Obeticholic acid pharmaceutical composition as well as preparation method and application thereof
A technology of obeticholic acid and its composition, which is applied in the field of obeticholic acid pharmaceutical composition and its preparation, can solve the problems of unfavorable large-scale industrial production, unstable preparation process, and low production efficiency, and achieve optimal dissolution rate and Uniformity of quality, shortened production cycle, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
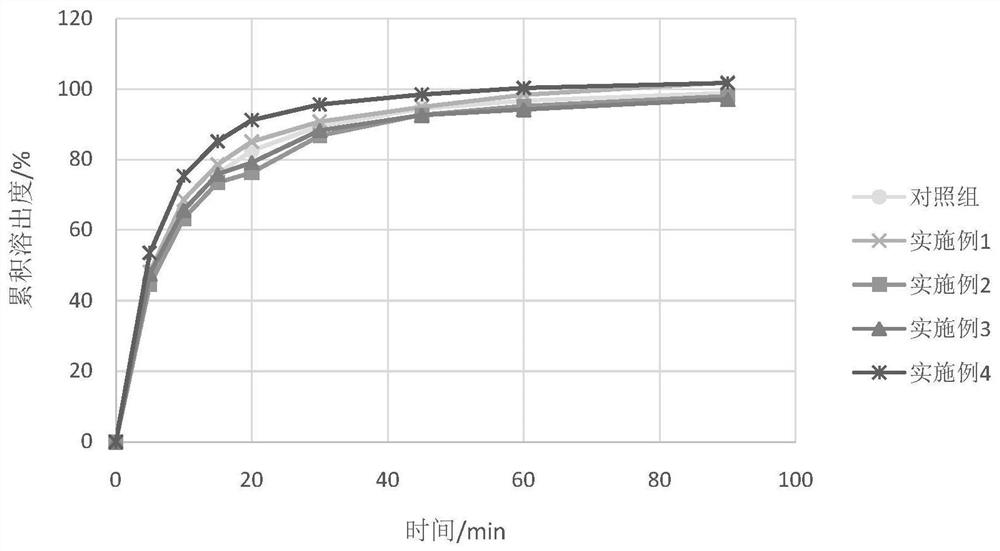
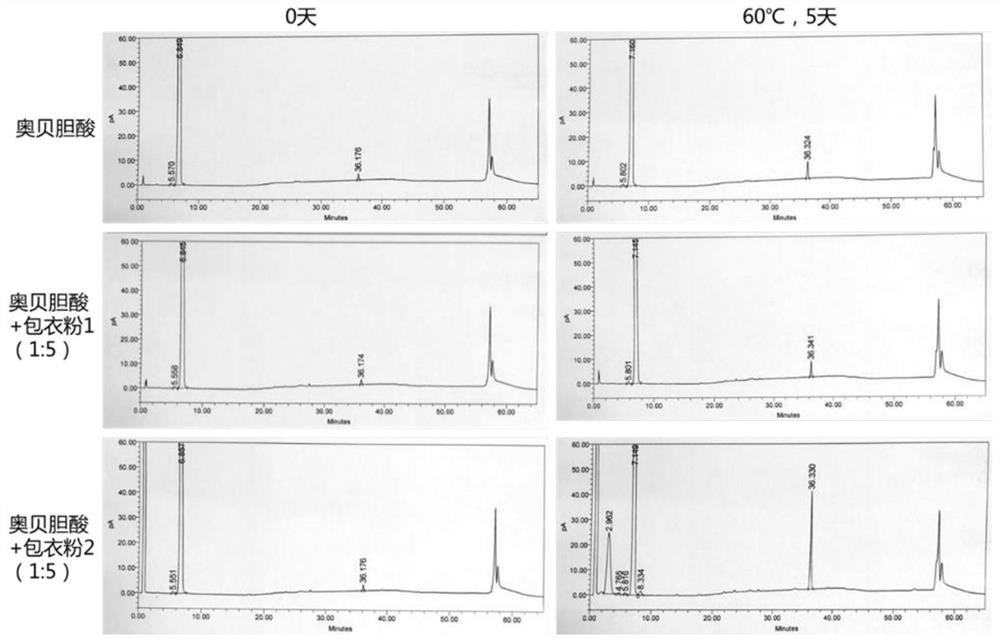
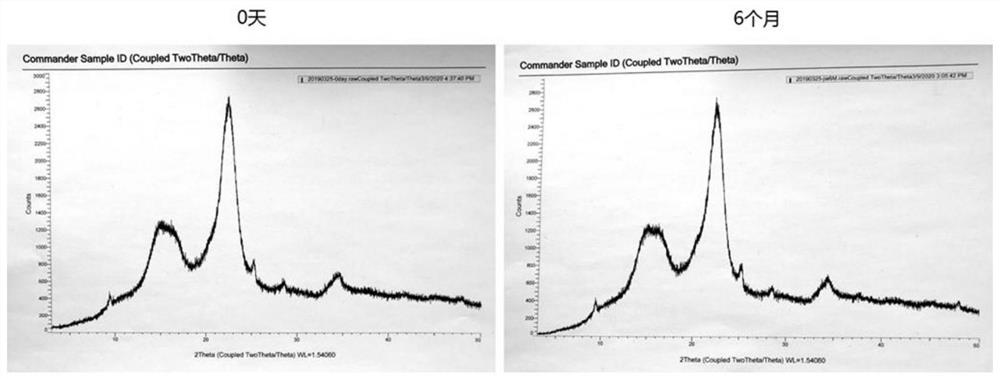
Examples
Embodiment 1
[0108] Embodiment 1 Preparation of obeticholic acid pharmaceutical composition of the present invention
[0109] The preparation method of obeticholic acid pharmaceutical composition comprises the following steps:
[0110] (1) sending the obeticholic acid crude drug into an ultra-high-speed jet mill for micronization, with a pressure of 0.3Mpa;
[0111] (2) Put obeticholic acid micronized granules, microcrystalline cellulose in granules, and sodium carboxymethyl starch in granules into a ziplock bag and mix for 5 minutes, pass through a vertical granulator equipped with a 1.0mm screen, and then transfer to After mixing in a ziplock bag for 8 minutes, add intragranular magnesium stearate, and mix for 5 minutes to prepare a premix;
[0112] Put the prepared premix into the dry granulator, set the feeding speed: 20rpm, the roller pressure: 25bar, the roller speed: 8rpm, the shear speed: 100rpm, the granulation speed: 100rpm, the screen aperture: 1.0 mm, run with the above par...
Embodiment 2
[0115] Embodiment 2 Preparation of obeticholic acid pharmaceutical composition of the present invention
[0116] The preparation method of obeticholic acid pharmaceutical composition comprises the following steps:
