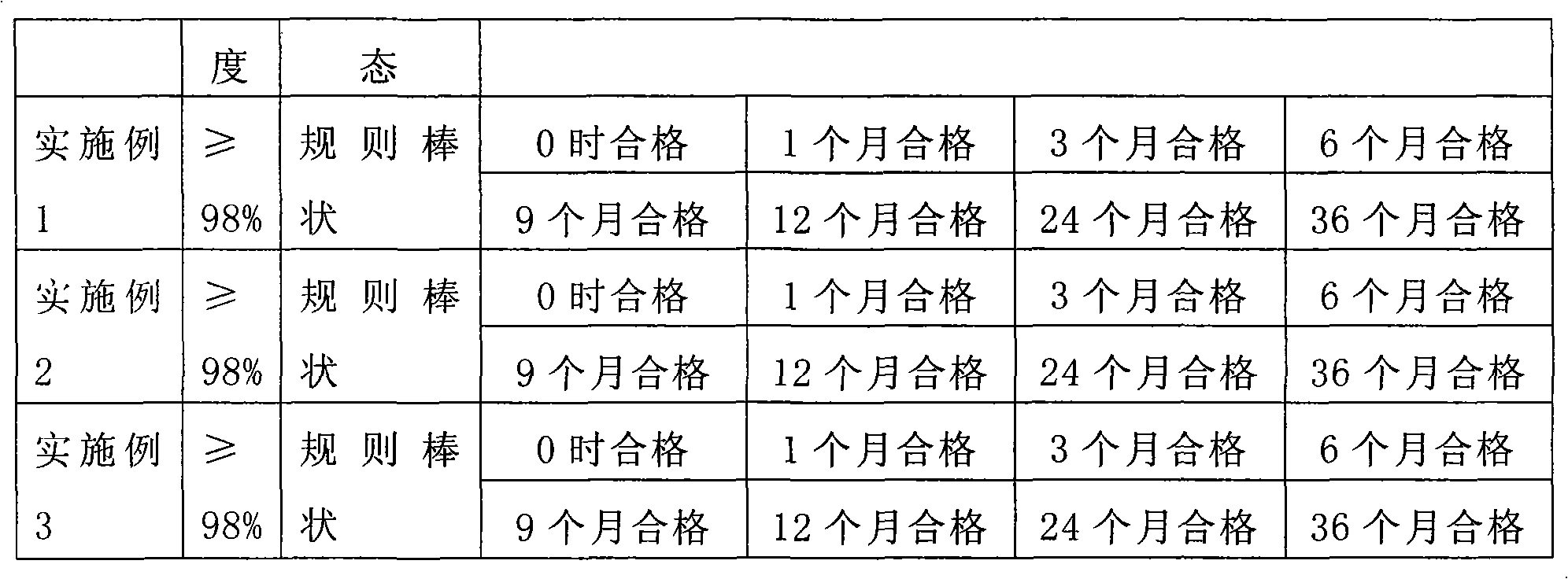
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
60 results about "Cefradine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
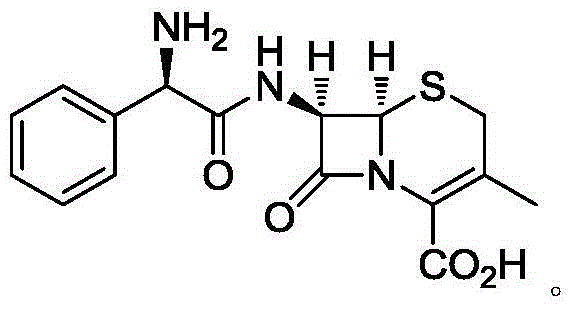
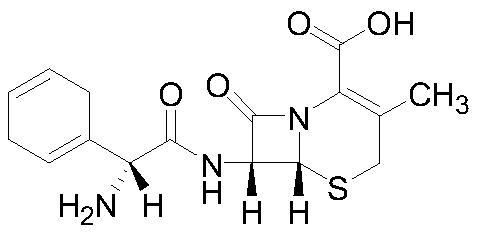
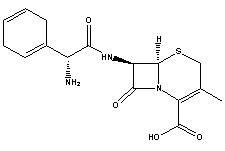
Cefradine (INN) or cephradine (BAN) is a first generation cephalosporin antibiotic.
Slow release injection containing cefradine
InactiveCN1843360AIncrease concentrationReduce concentrationAntibacterial agentsOrganic active ingredientsAdjuvantTreatment effect
Disclosed is a slow release injection containing cephalosporin, which comprises slow release microballoons and dissolvent, wherein the slow release microballoons include slow release auxiliary materials and cephalosporin, the dissolvent being specific one containing suspension adjuvant such as sodium carboxymethyl cellulose, the slow release auxiliary materials are selected from EVAc, PLA, PLGA, sebacylic acid copolymer, albumen glue or gelatin, the slow release microballoons can also be made into slow release implantation agent or ointment.
Owner:SHANDONG LANJIN PHARMA +1
Synthesis method of cefradine
ActiveCN101643477AGood crystal formImprove stabilityOrganic chemistryAntiinfectivesSynthesis methodsCefradine
The invention relates to a synthesis method of cefradine, comprising the following steps: using dihydro phenylglycine sodium dane saltand carbonyldiimidazole to prepare active amide in anhydrous solvent, then performing a reaction between active amide and 7-ADCA and finally hydrolyzing and crystallizing after the reaction to obtain cefradine.
