Voriconazole composition and preparation method thereof
A technology of voriconazole and voriconazole tablets, which is applied in the direction of pill delivery, antifungal agents, etc., and can solve the problems of shortening the time to maintain the drug effect, problems with the speed or degree of drug absorption, and small safety index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
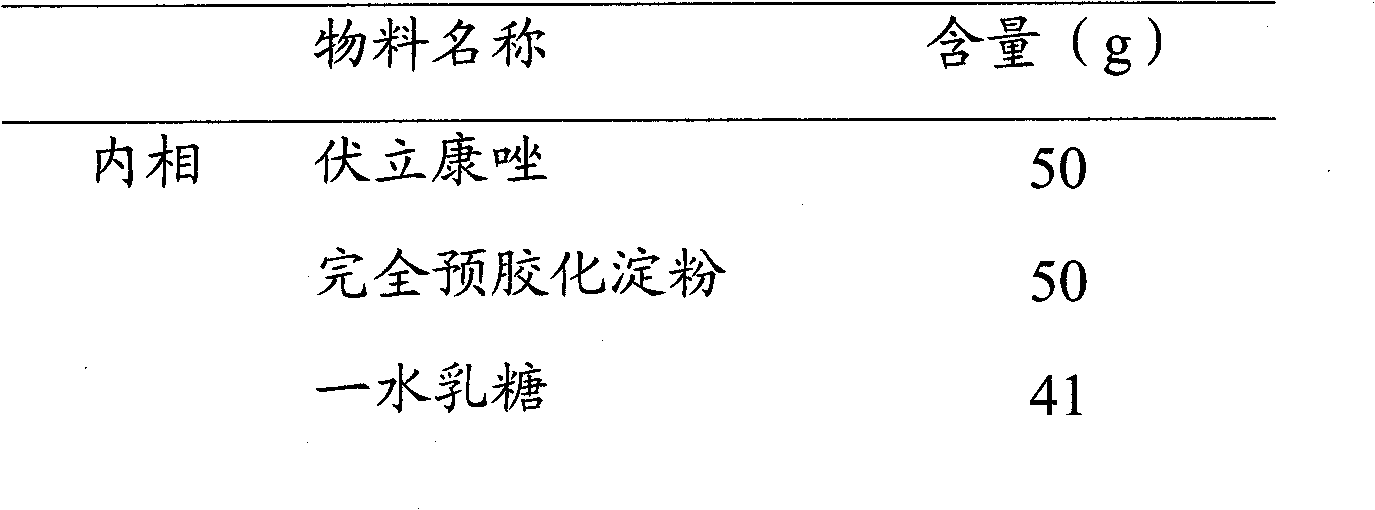
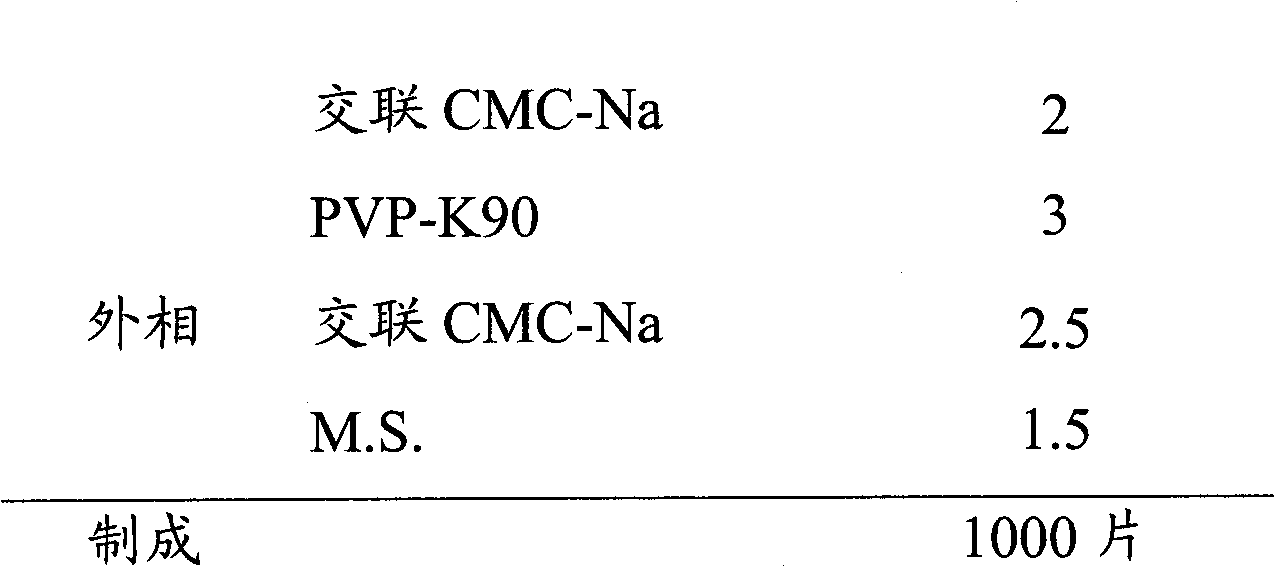
[0045]
[0046] Preparation Process:
[0047] (i) Weigh the internal phase material and add it to the high-speed stirring granulator, start stirring to make the mixture uniform;
[0048] (ii) PVP-K90 is formulated into a 5g / 100g aqueous solution, added to the inner phase material, and stirred in a granulator at high speed to prepare a soft material;
[0049] (iii) swinging granulator to prepare 24 mesh granules;
[0050] (iv) drying the obtained particles using a fluidized bed, and the drying temperature is controlled at 30-40°C;
[0051] (v) The dried particles are granulated through a 20-mesh sieve;
[0052] (vi) The particles are evenly mixed with the external phase material;
[0053] (vii) Compression into tablets.
[0054] (viii) Coating with a high-efficiency coating machine, and the weight gain of the coating is 3.5%.
Embodiment 2
[0056]
[0057]
[0058] Preparation Process:
[0059] (i) Weigh the internal phase material and add it to the high-speed stirring granulator, start stirring to make the mixture uniform;
[0060] (ii) PVP-K90 is formulated into a 5g / 100g aqueous solution, added to the inner phase material, and stirred in a granulator at high speed to prepare a soft material;
[0061] (iii) swinging granulator to prepare 24 mesh granules;
[0062] (iv) drying the obtained granules using a fluidized bed, and the drying temperature is controlled at 30-40°C;
[0063] (v) The dried particles are granulated through a 20-mesh sieve;
[0064] (vi) The particles are evenly mixed with the external phase material;
[0065] (vii) Compression into tablets.
[0066] (viii) Coating with a high-efficiency coating machine, and the weight gain of the coating is 5.0%.
Embodiment 3
[0068]
[0069] Preparation Process:
[0070] (i) Weigh the internal phase material and add it to the high-speed stirring granulator, start stirring to make the mixture uniform;
[0071] (ii) PVP-K90 is formulated into a 5g / 100g aqueous solution, added to the inner phase material, and stirred in a granulator at high speed to prepare a soft material;
[0072] (iii) swinging granulator to prepare 24 mesh granules;
[0073] (iv) drying the obtained granules using a fluidized bed, and the drying temperature is controlled at 30-40°C;
[0074] (v) The dried particles are granulated through a 20-mesh sieve;
[0075] (vi) The particles are evenly mixed with the external phase material;
[0076] (vii) Compression into tablets.
[0077] (viii) Coating with a high-efficiency coating machine, and the weight gain of the coating is 3.5%.
[0078] The following comparative examples are voriconazole tablets prepared by adopting several concentrations of PVP-K30 and several concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com