[0117] (1) Send the obeticholic acid crude drug into a jet mill for micronization, with a pressure of 0.5Mpa;
[0118] (2) Put obeticholic acid micronized granules, intragranular microcrystalline cellulose, and intragranular sodium carboxymethyl starch into a 5L mixing hopper, mix at 30rpm for 25min, and pass through a vertical granulator equipped with a 1.2mm screen. , then transferred to the mixing hopper and mixed at the same speed for 10 minutes, then added intragranular magnesium stearate, and mixed for 8 minutes to obtain a premix;
[0119] Put the prepared premix into the dry granulator, set the feeding speed: 20rpm, the roller pressure: 40bar, the roller speed: 8rpm, the shear speed: 100rpm, the granulation speed: 100rpm, the screen aperture: 1.5 mm, ru...
Embodiment 3
[0122] Embodiment 3 Preparation of obeticholic acid pharmaceutical composition of the present invention
[0123] The preparation method of obeticholic acid pharmaceutical composition comprises the following steps:
[0124] (1) Send the obeticholic acid crude drug into a jet mill for micronization, with a pressure of 0.6Mpa;
[0125] (2) Put obeticholic acid micronized granules, microcrystalline cellulose in granules, and sodium carboxymethyl starch in granules into a 50L mixing hopper for mixing, mix at a speed of 25rpm for 60min, and then pass through a vertical mixer equipped with a 1.5mm sieve. The granulator and the granulator are also equipped with rounded granulation knives, set the speed at 300rpm, and then transfer the undersieve to a 50L mixing hopper, and mix at a speed of 45rpm; after 30min, add magnesium stearate in the granule and mix for 20min. Get the premix;
[0126] Put the prepared premix into the dry granulator, set the feeding speed: 60rpm, the roller p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com