Owner:哈药集团股份有限公司 +1
Cefradine preparing process
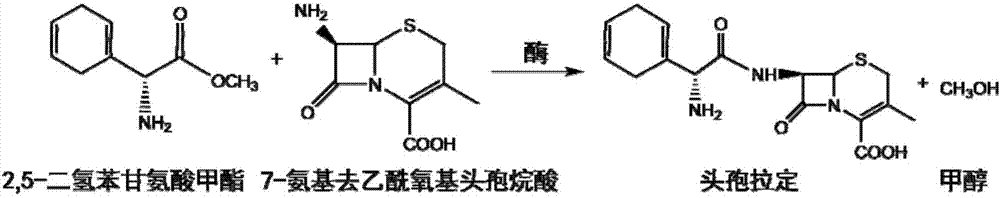
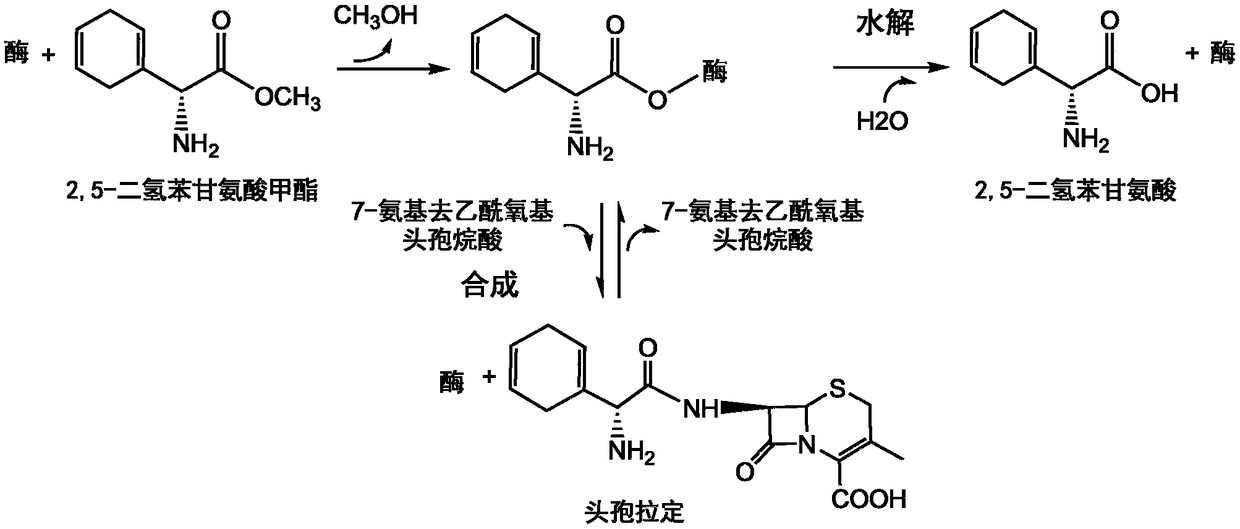
The present invention discloses cefradine preparing process. Under the catalysis of enzyme, 7-ADCA and methyl dihydrogen benzene glycinate are reacted in a double water phase system inside an enzyme catalyzed reactor to form cefradine. Cefradine is obtained through separating the upper phase and the lower phase and filtering, and the mother liquid is returned to the enzyme catalyzed reactor for circular reaction. The enzyme catalyzed cefradine synthesizing process has low solvent consumption, reduced environmental pollution, timely separation of the reaction product from side product and high reaction conversion rate.
Owner:ZHEJIANG ANGLIKANG PHARMA
Cefradine compound and capsule preparation method thereof
InactiveCN101550145AHigh puritySmall toxicityAntibacterial agentsOrganic active ingredientsAlcoholMagma
The invention provides a cefradine compound and a capsule preparation method thereof. The preparation method of the cefradine compound includes the following steps: cefradine is added into an acid solution and dissolves fully in ice water bath; active carbon is added for discoloration and the mixed solution is filtered and the filtrate is combined and then put into a container; the filtrate is stirred at the initial temperature of crystallization to constant temperature; alkaline liquor is gradually added; the magma is filtered after the temperature of the magma reduces to the crystallization end temperature, washed with alcohol and dried in vacuum to obtain the compound. Based on the total weight of capsule, cefradine capsule comprises 20-70wt of cefradine, 0-60wt of filling agent, 0-10wt of disintegrating agent, 1-10wt of adhesive and 0.5-3wt of lubricant.
Owner:HAINAN MEIDA PHARMA
Preparation method for cefradine
The invention discloses a preparation method for cefradine. The method comprises the following steps: (1) condensation reaction: reacting 7-amino-deacetoxycephalosporanic acid with a silane protective agent in an anhydrous solvent so as to generate silicon-esterified amino-deacetoxycephalosporanic acid, dissolving activated dihydrophenylglycine, and mixing the dissolved dihydrophenylglycine and the silicon-esterified amino-deacetoxycephalosporanic acid to conduct condensation reaction; and (2) hydrolysis, decoloring and crystallization. The preparation method for the cefradine, disclosed by the invention, is simple and easy; the use of the protective agent in the activated dihydrophenylglycine preparing process is avoided; the whole process has the advantages of comparatively mild reaction condition, low raw material cost and less pollution; and the quality of the prepared product is stable.
