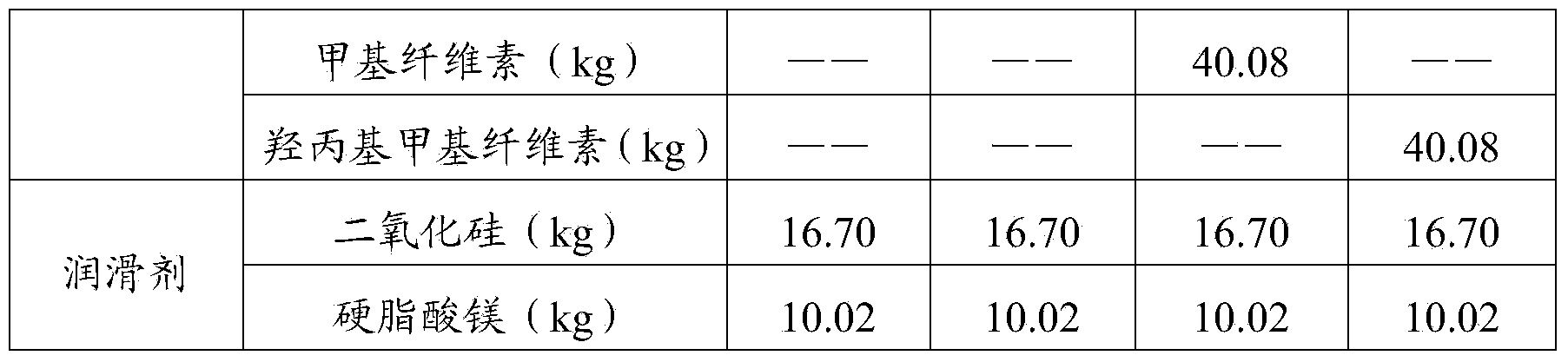Spirulina composition and preparation method and preparation thereof
A technology of spirulina and composition, applied in the field of pharmaceutical preparations, can solve problems such as splinter, insufficient adhesiveness, influence on product quality, etc., and achieve the effects of small influence, no appearance, and white spots on appearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Compatibility test of raw materials and auxiliary materials
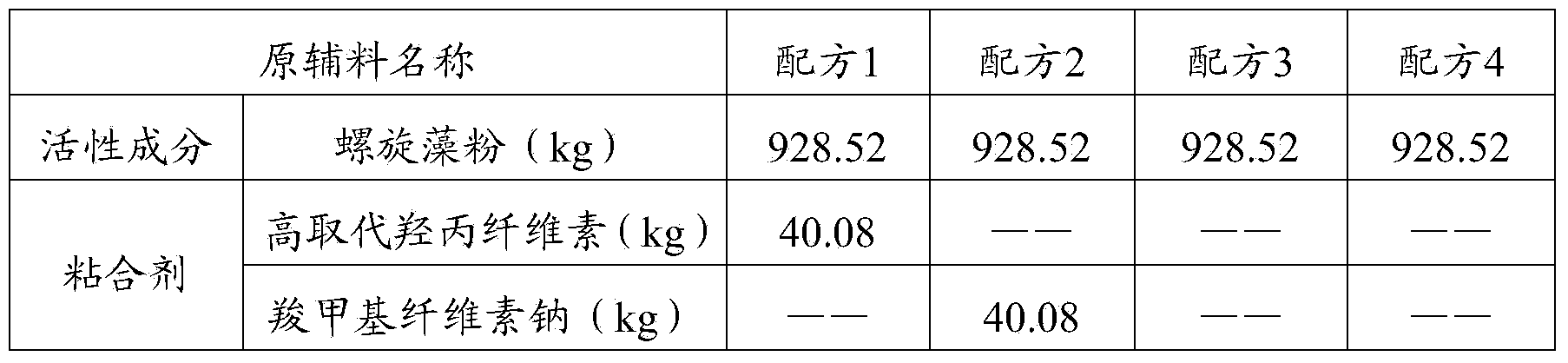
[0032] The formula of spirulina health product tablet is as shown in table 1.
[0033] The formula of table 1 spirulina health product tablet
[0034]
[0035]
[0036] The preparation process of the spirulina health product tablets of the above formula 1 to formula 4 is: take the binder of the formula amount, mix it with the spirulina powder 4 times the amount of the binder formula, and sieve (40 mesh) to obtain the premixed material;
[0037] The premixed material prepared above is mixed with the remaining amount of spirulina powder, then mixed with a lubricant, and compressed into tablets to obtain the product.
