latter from propagating into the treatment-sensitive portions of the device.
Owner:ABB RES LTD +1
Variable size intragastric implant devices
Transoral obesity treatment devices and related methods for operation thereof are described which occupy space within a stomach and / or stimulate the stomach wall. The transoral obesity treatment devices and related methods are intended to assist a patient in maintaining a healthy body weight. Features of the devices include insertion transorally and without invasive surgery, without associated patient risks of invasive surgery, and without substantial patient discomfort. The life span of these devices may be material-dependent upon long-term survivability within an acidic stomach, but is intended to last one year or longer. The devices have the capacity to vary in size and are desirably self-actuating in that they change shape and / or volume using internal motors or actuators. The changing character of the devices helps prevent the person's stomach from compensating for the implant, such as sometimes happens with static intragastric devices.
Owner:APOLLO ENDOSURGERY INC
Portable sputum collector
PendingCN107929085AEasy to disinfectRapid DisinfectionMedical waste disposalSpittle receiving devicesPatient riskMedical ward
The invention relates to a portable sputum collector, comprising an outer container and an inner container, the inner container can be accommodated in the outer container, the outer container is provided with a laterally opened side cover of the outer container, a spitting port is opened on the upper part of the outer container and The top of the inner container is provided with a top cover of the inner container; a liquid injection pipe is fixedly connected to the collector through the wall of the outer container, and its liquid inlet is arranged on the outside of the collector, and the liquid outlet is arranged on the outside of the collector. inside of the collector. The sputum collector is simple and lightweight, can be taken at hand, has multiple airtight immediate disinfection functions, can effectively reduce the secondary pollution of sputum to the ward environment, and reduce the risk of cross-infection of patients' family members, patients or staff. It solves the difficult problem of collecting sputum from patients, and has very high clinical use value.
Owner:JINHUA MUNICIPAL CENT HOSPITAL
Method and apparatus for predicting arrhythmias using diurnal heart rate
Owner:MEDTRONIC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com