Owner:ZHEJIANG ZHEBANG PHARMA
A sustained release injection containing cephalosporin and application thereof
InactiveCN1879627AEasy to operateGood repeatabilityAntibacterial agentsOrganic active ingredientsBacillus acnesWhole body
The invention relates to a release injection which contains cefradine, wherein it is formed by release micro ball and dissolvent; the release micro ball contains release findings and penicillin antibiotic; the dissolvent is the special one contains sodium carboxymethyl cellulose as suspension agent; the viscosity is 100cp-3000cp (20Deg. C-30Deg. C); the release agent is selected from EVAc, polyphenyl, PLA, PLGA, sebacic acid polymer, protein pastern, and dope; the release micro ball can be made into release implant agent; the release implant agent and release injection is laid on bacteria stove or injected, to release the medicine for 5-30 days, to keep the effective medicine density and reduce the whole toxicity. The invention can be used to cure the infection caused by staphylococcus, streptococcus, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Preparation method and application of photocatalyst
ActiveCN108906110AHigh specific surface areaHigh catalytic activityWater/sewage treatment by irradiationWater treatment compoundsHeterojunctionTetracycline Hydrochloride
The invention discloses a preparation method and application of a photocatalyst, and belongs to the field of photocatalysis. According to the preparation method, guanidine hydrochloride, ammonium chloride and bismuth nitrate are used as raw materials, and high-energy ball milling treatment is carried out in a closed container under a protective gas atmosphere, then roasting is carried out, and theBiOCl / g-C3N4 layered heterojunction photocatalyst is synthesized. The preparation method disclosed by the invention is simple in steps, the obtained catalyst is good in stability, and the degradationrate of tetracycline hydrochloride and / or cefradine in water through the use of the obtained catalyst is up to 99% or above. In the method disclosed by the invention, no solvent is used, so that themethod is environmentally friendly, economic and practical, meets the actual production requirements, and can be popularized and used in a large scale.
Owner:BINZHOU UNIV
Cefradine capsule and preparation method thereof
InactiveCN104688713AImprove liquidityHigh dissolution rateAntibacterial agentsOrganic active ingredientsDissolutionCefradine
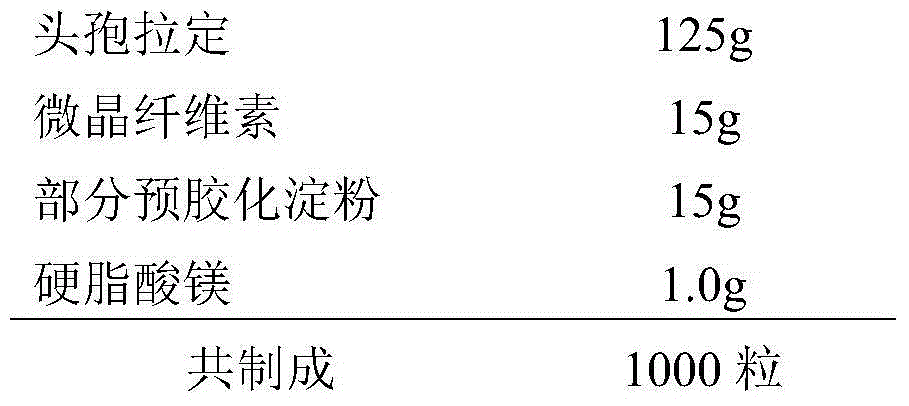
The invention provides a cefradine capsule and a preparation method thereof. The capsule comprises the following components in weight percentage: 70-90 wt% of cefradine, 5-15 wt% of partial pregelatinized starch, 5-15 wt% of microcrystalline cellulose and 0.1-1 wt% of magnesium stearate. The components in the capsule have good compatibility; the prepared cefradine capsule is good in dissolution and high in stability; the provided preparation method is simple in process and easy in quality control.
Owner:JIANGSU YABANG QIANGSHENG PHARMA +1
Monoclonal antibody, enzyme-linked immunosorbent assay method and kit for detecting cefalexin, cefadroxil and cefradine
ActiveCN104558189AHigh recognition sensitivityExcellent recognition sensitivityMicroorganism based processesTissue cultureCefalexinCefradine
The invention discloses a specific monoclonal antibody capable of distinguishing cefalexin, cefadroxil and cefradine, and an enzyme-linked immunosorbent assay method and kit for detecting the cefalexin, the cefadroxil and the cefradine. According to the invention, the monoclonal antibody is secreted by a hybridoma cell strain 3A6 of which the preservation number is CCTCC No.C201340. Compared with the prior art, the monoclonal antibody, prepared by the invention, can be used for distinguishing the cefalexin, the cefadroxil and the cefradine at the same time. The enzyme-linked immunosorbent assay method and kit disclosed by the invention have the advantages of high detection efficiency, high sensitivity, high precision, high accuracy and the like.
