Cefradine compound and capsule preparation method thereof
A cefradine and compound technology, which is applied to the preparation of cefradine capsules and the field of cefradine refining, can solve the problems of residual organic solvent, mixing of water-insoluble impurities, affecting refining effect, etc., and achieve simple refining process, reduce toxic and side effects, and improve product quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
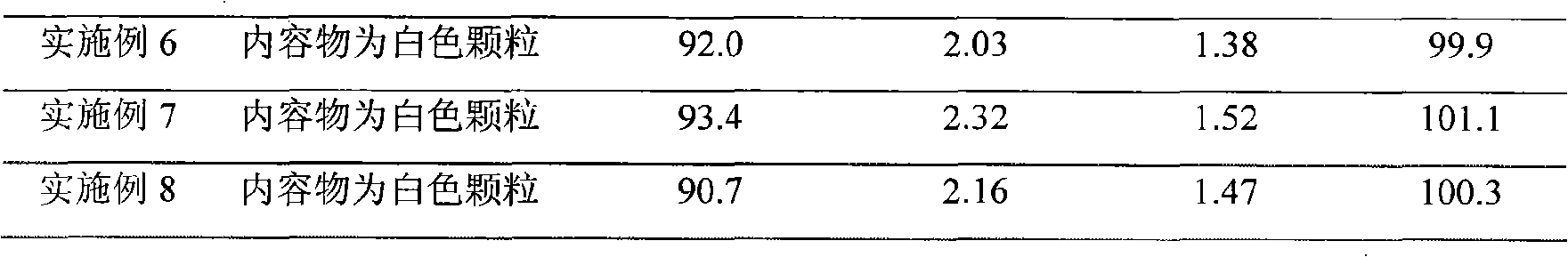
Embodiment 1
[0035] The refining of embodiment 1 cefradine
[0036] Add 1000g of cephradine to 4000ml of water containing 10ml of concentrated hydrochloric acid for hydrolysis, stir at 10°C for 15 minutes, add 13.5g of activated carbon to the water layer for decolorization, filter, and collect the filtrate. Add 25ml of triethylamine to adjust the pH value to 3.0, add 0.1g of seed crystals, and continue to adjust to 5.4 with 15ml of triethylamine. Cool to 5°C and let stand overnight. Crystals were precipitated, washed with 60% acetone solution, and vacuum-dried at 50° C. to obtain 648.4 g of refined cephradine with a purity of 96.8%.
Embodiment 2
[0037] The refining of embodiment 2 cefradine
[0038] Add 1000g of cephradine to 2000g of 0.1mol / L hydrochloric acid solution, dissolve completely in an ice-water bath, add 13.3g of activated carbon for decolorization for 30 minutes, filter for decarbonization, wash the filter cake with 100ml of water, combine the filtrates, and transfer them to a three-necked flask. Stir to constant temperature, slowly add triethylamine / DMF solution with a volume ratio of 1.2:1, control the volume and speed of addition, monitor the pH change of the crystallization solution online with an acidity meter, control the pH value of the crystallization end point to 5.2, and stop adding alkali , the dosage is 300ml, the crystal slurry is lowered to 5°C in 1.5h, filtered after constant temperature, washed with 100ml of 95% ethanol, and dried in vacuum at 45°C to obtain 886.4g of cephradine refined product, and the detection purity is 99.1%.
Embodiment 3
[0039] The refining of embodiment 3 cefradine
[0040] Add 2000g of cephradine into 4000g of 0.5mol / L hydrochloric acid solution, dissolve completely in an ice-water bath, add 28.2g of activated carbon for decolorization for 15 minutes, filter for decarburization, wash the filter cake with 100ml of water, combine the filtrates, and transfer them to a three-necked flask. Stir down to a constant temperature, slowly add 10% sodium bicarbonate solution, control the volume and speed of addition, monitor the pH change of the crystallization solution online through an acidity meter, control the crystallization terminal pH value to 5.1, stop adding alkali, and the dosage is 500ml. The crystal slurry was lowered to 5°C within 1h, filtered after constant temperature, washed with 100ml of 95% ethanol, and vacuum-dried at 45°C to obtain 1684.7g of cephradine refined product with a detection purity of 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com