Cefradine compound prepared by adopting high-flux medicine crystal form rapid screening technology and preparation thereof
A cefradine and compound technology, which is applied in the field of cefradine compounds and preparations thereof, can solve the problems of poor thermodynamic stability, high impurity content, low crystallization yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of cefradine compound
[0029] Add 50ml of water and 15ml of N-methylacetamide into the reactor, and control the temperature of the water bath at 40°C. Add 10.01 g of crude cephradine to the above mixed solution, stir continuously until dissolved, and then adjust the pH value to 3.7 with hydrochloric acid. After the reaction was complete, the liquid was transferred to a crystallizer, cooled to 25° C. in a water bath, and elliptical seed crystals were added to grow the crystals for 3 hours. Slowly add tetrachloromethane to the above solution, and filter; dissolve the above filtrate in 100ml of water, add 0.15g of activated carbon, stir and adsorb for 30min, and filter. Add 200ml of ethanol to the filtrate, stir until it is completely precipitated, and filter; wash the filtrate with 30ml of ethanol×2, and dry it in vacuum at 35°C for 3h to obtain 9.81g of white crystalline powder.
[0030] The X-ray powder diffraction pattern of the product...
Embodiment 2
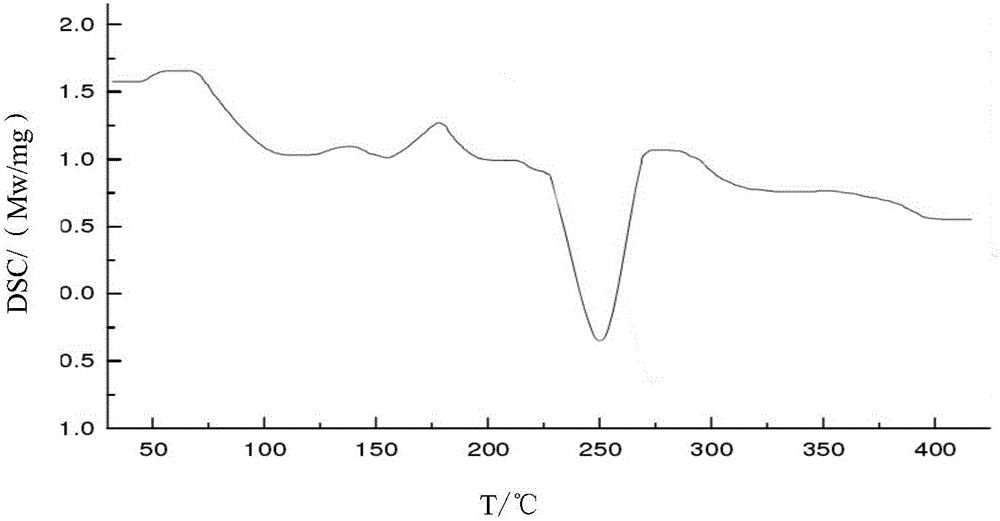
[0030] The X-ray powder diffraction pattern of the product has characteristic peaks at diffraction angles 2θ of 11.46°, 18.47°, 20.85°, 23.21°, and 29.32°. Solid Fourier Transform Infrared Spectrum at a wavenumber of 3311.2cm -1 , 3021.1cm -1 , 1764.2cm -1 , 1684.5cm -1 , 1604.1cm -1 , 1390.4cm -1 , 1351.2cm -1 , 1276.1cm -1 , 1189.1cm -1 , 1060.4cm -1 ,972.4cm -1 ,827.1cm -1 ,774.1cm -1 , 675.4cm -1 There are characteristic peaks. The DSC decomposition temperature is 250.1°C. It is still a white crystalline powder after being placed at a high temperature of 60°C for 10 days. The thermal stability test result at 60°C shows that the weight change rate is 2.1% in 10 days, and the thermal stability is good. Embodiment 2: the preparation of cefradine compound
[0031]Add 50ml of water and 15ml of N-methylacetamide into the reactor, and control the temperature of the water bath at 40°C. Add 10.02 g of crude cephradine into the above mixture, stir continuously until...
Embodiment 3
[0032] The X-ray powder diffraction pattern of the product has characteristic peaks at diffraction angles 2θ of 11.42°, 18.44°, 20.86°, 23.20°, and 29.35°. Solid Fourier Transform Infrared Spectrum at a wavenumber of 3312.3cm -1 , 3021.7cm -1 , 1764.3cm -1 , 1684.4cm -1 , 1604.2cm -1 , 1390.7cm -1 , 1351.3cm -1 , 1276.3cm -1 , 1189.2cm -1 , 1060.5cm -1 ,972.6cm -1 ,827.3cm -1 ,774.4cm -1 , 675.7cm -1 There are characteristic peaks. The DSC decomposition temperature is 250.2°C. It is still a white crystalline powder after being placed at a high temperature of 60°C for 10 days. The result of thermal stability test at 60°C shows that the weight change rate in 10 days is 2.2%, and the thermal stability is good. Embodiment 3: the preparation of cefradine compound
[0033] Add 50ml of water and 15ml of N-methylacetamide into the reactor, and control the temperature of the water bath at 40°C. Add 10.14 g of crude cephradine to the above mixed solution, stir continuou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com