Owner:HUAZHONG AGRI UNIV
Lung-targeting cefradine microsphere for animal and birds and its preparing method
InactiveCN1985831AReduce releaseGood curative effectAntibacterial agentsOrganic active ingredientsSide effectMicrosphere
The present invention belongs to the field of veterinary medicine technology, and is especially lung targeting cefradine microsphere for animal and its preparation process. The cefradine microsphere is prepared with cefradine as medicine component and gelatin as carrier in the weight ratio of 1 to 2, and through dissolving cefradine in gelatin solution and adding Span-80 and liquid paraffin through stirring to obtain emulsion; cooling in icy bath to below 5 deg.c and adding glutaraldehyde through stirring for cross-linking and curing; dewatering with isopropyl alcohol and suction filtering; washing with isopropyl alcohol and ethyl ether to eliminate glutaraldehyd, washing with petroleum ether to eliminate liquid paraffin in the surface of microsphere and vacuum drying at room temperature to obtain cefradine microsphere. The medicine has raised tissue selectivity, delayed release, raised curative effect and lowered toxic side effect.
Owner:TIANJIN RINGPU BIO TECH
Medicine for treating epistaxis through oral administration and external use and method thereof
ActiveCN103316015AEnhance coagulation and hemostasisOrganic active ingredientsRespiratory disorderCefradinePharmacologic therapy
The invention provides a medicine for treating epistaxis through oral administration and external use and a method thereof. The following components are combined for use as the medicine in parts by weight: 0.5 part of chloramphenicol tablets, 0.5 part of cefradine injection powder, 0.5 part of furazolidone tablets, 30 parts of radix rehmanniae, 30 parts of prepared rhizome of rehmannia, 25 parts of garden burnet root, 30 parts of golden cypress, 30 parts of fructus forsythiae, 25 parts of caulis spatholobi and 25 parts of liquorice, wherein western medicines, namely the chloramphenicol tablets, the cefradine and the furazolidone tablets, are bactericidal medicines for external use, and wherein traditional Chinese medicines, namely radix rehmanniae, prepared rhizome of rehmannia, garden burnet root, golden cypress, 30 parts of fructus forsythiae, caulis spatholobi and liquorice are decocted with water and used as medicines for oral administration. The medicine provided by the invention is obvious in medicine treatment effect, quick to stop bleeding and worthy of popularization and utilization.
Owner:ZHENAO JINYINHUA PHARM
Cefradine compound prepared by adopting high-flux medicine crystal form rapid screening technology and preparation thereof
InactiveCN106432269AHigh purityLow impurity contentAntibacterial agentsOrganic active ingredientsSolubilityTechnology development
The invention discloses a cefradine compound prepared by adopting a high-flux medicine crystal form rapid screening technology. The 'high-end medicine product refined crystallization technology development and industrialization project' won the second prize of the national scientific and technological process in 2015, and the high-flux medicine crystal form rapid screening technology belongs to one of the high-end medicine product refined crystallization technology. The cefradine compound is measured by adopting X-ray powder diffraction, and main characteristic peaks represented by a diffraction angle 2 theta in an atlas are 11.46+ / -0.2 degrees, 18.47+ / -0.2 degrees, 20.85+ / -0.2 degrees, 23.21+ / -0.2 degrees and 29.32+ / -0.2 degrees. The compound is high in purity, low in impurity content and good in flowability and stability. Meanwhile, the invention discloses a preparation prepared by adopting cefradine, and the preparation is the cefradine for injection. The preparation is simple in preparation process, does not need any excipient, has the better solubility and stability and is smaller in side effect compared with an existing preparation.
Owner:陕西顿斯制药有限公司
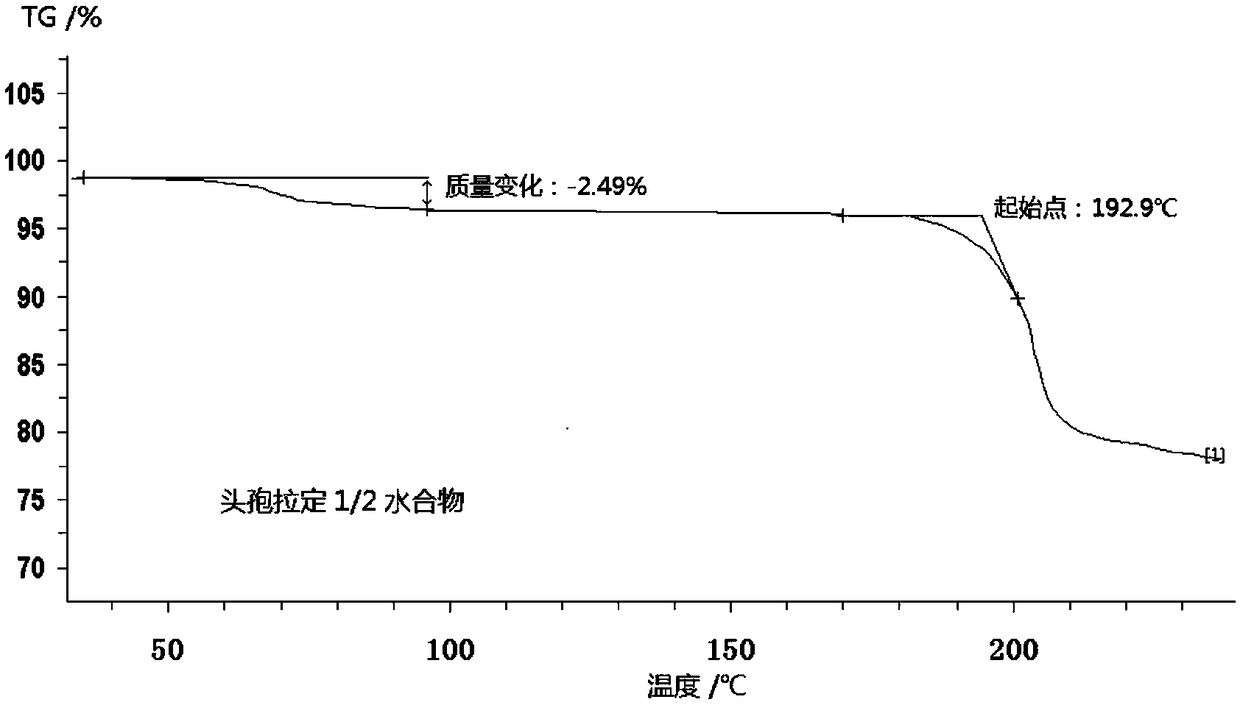
Cefradine compound containing half water