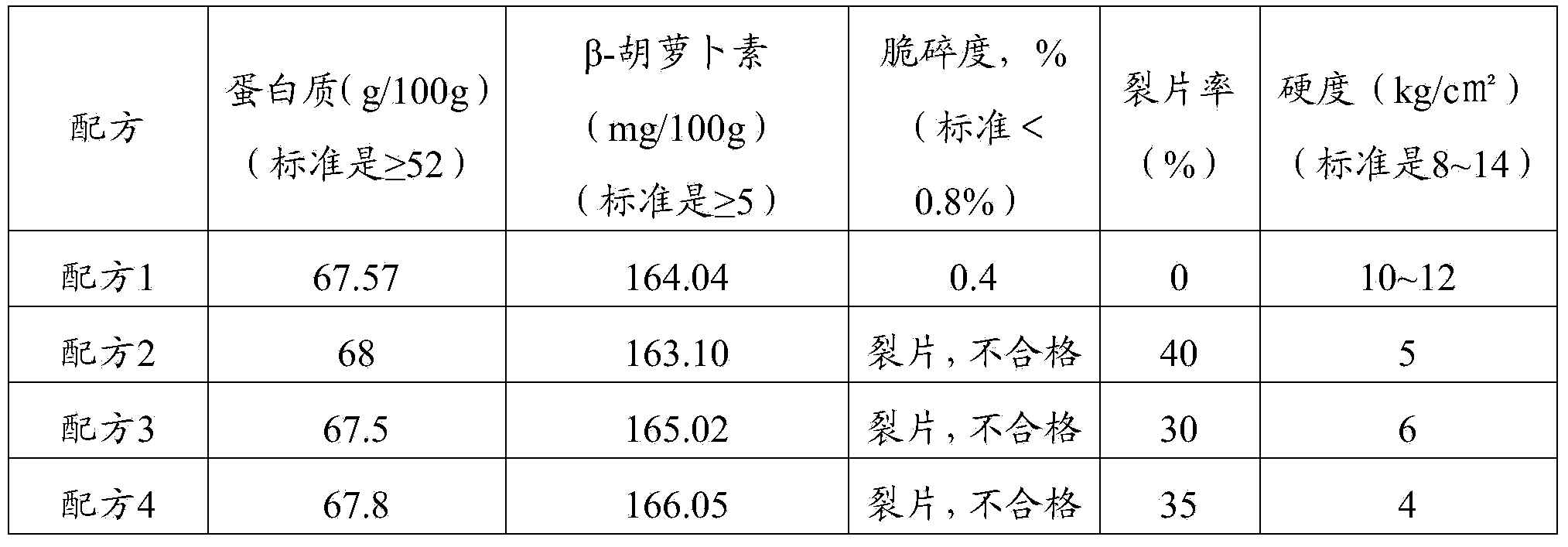
[0038] Get the above-mentioned prepared spirulina health product tablet and carry out quality inspection. The results of quality testing are shown in Table 2.
[0039] Table 2 Quality Test Results of Spirulina Health Products Tablets
[0040]
[0041] As can be seen from...
Embodiment 2
[0042] Embodiment 2 preparation process comparison test
[0043]The formula of spirulina health product tablet is identical with embodiment 1 formula 1. Among them, the preparation process 1 is completely consistent with the embodiment 1; in the preparation processes 2-7, except that the ratio of the binder and the spirulina powder in the premix is different, other process steps and parameters are consistent with the embodiment 1; the preparation process There is no pre-mixing step in 8, namely: directly mix the spirulina powder with the binder in the formula amount, then mix with the lubricant, and press into tablets. See Table 3 for the ratio of binder and spirulina powder in premixes with different preparation processes.
[0044] The proportioning ratio of binder and spirulina powder in the premix of different preparation processes of table 3
[0045] Preparation Process
The ratio of adhesive to spirulina powder
Preparation process 1
1:4
...
Embodiment 3
[0053] The preparation of embodiment 3 spirulina health product tablet
[0054] The formula of spirulina health product tablet is as shown in table 5.
[0055] The formula of table 5 spirulina health product tablet
[0056]
[0057] Get the high-substituted hydroxypropyl cellulose of formula quantity and the spirulina powder of 4 times of high-substituted hydroxypropyl cellulose formula quantity mix, sieve (40 order), obtain premix;
[0058] The above-prepared premix is mixed with the remaining amount of spirulina powder, then mixed with silicon dioxide and magnesium stearate, compressed into tablets, and coated.
[0059] Get the above-mentioned prepared spirulina health product tablet and carry out quality inspection. At the same time, commercially available common spirulina tablets were used as a control.
[0060] The formula of commercially available common spirulina tablet is: spirulina powder 928.52kg, silicon dioxide 16.70kg, magnesium stearate 10.02kg, transpare...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com