The invention discloses a cefradine compound containing half water and a preparation method thereof. Each mole of cefradine comprises a half mole of water. The cefradine compound has characteristic peaks at the positions with the X-ray diffraction spectrum diffraction angle 2 theta of 7.25+ / -0.2 degrees, 10.98+ / -0.2 degrees, 14.53+ / -0.2 degrees, 16.28+ / -0.2 degrees, 17.80+ / -0.2 degrees, 19.38+ / -0.2 degrees, 20.21+ / -0.2 degrees, 22.06+ / -0.2 degrees and 22.90+ / -0.2 degrees. A crude cefradine product is dissolved in water and adsorbed by activated carbon, and a dissolving and precipitation agentis added for crystallization to obtain a cefradine crystal; the crystal is dissolved in a mixed solution of water and methanol, seed crystals are added, and crystallization is performed to obtain thecefradine compound containing half water. The operation is simple, reactants are easy to obtain, the reaction conditions are mild, and the yield is high. The cefradine compound containing half water has great particle size distribution, high fluidity, low impurity content, high stability and a wider application prospect.
Owner:陕西顿斯制药有限公司 +1
Medicine with resistance to staphylococcus aureus as well as preparation method and application of medicine
InactiveCN106619829AIncreased sensitivityReduce dosageAntibacterial agentsOrganic active ingredientsAntibiotic YStaphylococcus aureus
The invention belongs to the field of biological medicines and particularly relates to a medicine with resistance to staphylococcus aureus as well as a preparation method and application of the medicine. The medicine with resistance to staphylococcus aureus comprises a traditional Chinese medicine composition and antibiotics, wherein the traditional Chinese medicine composition is prepared from flos lonicerae, radix scutellariae and fructus forsythiae according to a weight ratio of 1 to (0.1-1) to (0.5-2); the antibiotics are selected from one of cefradine, levofloxacin and gentamicin. With combination of the traditional Chinese medicine composition and the antibiotics, the sensibility to antibiotics of staphylococcus aureus can be improved; the medicine resistance is difficultly generated when frequently taking the medicine; meanwhile, the dosage of the antibiotics can be effectively reduced; the occurrence of adverse effect of medicinal hematuria can be reduced; the medicine has a good application prospect; the invention further provides application of the medicine with resistance to staphylococcus aureus to preparation of medicines or antibacterial products.
Owner:SOUTH CHINA SEA FISHERIES RES INST CHINESE ACAD OF FISHERY SCI
Cefradine synthase mutant and coding gene thereof
ActiveCN107099523AIncrease enzyme activityIncrease vitalityHydrolasesFermentationEscherichia coliCefradine
The invention discloses a cefradine synthetase mutant and a coding gene thereof. A protein shown as A) or B) or C) is provided, wherein A) is obtained by substitution of twenty-fourth-site phenylalanine of the beta chain of Escherichia coli natural penicillin G acylase into alanine and substitution of sixty-seventh-site serine of the beta chain of the Escherichia coli natural penicillin G acylase into the alanine; B) is obtained by substitution of 142nd-site methionine the alpha chain of the Escherichia coli natural penicillin G acylase into leucine, substitution of twenty-fourth-site phenylalanine of the beta chain into the alanine, and substitution of sixty-seventh-site serine of the beta chain into the alanine; and C) is a protein derived from the A) or B) by substitution and / or deletion and / or addition of one or a plurality of amino acid residue of the protein defined by the A) or B), and has the ability of synthesis of cefradine. The protein provided on has high activity for synthesis of the cefradine and Vs / Vh and lower alpha, and lays a foundation for the industrialization of the enzyme method synthesis of the cefradine.
Owner:TSINGHUA UNIV
Antibacterial composite medicine
An antibacterial composite medicine is prepared from ceftriaxone and cefradine in the radio of 1:(0.1-10) and they have cooperation effect. Its medical application is also disclosed.
Owner:GUANGZHOU PUIS PHARMA FACTORY
Cefradine pharmaceutical composition
InactiveCN107496430ASimple recipeSimple preparation processAntibacterial agentsOrganic active ingredientsSide effectPolyvinyl chloride
The invention discloses a cefradine pharmaceutical composition, which is prepared from the following raw materials in parts by weight: 40 to 50 parts of cefradine, 10 to 20 parts of cyclodextrin, 10 to 20 parts of pregelatinized starch, 2 to 8 parts of sodium hydrogen carbonate, 0.5 to 1.1 parts of citric acid, 0.4 to 1 part of magnesium stearate, 2 to 8 parts of fatty acid sorbitan, 1 to 5 parts of lecithin, 2 to 6 parts of PVC (polyvinyl chloride) and 1 to 5 parts of aerosil. The cefradine pharmaceutical composition is simple and scientific in prescription and high in stability, and has little pharmacological toxic or side effects; in addition, a preparation process is simple and suitable for large-scale production.
Owner:江苏宏梓新能源科技有限公司
Synthetic intermediate compound of cephradine or cefroxadine and its preparation method and application
InactiveCN102267953AEasy to purifyAvoid isomerizationOrganic chemistryBulk chemical productionChemical industryTert-Butyloxycarbonyl protecting group
The invention relates to an intermediate compound for synthesizing cefradine or cefroxadine, and a preparation method and application thereof, belonging to the fields of medicine and chemical industry. The chemical name of the intermediate compound for synthesizing cefradine or cefroxadine is D-2-(tert-butoxycarbonyl)amino-2-(1,4-cyclohexadiene)acetate; and the structural formula is disclosed as chemical structural formula I, wherein R is disclosed in the specification.
Owner:NANJING FROCHEM TECH
Application of cefradine as bovine enterovirus inhibitor
The invention belongs to the technical field of prevention and treatment of bovine enterovirus, and particularly relates to application of cefradine as a bovine enterovirus inhibitor. The bovine enterovirus can cause gastrointestinal diseases and respiratory symptoms of cattle, and has serious harm to the breeding industry, research on the bovine enterovirus is little at present, and moreover, effective prevention and treatment drugs have are not disclosed yet. The invention provides application of the cephradine in inhibiting and killing the bovine enterovirus. The research shows that the cefradine can inhibit and kill the bovine enterovirus on an in-vitro MDBK cell model, can effectively inhibit invasion and replication of the bovine enterovirus, has low cytotoxicity, can be used as a new class of bovine enterovirus resisting drugs, lays a foundation for prevention and control of the bovine enterovirus and research and development of the drugs, and also lays an experimental foundation and provides a new visual field for development of high-efficiency specific bovine enterovirus resisting drugs.
Owner:DAIRY CATTLE RES CENT SHANDONG ACADEMY OF AGRI SCI +1
Slow released capsule of cefradine and bletilla tuber glue for animal and birds
InactiveCN1985949AExtended stayGood treatment effectAntibacterial agentsOrganic active ingredientsDiseaseBletilla striata
The present invention belongs to the field of veterinary medicine technology, and is especially slow released veterinary medicine capsule prepared with cefradine and bletilla tuber as material. The preparation process includes the following steps: water extracting and alcohol precipitation to extract bletilla tuber glue; mixing cefradine and bletilla tuber in the ratio of 1 to 3 and 10 % concentration alcohol solution of PVP as adhesive; making pellet of 20 meshes and encapsulating. The slow released veterinary medicine capsule has long medicine retaining time in the disease focus, high local medicine concentration, excellent bioadhesion and obvious medicine slow releasing characteristic.
Owner:TIANJIN RINGPU BIO TECH
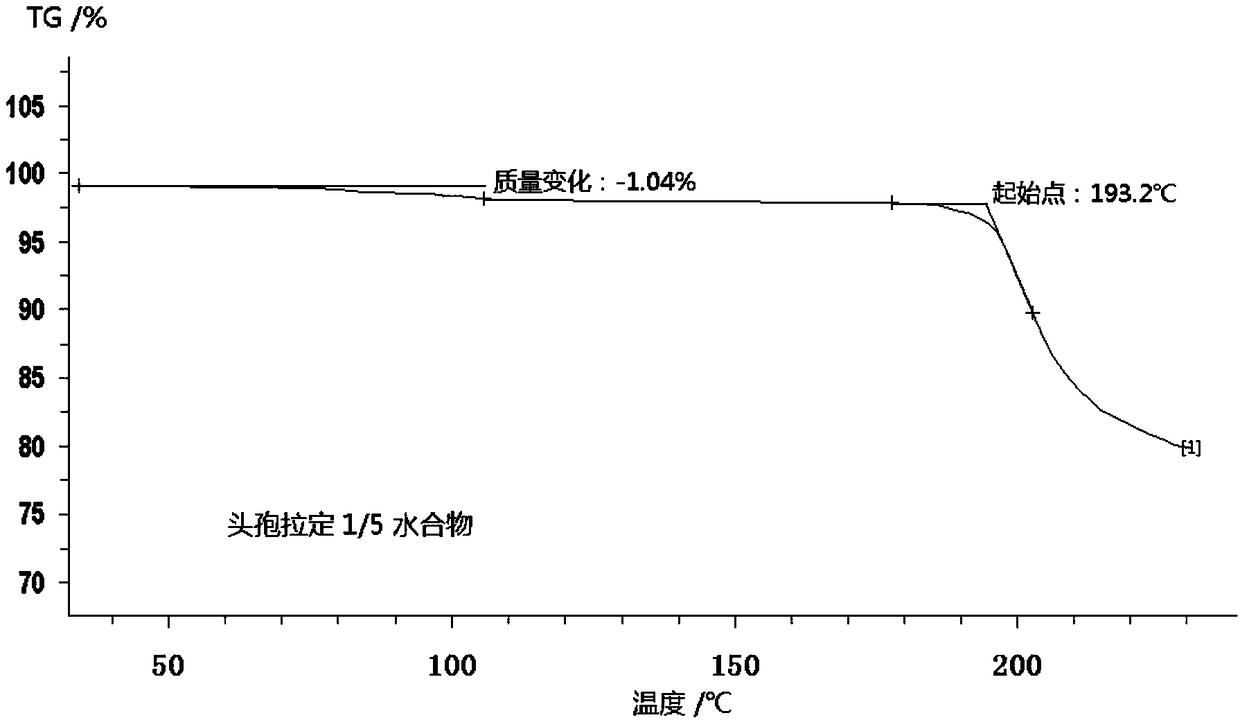
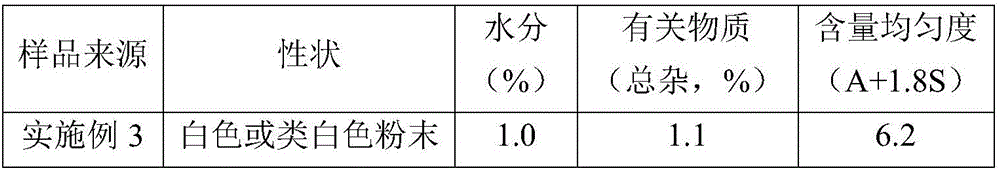
Cefradine compound containing one-fifth water and pharmaceutical composition preparation thereof
The invention discloses a cefradine compound containing one-fifth water and a pharmaceutical composition preparation thereof. Each mole of cefradine contains one-fifth mole of water. The cefradine compound has characteristic peaks at the positions with the X-ray diffraction spectrum diffraction angle 2 theta of 5.50+ / -0.2 degrees, 7.21+ / -0.2 degrees, 14.49+ / -0.2 degrees, 15.69+ / -0.2 degrees, 16.24+ / -0.2 degrees, 16.66+ / -0.2 degrees, 20.16+ / -0.2 degrees and 22.04+ / -0.2 degrees. First, a dichloromethane solution and mixed anhydride of 7-ADCA tetramethylguanidine are separately synthesized and then reacted with each other to obtain the cefradine compound containing one-fifth water. The operation is simple, reactants are easy to obtain, the reaction conditions are mild, and the yield is high.The cefradine compound containing one-fifth water has low hygroscopicity and impurity content, high fluidity and thermodynamic stability and a wider application prospect.
Owner:赵建宇
Preparation method and composition of cefradine with original research quality
ActiveCN105713011ASimple manufacturing methodMild process reaction conditionsAntibacterial agentsOrganic active ingredientsCefradineSolvent
The invention discloses a preparation method of cefradine with an original research quality on the basis of KR840002043 (B1) and US4235900 (A). The preparation method comprises steps as follows: (1) dihydrophenylglycine chloride hydrochloride is added to a solvent, the mixture is cooled, protected 7-ADCA is added to the mixture, and the mixture is subjected to a condensation reaction after being mixed; (2) water and hydrochloric acid are added after the reaction ends, the mixture is subjected to a hydrolysis reaction, an organic phase layer is left to stand for use, a water layer is subjected to vacuum distillation, and non-distillate is retained; (3) the non-distillate is crystallized and filtered, filtrate is left to stand for use, a filter cake is washed and dried, and cefradine is obtained; (4) the organic phase layer and the filtrate are combined, and cefradine is recycled and extracted. The invention further discloses composition of cefradine with the original research quality. The prepared cefradine and the cefradine composition have the original research quality, the preparation method is simple, the reaction conditions of the whole procedure are relatively mild, the cost of raw materials is low, little pollution is caused, and the prepared cefradine composition has stable and high quality.
Owner:广东金城金素制药有限公司 +1
Method for detecting methyl acetoacetate in cefradine
InactiveCN111650322AQuantitatively accurateExact applicabilityComponent separationMethyl acetateCefradine
The invention provides a method for detecting impurity methyl acetoacetate in cefradine. A GC external standard method is adopted to qualitatively and quantitatively analyze methyl acetoacetate in cefradine, experimental operation is simple, water is used as a solvent in the analysis and detection process, pollution is avoided, chromatographic peaks are free of interference, peak patterns are symmetrical, methodological verification results all meet requirements, and it is shown that the detection method is good in specificity, high in sensitivity and accurate in quantification. The detectionmethod provided by the invention is suitable for detection of methyl acetoacetate in cefradine, and is convenient for large-scale production and control of product quality in a quality control process.
Owner:SHANDONG XINHUA PHARMA CO LTD
Method for determining concentration of cefradine in blood plasma by liquid chromatography-tandem mass spectrometry
The invention discloses a method for determining the concentration of cefradine in blood plasma through liquid chromatography-tandem mass spectrometry. The method comprises the following steps: (1) carrying out pretreatment on a blood plasma sample; (2) determining the concentration of cefradine in the plasma sample subjected to pretreatment in the step (1) by adopting a liquid chromatography-tandem mass spectrometry method, wherein the liquid chromatography-tandem mass spectrometry comprises chromatographic conditions and mass spectrometry conditions, the chromatographic conditions comprise that an aqueous solution containing 0.1% formic acid and 5mM ammonium acetate is adopted as a mobile phase A, acetonitrile is adopted as a mobile phase B, and a volume ratio of the mobile phase A to the mobile phase B is 70: 30; performing isocratic elution, and the elution time is 3.0 min. By adopting the method disclosed by the invention, the rapid and sensitive detection of the cefradine in theblood plasma can be realized; the plasma consumption is low, and the sample pretreatment is simple.
Owner:苏州必宜生物科技有限公司
Cefradine composition
InactiveCN104107171AGood treatment effectPyrogenic reaction noAntibacterial agentsOrganic active ingredientsCurative effectCefradine
The invention provides a cefradine composition. The cefradine composition is prepared by using cefradine, borneol, vitamin E and sodium carbonate according to a weight part ratio of 60-140:2-14:6-20:3-12. The invention also provides a preparation method of the composition. The cefradine composition is better than cefradine compositions in the prior art in safety, stability and curative effects, and the preparation method of the cefradine composition has the advantages of energy saving and environmental protection.
Owner:邓学峰
High-selectivity mutant of cefradine synthesizing enzyme and coding gene thereof
The invention discloses high-selectivity mutant of cefradine synthesizing enzyme and a coding gene thereof. The invention provides the following protein obtained by the steps of substituting 142-sitemethionine of an alpha chain of natural penicillin G acylase of escherichia coli with phenylalanine, substituting 24-site phenylalanine of a beta chain with alanine and substituting 67-site serine ofthe beta chain with alanine. The protein provided by the invention has higher activity and Vs / Vh for synthesizing the cefradine and lower alpha and lays foundation for industrialization of enzymatic synthesis of the cefradine.
Owner:TSINGHUA UNIV
Process method for preparing cefradine through microreactor
InactiveCN112321608APrecisely control the ratioControl ratioOrganic chemistryMicroreactorPhysical chemistry
The invention discloses a process method for preparing cefradine through a microreactor. The process method comprises the following main raw materials: mixed anhydride and 7-ADCA. According to the process, a mixed anhydride solution with a certain temperature and a certain flow rate is used as a mobile phase, a 7-ADCA solution with a certain temperature and a certain flow rate is used as a dispersion phase, uniform and rapid mixing and reaction are performed through a membrane dispersion microreactor, and a cefradine solution is prepared. Compared with the prior art, the method has the advantages that various conditions in production can be more accurately controlled, the mass transfer rate is increased, a fine, rapid and controllable continuous production mode is achieved, the productionefficiency is improved, meanwhile, zero application of liquid nitrogen in the production process is achieved, the production cost is reduced, the discharge amount of dichloromethane is reduced, and the pollution to the environment is reduced.
Owner:河北载和新材料科技有限公司
A kind of preparation method and composition of original developed quality cephradine
ActiveCN105713011BSimple manufacturing methodMild process reaction conditionsAntibacterial agentsOrganic active ingredientsCefradineSolvent
The invention discloses a preparation method of cefradine with an original research quality on the basis of KR840002043 (B1) and US4235900 (A). The preparation method comprises steps as follows: (1) dihydrophenylglycine chloride hydrochloride is added to a solvent, the mixture is cooled, protected 7-ADCA is added to the mixture, and the mixture is subjected to a condensation reaction after being mixed; (2) water and hydrochloric acid are added after the reaction ends, the mixture is subjected to a hydrolysis reaction, an organic phase layer is left to stand for use, a water layer is subjected to vacuum distillation, and non-distillate is retained; (3) the non-distillate is crystallized and filtered, filtrate is left to stand for use, a filter cake is washed and dried, and cefradine is obtained; (4) the organic phase layer and the filtrate are combined, and cefradine is recycled and extracted. The invention further discloses composition of cefradine with the original research quality. The prepared cefradine and the cefradine composition have the original research quality, the preparation method is simple, the reaction conditions of the whole procedure are relatively mild, the cost of raw materials is low, little pollution is caused, and the prepared cefradine composition has stable and high quality.
Owner:广东金城金素制药有限公司 +1
Cefradine-borneol composition
InactiveCN102670620AHigh antibacterial activityAntibacterial activity does not changeAntibacterial agentsAntimycoticsResistant bacteriaConventional medicine
The invention discloses a cefradine-borneol composition belonging to the technical field of the preparation of medicines. The cefradine-borneol composition comprises cefradine and borneol according to the mass ratio of 1:0.1 to 1:10, wherein the optimal mass proportion is 1:1. The composition can be prepared into various medicament forms, such as tablets, capsules, granules, slow release preparations and injections, by using a conventional medicine preparation method and auxiliary materials which are universal pharmaceutically. The composition has a very good inhibiting action to various medicine-resistant bacteria, and the minimal inhibitory concentration (MIC) value is lowered at an order of magnitude. Therefore, the phenomenon of medicine resistance of common antibiotics is greatly improved, and the application prospect is extensive.
Owner:GUANGDONG PHARMA UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com